Abstract
Background:
In traditional Indian medicine, azadirachta indica (neem) is known for its wide range of medicinal properties. Various parts of neem tree including its fruit, seed, bark, leaves, and root have been shown to possess antiseptic, antiviral, antipyretic, anti-inflammatory, antiulcer, antimalarial, antifungal and anticancer activity.
Materials and Methods:
MCF-7 and MDA MB-231 cells were exposed to various concentrations of 2% ethanolic solution of NSO (1-30 µl/ml) and further processed for cell viability, cell cycle and apoptosis analysis. In addition, cells were analyzed for alteration in Mitochondrial Membrane Potential (MMP) and generation of Reactive Oxygen Species (ROS) using JC-1 and DCFDA staining respectively.
Results:
NSO give 50% inhibition at 10 µl/ml and 20 µl/ml concentration in MCF-7 and MDA MB-231 cells respectively and, arrests cells at G0/G1 phase in both the cell types. There was a significant alteration in mitochondrial membrane potential that leads to the generation of ROS and induction of apoptosis in NSO treated MCF-7 and MDA MB-231 cells.
Conclusion:
The results showed that NSO inhibits the growth of human breast cancer cells via induction of apoptosis and G1 phase arrest. Collectively these results suggest that NSO could potentially be used in the management of breast cancer.
Keywords: Neem seed oil, breast cancer, apoptosis, reactive oxygen species, cell cycle
Introduction
Even after improved intensive treatment, breast cancer is one of the most vital problems and a major cause of mortality in woman worldwide (Siegel et al., 2016). Limitations of modern therapy cannot be ignored because of its substantial side effects, and it is therefore fundamental visualization to investigate the novel agent(s) for breast cancer treatment. MCF-7 (estrogen receptor positive) cells are used not only for basic studies but also a well-established in vitro model system for evaluation of estrogen responsive antineoplastic drugs. MDA MB-231 cell lines are estrogen receptor negative cells, derived from breast adenocarcinoma whose growth is estrogen independent. MDA MB-231 cells are an excellent model system that mimics estrogen independent tumor (Kaushik et al., 2016).
Neem (Azadirachta indica) is the ancient medicinal plant having tremendous potential for various kinds of human ailments including anti-cancer efficacy (Subapriya and Nagini, 2005; Prashant et al., 2007). Neem has been proven effective in several health disorders viz. skin ailments, diabetes, dental and oral problems and gastric ulcers etc. (Charles and Charles, 1992; Bandyopadhyay et al., 2004; Bose et al., 2007; Kochhar et al., 2009). Derivatives of neem such as limonoids, azadirachtin and flavonoids isolated from its various parts are drawing attention due to their antineoplastic properties and immune-modulatory effects (Paul et al., 2011; Babykutty et al., 2012).
Induction of apoptosis is an important characteristic for antitumor activity of several chemotherapeutic agents (Kastan and Bartek, 2004). It has been demonstrated that neem alters cell cycle and induces apoptosis in various carcinoma via both extrinsic and intrinsic apoptotic pathways (Arakaki et al., 2006; Priyadarsini et al., 2010). In the present study, an attempt has been made to evaluate the efficacy of Neem Seed Oil (NSO) on MCF-7 and MDA MB-231 Human Breast Cancer Cells (HBCCs).
Materials and Methods
Reagents
DMEM, streptomycin sulfate, gentamicin sulfate, penicillin G, propidium iodide (PI), Annexin V-FITC apoptosis detection kit, sulforhodamine B (SRB), trypsin, phosphate buffer saline (PBS) were procured from Sigma Chemical Co. (St. Louis, MO, USA). 5,5’6,6’-tetrachloro-1,1’3,3’-tetraethyl-benzimidazolyl carbocyanine chloride (JC-1) was purchased from BioVision Research Products (Mountain View, CA 94043, USA). 2’,7’-Dichlorofluorescein diacetate (DCFDA) was procured from Merck-Calbiochem. Fetal Bovine Serum (FBS) was purchased from GIBCO BRL Laboratories (New York). Neem Seed Oil was purchased from Tansukh Herbals (P). Ltd, Lucknow, India. All the other chemicals and reagents used were of analytical grade.
Cell Culture
MCF-7 and MDA MB-231 cells were procured from the National Centre for Cell Sciences (NCCS), Pune, India. Non-tumorigenic human mammary epithelial cells (HMECs) MCF-10A cells were acquired from American Type Culture Collection (ATCC, Manassas, VA). All the cells were cultured as described previously (Kaushik et al., 2016).
For the experimental purposes, ~70-80% confluent cells were trypsinized and plated in DMEM medium containing antibiotics, FBS and HEPES for 24 h in 5% humidified CO2 incubator at 37 °C. Thereafter, cells were treated with 2% ethanolic solution of Neem Seed Oil (NSO) at various concentrations, as described individually.
Cytotoxicity assay
Sulforhodamine-B (SRB) assay was performed to determine the cytotoxicity of NSO in HBCCs. Briefly, 1.0×104 cells/well were plated in 96 well plate and treated with NSO (1-30 µl/ml) for 48 h. Cells were fixed with 10% chilled Trichloroacetic Acid (TCA), washed with deionized water and air dried. Subsequently, 0.4% SRB solution in 1% glacial acetic acid was added in each well and incubated at room temperature for 30 min. The cells were washed with 1% glacial acetic acid and air dried. Afterward, 10mM Tris was added in each well to solubilize the bound SRB and absorbance was read at 560 nm using SpectraMax M2e Elisa Microplate Reader (Molecular Devices Inc.) (Kaushik et al., 2016).
Cell/Nuclear morphological analysis
For cellular morphological analysis, 0.2×106 cells of each type were plated in 6 well plate in DMEM. After 24 h, MCF-7 cells were treated with 1-20 µl/ml NSO however, MDA MB-231 and MCF-10A cells were treated with 5-25 µl/ml NSO for next 48 h. Thereafter, cells were observed under phase contrast microscope (Nikon Eclipse Ti, Japan) and photographed. To determine nuclear changes, the cells were harvested using 0.1% trypsin-EDTA solution and washed with chilled phosphate buffer saline (PBS, pH 7.4). Subsequently, cells were stained with 1:1 ratio of 10 µg/ml acridine orange (AO) and propidium iodide (PI). Freshly stained cells were transferred onto a glass slide and observed under fluorescence microscope and photographed (Wilkins et al., 2010).
Quantification of cell cycle kinetics and apoptosis
To study cell cycle kinetