Abstract
Background and objectives:
Imatinib mesylate is approved for the treatment of Chronic Myeloid Leukemia (CML). About 20% of patients with CML do not respond to treatment with Imatinib either initially or because of acquired resistance. In addition to mutated BCR-ABL1 kinase, the organic cation transporter1 (OCT1, encoded by SLC22A1) has been considered to contribute to Imatinib resistance in patients with chronic myeloid leukemia (CML). OCT1 has been reported to be the main influx transporter involved in Imatinib uptake into CML cells. To date, only a few studies have been reported on involvement of influx transporters in development of Imatinib resistance. Therefore this study was aimed to determine the expression level of Imatinib uptake transporter (OCT1) in CML patients and to correlate this level with molecular response.
Methods:
One hundred fifty eight patients on Imatinib were considered for gene expression analysis study for OCT1 gene. Total RNA was extracted from peripheral blood mononuclear cells. Complementary DNAs (cDNAs) were synthesized and Real Time Polymerase Chain Reaction (RQ-PCR) was performed.
Results:
High OCT1 expression was present in 81 (51.8%) patients and low OCT1 expression was in 77 (48.7%) patients. Low Sokal risk score group have a significantly high OCT1 expression (p=0.048). The rate of molecular response was higher in those with high OCT1 expression than in those with low OCT1 expression (p=0.05). Both event-free survival and median overall survival were significantly shorter in patients with low OCT1 expressions when compared to the patients with high OCT1 expression (p=0.03 and p=0.05).
Conclusions:
Our findings demonstrated that the mRNA expression level of OCT1 was significantly correlated with molecular response in CML patients. Based on these findings, present study believes that the pre-therapeutic higher expression of OCT1 may help to predict response to imatinib therapy in CML patients.
Keywords: CML, Imatinib, MMR
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder, and it was one of the first diseases to be associated with a chromosomal alteration, the Philadelphia (Ph) chromosome. BCR-ABL fusion gene arises from the reciprocally translocation between the chromosome 9 and 22, which encodes an oncoprotein that exhibits constitutively active tyrosine kinase activity. Imatinib mesylate (trade name Glivec, formerly STI571; Novartis AG, Basel, Switzerland) is the first-line therapy for CML that potently inhibits the tyrosine kinase activity of BCR-ABL (Schindler et al., 2000; Frazer et al., 2007).
Besides the good results obtained with Imatinib, the number of cases of resistance to this TKI has increased, due to several causes. The mechanisms involved in resistance to Imatinib are usually divided according to its dependence on BCR-ABL fusion gene. Point mutations, over-expression and gene amplifications are included in the group of BCR-ABL dependent mechanisms. The other mechanisms comprise those independents of fusion gene, in particular those factors associated to drug metabolism and drug transport to inward and outward of the target cells (Milojkovic et al., 2009; Quintás-Cardama et al., 2009). Particularly, the P-glycoprotein (P-gP, ABCB1 or MDR1-Multi Drug Resistance) and the BCRP –breast cancer related protein (ABCG2) is the efflux drug transporters that extrude Imatinib from the interior of the cell (Thomas et al., 2004). Additionally to drug efflux, the entrance of Imatinib into the cell is a membrane transporter dependent process, namely OCT1 (organic cation transporter 1) and OCTN2 (organic cation/carnitine transporter). Human organic cation transporter (hOCT1), is a member of solute carrier 22 (SLC22) superfamily is one of the influex transporters involved in the entrance of Imatinib inside the cells (White et al., 2006). OCT1 proteins are involved in the transportation of endogenous and exogenous cationic substrates at physiologic pH levels. A previous study reported that Imatinib mainly enters the cell through OCT1 (Thomas et al., 2004). An altered expression of OCT1 and other drug transporters may be an important factor in determining intracellular levels of the drug and, hence, affecting cell response to Imatinib (Crossman et al., 2005).
Since OCT1 actively transports Imatinib into cells, patients with low baseline expression of OCT1 may be unable to achieve adequate intracellular concentrations of Imatinib, and hence fail to achieve a cytogenetic and molecular response (Hu et al., 2008). It is also found that the patients with high pre treatment OCT1 expression had superior CCyR rates, progression free and overall survival. The pretreatment OCT1 expression was the most powerful predictor of CCyR achievement at six months (Wang et al., 2008). This study aimed to investigate the expression levels of OCT1 gene in CML patients by quantitative real time polymerase chain reaction (RQ-PCR) and correlate with molecular response.
Materials and Methods
Patients: A total of 158 consecutive Chronic Myeloid Leukemia (CML) patients attending the Haematology Out Patient Department (OPD) of the AIIMS, New Delhi from July 2010 to July 2014 were the subjects of the study. The baseline characteristics included age, sex, haemoglobin level, TLC, DLC with blast count and basophils, platelet count, and Sokal score. Twenty healthy individuals from the laboratory staff were taken as controls. Informed written consent was taken from all the patients and controls for participation in this study. The study protocol was approved by the Ethics Committee of the institution.
All the patients were divided into two groups: responders (n=108) and non-responders (n=50). Responders: Patients who achieved a complete molecular response (CMR) or a major molecular response (MMR) (BCR/ABL: ABL ratio <1% as assessed by RQ-PCR). Non-responders: Those without CMR or MMR (BCR/ABL: ABL ratio ≥ 1% as assessed by RQPCR). For BCR-ABL quantification, the plasmid DNA standards having copy numbers 5x103, 5x104, 5x105, 5x106, 5x107 were used as calibrators for generation of standard curves for both BCR-ABL and ABL. RQ-PCR results were analyzed using the standard curves which were used to calculate the quantity of BCR-ABL and ABL transcript (copy numbers) in the samples. Change in the BCR-ABL transcript level was expressed as the ratio of BCR-ABL/ABL (Chuah et al., 2009).
RNA Extraction and Complementary DNA (cDNA) Synthesis: Mononuclear cells were obtained from 5- to 8-mL peripheral blood samples using Ficoll Hypaque density gradient centrifugation. Total RNA was extracted from 4 to 6 × 106 cells per sample using trizol reagent (Life Techonologies Corporation, Carlsbad, CA). The quality and quantity of extracted RNAs were analyzed using electrophoresis and optical density (OD) measurement at 260 to 280 nm; cDNA synthesis was performed via a RevertAid first strand cDNA synthesis kit (Fermentas International Inc, Burlington, ON, Canada). Afterwards, 1 μL of RNA was reverse transcripted to cDNA and added to the 20-μL final volume, which included 1μl M-MLV RT (200 ug/μL), 4 μL 1 × buffer, 2 μL random hexamer primer (500 ng/μL), 2 μl dNTP mix (10 mmol), 1μl RNase inhibitor (40 ug/μL), and 9μl diethylpyrocarbonate (DEPC)–treated water.
SYBER-Green Real-time RT-PCR: For assaying gene expression Fast Start SYBR Green Master Mix (Roche Diagnostics, Indianapolis, IN) and the Corbett Rotor-Gene 6000 system (QIAGEN, Valencia, CA) were used. PCR was performed according to the protocol specified by the manufacturer. PCR amplification was performed with the following primers OCT1 (Forward) 5’GGGCAGCCTGCCTCGTC ATG-3’, OCT1 (Reverse) 5’ACCTCCCTC AGCCTGAA GAC-3’, β-actin (Forward) 5’-GCTGTGCTACGTCG CCCTG-3’,β-actin (Reverse) 5’-GGAGGAGCTG GAAGCAGCC-3’ (White et al., 2005). Each reaction had a final volume of 20 μL, containing 10 μL of SYBR Green PCR Master Mix, 300 nM of each type of primer, 2 μL cDNA, and 6.4 μL DEPC-treated water. To optimize the conditions, serial dilution of cDNA in distilled water was performed. PCR was performed at 95°C for 15 min and was followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 57°C for 30 sec, and extension at 60°C for 1 min. The specificity of the amplified product was verified by melting curve analysis. β-actin gene expression was used as endogenous control for data normalization.
Statistical Analysis
All statistical analysis was performed using STATA 11 (College Station, TX, USA). Survival curves were estimated according to the method of Kaplan and Meier, and statistical differences between curves were assessed by the log-rank test. In case of categorical parameters, chi-square, Fisher’s exact test was used to compare the groups. p≤0.05 was regarded to have statistical significance. Overall survival was defined as the time elapsed between diagnosis and death or last follow-up and event free survival was defined as loss of response, progression to AP/BP on Imatinib and death or last follow-up.
Results
A total of 158 CML patients were analyzed. There were 98 (62%) male and 60 (37%) female patients (M:F::1.6:1). Median age at the time of presentation was 35 years with age ranging from 18-70 years. All these patients received Imatinib provided free by the Max Foundation as part of the Imatinib International Patient Assistance Program (GIPAP). Patients in the chronic phase received Imatinib 400 mg daily, and those with advanced disease received 600–800 mg daily All these patients were diagnosed as having CML according to the WHO criteria. CML-CP was diagnosed in 124, CML-AP in 20 and CML-BC in 14 patients. Median haemoglobin (Hb) was 9.5 g/L (range of 4.2–16.3 g/L), median total leukocyte count (TLC) was 174 x109/L (range 0.9-653x109/L) and median platelet count was 289 x109/L (range 13–1056 x109/L). The average bone marrow blast percentage was 2 (range 0-60) in all the patients According to Sokal score based on age, spleen, platelets and blast count, 46% patients were categorised in low, 36% in intermediate and 17% patients were in high-risk groups respectively Low LAP (Leukocyte Alkaline Phosphatase) score was present in 73% patients. The median follow-up of all patients was median 38 months.
Of 158 cases analyzed, 108 patients who achieved either CMR or MMR were included in responders category, while 50 patients who did not achieved either CMR or MMR were included in non-responders. The transcript expression levels of OCT1 genes were compared between responders and non-responders categories of CML patients. OCT1 expression was analyzed by RQ- PCR (Figure 1(a) and Figure 1(b). Out of 158 patients 81 (51.8%) had high OCT1 expression and 77 (48.7%) had low OCT1 expression.
Figure 1.
Amplification Plot of OCT1 Gene of CML Patients
There was no difference in the hematological parameters in the patients with high OCT1 expression and in patients with low OCT1 expression. Based on Sokal risk score 81 patients were evaluated for prognosis in high OCT1expression group. In the high OCT1 expression group, 11 (13%) belonged to high-risk group, 25 (30%) patients to intermediate-risk group and 45 (55%) patients to low- risk group. Patients with high OCT1 expression had significant higher Sokal scores than those with low OCT1 expression (p < 0.048) (Table 1).
Table 1.
OCT1 Expression and Its Correlation with Hematological Characteristics
| Characteristics | High expression (n=81) (%) | Low expression (n=77) (%) | p value |
|---|---|---|---|
| Median age (years) (Range)* | 35 (20-70) | 35 (18-70) | 0.167 |
| Age Category | 0.62 | ||
| ≤40 | 54 (66) | 48 (62) | |
| ≥40 | 27 (33) | 29 (37) | |
| *Hemoglobin (g/dl) (Range) | 9 (4.8-13.1) | 8.9 (4.2-16.3) | 0.142 |
| Hb Category | |||
| ≤12 (g/dl) | 75 (92) | 71 (92) | 0.581 |
| ≥ 12 (g/dl) | 6 (7) | 6 (8) | |
| *WBC (X109/ L) (Range) | 174 (.9-520) | 173 (4.3-653) | 0.698 |
| WBC Category | 0.151 | ||
| ≤50 | 9 (11) | 14 (18) | |
| ≥ 50 | 72 (88) | 63 (81) | |
| *Platelet (X109/ L) (Range) | 289 (30-974) | 206 (13-1056) | 0.324 |
| Platelet Category | 0.103 | ||
| ≤450 | 56 (69) | 61 (79) | |
| ≥450 | 25 (30) | 16 (20) |
There was a significant difference observed between patients with higher OCT1 had significantly high event free survival as compared to patients with low OCT1 expression (p= 0.0315). The probability of event free survival of patients with high OCT1 expression at 24 and 36 months was 0.65 (95% CI, 0.53-0.75) and 0.59 (95% CI, 0.46-0.70) respectively. Similarly, the probability of event free survival of patients with low OCT1 expression at 24 and 36 months was 0.75 (95% CI, 0.63-0.84) and 0.64 (95% CI, 0.39-0.81) respectively (Figure 2).
Figure 2.
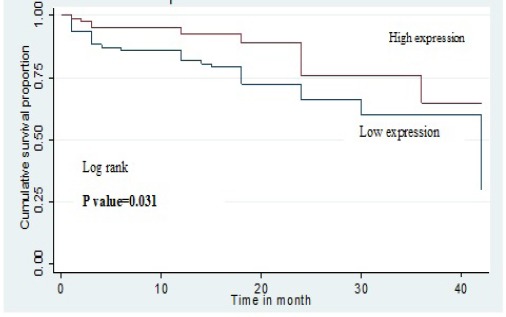
Kaplan–Meier Curve Shows the Comparison of Event Free Survival (EFS) with OCT1 Expression
Out of 158 patients studied 15 patients died during the follow up. The median overall survival was significantly shorter in patients with low OCT1 expression when compared to the patients with high OCT1 expression. The death of the patients in different subgroups is as follows: CP-3/124 (2.4%), AP-2/20 (10%) and BC-10/14 (71.4%). The patients with higher OCT1 had significantly higher overall survival as compared to patient with low OCT1 expression (p= 0.05). The probability of overall survival of patients with high OCT1 expression at 24 and 36 months was 0.87 (95% CI, 0.77-0.93) and 0.81 (95% CI, 0.64-0.91) respectively. Similarly, the probability of overall survival of patients with low OCT1 expression at 24 and 36 months was 0.96 (95% CI, 0.88-0.98) and 0.88 (95% CI, 0.62-0.97) respectively (Figure 3).
Figure 3.
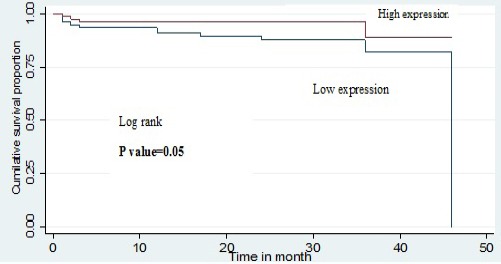
Kaplan–Meier Curve Shows the Comparison of Overall Survival (OS) with OCT1 Expression
We observed that patients who had higher OCT1 expression showed significantly better molecular response as compared to those with low OCT1 expression (p=0.05). Moreover Responder patients had significantly high OCT1expression as compared to non responders (p=0.018) (Table 2).
Table 2.
OCT1 Expression According to Treatment Outcome of Imatinib
| Characteristics | High expression (n=81) (%) | Low expression (n=77) (%) | p value |
|---|---|---|---|
| Gender | |||
| Male | 52 (64) | 46(59) | 0.34 |
| Female | 29 (35) | 31 (40) | |
| CML Phase | |||
| CP | 68 (83) | 56 (72) | 0.145 |
| AP | 9 (11) | 11 (14) | |
| BC | 4 (4) | 10 (12) | |
| Sokal score | |||
| High | 11 (13) | 17 (22) | 0.048 |
| Intermediate | 25 (30) | 32 (41) | |
| low | 45 (55) | 28 (36) | |
| Hematological Response | 0.062 | ||
| Complete | 74 (91) | 63 (81) | |
| Partial | 7 (8) | 14 (18) | |
| Molecular Response | 30 (37) | 18 (23) | 0.05 |
| Complete | 32 (39) | 28 (36) | |
| Major | 19 (23) | 31 (40) | |
| Poor | |||
| Gleevec (mg/day) | 0.283 | ||
| 400 | 70 (86) | 63 (81) | |
| 600 or 800 | 11 (13) | 14 (18) | |
| Response to Therapy | 0.018 | ||
| Responder | 62 (76) | 46 (59) | |
| Non Responder | 19 (23) | 31 (40) | |
* Wilcoxon rank sum test applied; Fisher’s exact /Chi-squire test applied.
Discussion
Introduction of Imatinib and other tyrosine kinase inhibitors has radically improved the outcome of patients with CML and some other diseases with BCR/ABL expression. However, a fraction of CML patients presents with resistance to this drug. Primary imatinib resistance occurs from the onset of therapy, where a lack of imatinib efficacy results in the failure to achieve adequate kinase inhibition, whereas secondary (or ‘acquired’) resistance occurs after the initial achievement of some degree of response, and can be a consequence of multiple factors, with BCR-ABL1 kinase domain mutations frequently having a substantial role (Kantarjian et al., 2003). Importantly, alterations to the expression and activity of key human drug transporters are increasingly recognized as playing a major role in the ability of TKIs to successfully enter and be retained within leukaemic cells and to be related to intrinsic or primary resistance (White et al., 2006; Crossman et al., 2005). One such transporter is the human organic cation transporter 1 (OCT1; SLC22A1), which has been associated with the active transport of imatinib into leukaemic cells (White et al., 2006). The involvement of OCT1 in mediating imatinib transport was first suggested by Thomas et al., (2004) who concluded that imatinib transport into CML cells was predominantly by a temperature-dependent, active transport mechanism, likely to be mediated by OCT1. Measuring the functional activity of the OCT1 protein in leukaemic patient cells at diagnosis (OCT1 activity) is an excellent prognostic indicator for both short- and long-term patient response (White et al., 2007; White et al., 2010). In addition, a number of studies have identified OCT1 mRNA levels to be predictive of patient outcome, with higher mRNA levels correlating with improved patient response (Crossman et al., 2005; Marin et al., 2010; Nardinelli et al., 2012; Zhong et al., 2012; Gromicho et al., 2013). To provide proof in principal of the role of OCT1 in imatinib uptake, Wang et al., (2008)generated an OCT1 overexpressing KCL22 cell line and demonstrated that the intracellular concentration of imatinib was increased in cells with high OCT1 mRNA expression, compared with control cells. The authors also identified a significant correlation between high pre-treatment OCT1 mRNA expression and superior imatinib response (Giannoudis et al., 2013).
This study demonstrated that the patients who had high OCT1 expression showed complete hematological response but the difference was not statistically significant. However, CML patients with high OCT1 transcript expression at the time of diagnosis had a rapid and considerably significant cumulative molecular response compared to those with low OCT1 mRNA expression (p=0.05). Similarly, Nardinelli et al. reported that high OCT1 mRNA expression significantly correlated with MMR achievement at 12, 18 and 60 months of Imatinib therapy (Nardinelli et al., 2012). Zhong et al., (2012)reported significantly increased OCT1 expression level in patients who achieved CCyR as compared to those who were resistant to Imatinib. Marin et al. reported that high levels of OCT1 transcript in untreated patients with early CP at the time of diagnosis significantly predicts the achievement of MMR and CMR within 6 years of Imatinib therapy. Crossman et al., (2005) also reported that high OCT1 mRNA expression prior to Imatinib therapy was associated with an improved 12-month CCyR rate as compared to patients with low OCT1 mRNA expression
In present study, no association was found between OCT1 expression and age, hemoglobin, TLC counts, and platelet counts, but it occurred more frequently in patients with high OCT1 expression. However, patients with high OCT1 expression had low Sokal risk score (p=0.048). The results reported in this current study that patients with high OCT1 expression had also significantly superior event free survival (p=0.03) and overall survival (p=0.05) as compared to those with low OCT1 mRNA. We also compared the expression levels of OCT1 transporter gene between responders and non responders group and found that responders group patients had significantly high OCT1 expression as compared to non responders group (p=0.018). Likewise results reported by Gromicho et al., (2013) who reported significantly increased OCT1 expression level in patients sensitive to Imatinib. Crossman et al., (2005) subsequently investigated whether OCT1 mRNA expression correlated with imatinib resistance, demonstrating increased OCT1 mRNA in imatinib responders, compared with non-responders.
Although not all studies found a significant correlation (White et al., 2007; White et al., 2010; Zhang et al., 2009; de Lima et al., 2014; Kim et al., 2014). De Lima et al., (2014) reported no significant correlation with Imatinib response when responders were compared to non-responders; however, high OCT1 mRNA expression within the responder cohort significantly correlated with MMR. Interestingly, although Kim et al., (2014) were unable to identify a correlation between imatinib response and OCT1 mRNA expression in imatinib-naïve patients, they did observe significantly decreased OCT1 mRNA expression at the time of imatinib resistance, compared with pre-imatinib therapy, suggesting a possible role for OCT1 expression in imatinib resistance. A study by Zhang et al., (2009) found no significant correlation with Imatinib response. Additionally, in a study by Nies et al., (2014) it has been reported that the cellular uptake of Imatinib is independent of OCT1. Thus, the studies investigating the impact of OCT-1 expression in predicting responses in CML patients present conflicting data.
OCT1 protein activity rather than OCT1 mRNA expression was found to be a strong determinant of responses in CML patients in some studies (White et al., 2007; White et al., 2010). In a study by White et al., (2007) OCT1 activity was measured in pre-therapy blood from CML patients and the patients were then categorized as having high and low OCT1 activity. Of patients with high OCT1 activity, 85 per cent achieved MMR by 24 months, versus 45 per cent with low OCT1 activity. The authors also concluded that OCT1 activity along with plasma Imatinib level that has been found to be a predictor of cytogenetic and molecular response in CML patients in previous studies may help in individualized optimization of Imatinib dose (Picard et al., 2007; Larson et al., 2008; Awidi et al., 2010; Malhotra et al., 2014).
Although we know that patients with low OCT1 expression who are treated with Imatinib frontline have a reduced prospect of achieving a deep molecular response and a higher risk of Imatinib resistance. Based on these findings, present study believes that the pre-therapeutic high OCT1 expression may be beneficial in the treatment of CML patients. Our study report that the decreased expression of OCT1 in CML is associated with a reduction in the intracellular concentration of Imatinib in leukemic cells, leading to poor treatment outcome in Imatinib treated patients. Further prospective studies in larger groups are needed to better define the effect of OCT1 expression on response to Imatinib treatment.
Acknowledgements
The first author Sunita Chhikara acknowledges the Indian Council of Medical Research, New Delhi for the research fellowship.
References
- Awidi A, Ayed AO, Bsoul N, et al. Relationship of serum Imatinib trough level and response in CML patients: long term follow-up. Leuk Res. 2010;34:1573–5. doi: 10.1016/j.leukres.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Chuah C, Melo JV. Targeted treatment of Imatinib -resistant chronic myeloid leukemia: Focus on dasatinib. Onco Targets Ther. 2009;2:83–94. doi: 10.2147/ott.s3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman LC, Druker BJ, Deininger MW, et al. hOCT 1 and resistance to Imatinib. Blood. 2005;106:1133–34. doi: 10.1182/blood-2005-02-0694. [DOI] [PubMed] [Google Scholar]
- de Lima LT, Vivona D, Bueno CT, et al. Reduced ABCG2 and increased SLC22A1 mRNA expression are associated with imatinib response in chronic myeloid leukemia. Med Oncol. 2014;31:851. doi: 10.1007/s12032-014-0851-5. [DOI] [PubMed] [Google Scholar]
- Frazer R, Irvine AE, McMullin MF, et al. Chronic myeloid leukaemia in the 21st cen-tury. Ulst Med J. 2007;76:8–17. [PMC free article] [PubMed] [Google Scholar]
- Giannoudis A, Wang L, Jorgensen AL, et al. The hOCT1 SNPs M420del and M408V alter imatinib uptake and M420del modifies clinical outcome in imatinib-treated chronic myeloid leukemia. Blood. 2013;121:628–37. doi: 10.1182/blood-2012-01-405035. [DOI] [PubMed] [Google Scholar]
- Gromicho M, Magalhaes M, Torres F, et al. Instability of mRNA expression signatures of drug transporters in chronic myeloid leukemia patients resistant to imatinib. Oncol Rep. 2013;29:741–50. doi: 10.3892/or.2012.2153. [DOI] [PubMed] [Google Scholar]
- Hu S, Franke RM, Filipski KK, et al. Interaction of Imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–8. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–5. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee SS, Jeong SH, et al. OCT-1, ABCB1, and ABCG2 expression in imatinib-resistant chronic myeloid leukemia treated with dasatinib or nilotinib. Chonnam Med J. 2014;50:102–11. doi: 10.4068/cmj.2014.50.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RA, Druker BJ, Guilhot F, et al. IRIS (International Randomized Interferon VSST1571 Study Group Imatinib pharmacokinetics and its association with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–8. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- Malhotra H, Sharma P, Bhargava S, et al. Correlation of plasma trough levels of Imatinib with molecular response in patients of chronic myeloid leukemia (CML): A single institution study from India. Leuk Lymphoma. 2014;55:2614–9. doi: 10.3109/10428194.2014.885515. [DOI] [PubMed] [Google Scholar]
- Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–88. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic D, Apperley J. Mechanisms of resistance to Imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–27. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- Nardinelli L, Sanabani SS, Didone A, et al. Pretherapeutic expression of the hOCT1 gene predicts a complete molecular response to imatinib mesylate in chronic-phase chronic myeloid leukemia. Acta Haematol. 2012;127:228–34. doi: 10.1159/000336610. [DOI] [PubMed] [Google Scholar]
- Nies AT, Schaeffeler E, van der Kuip H, et al. Cellular uptake of Imatinib into leukemic cells is independent of human organic cation transporter 1 (OCT1) Clin Cancer Res. 2014;20:985–94. doi: 10.1158/1078-0432.CCR-13-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard S, Titier K, Etienne G, et al. Trough Imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose Imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronicmyeloid leukemia. Blood. 2009;113:1619–30. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, et al. Struc-tural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–42. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Thomas J, Wang L, Clark RE. Active transport of Imatinib intoand out of pcells: implications for drug resistance. Blood. 2004;104:3739–45. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- Wang L, Giannoudis A, Lane S, et al. Expression of the uptake drug transporter hoct1 is an important clinical determinant of the response to Imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008;83:258–64. doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28:2761–67. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of Imatinib but not nilotinib (AMN107): Reduced OCT-1 activity is the cause of low in vitro sensitivity to Imatinib. Blood. 2005;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–72. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Cortes JE, Yao H, et al. Predictors of primary imatinib resistance in chronic myelogenous leukemia are distinct from those in secondary imatinib resistance. J Clin Oncol. 2009;27:3642–49. doi: 10.1200/JCO.2008.19.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JS, Meng FY, Xu D, Zhou HS, Dai M. Correlation between imatinib trough concentration and efficacy in Chinese chronic myelocytic leukemia patients. Acta Haematol. 2012;127:221–7. doi: 10.1159/000336244. [DOI] [PubMed] [Google Scholar]


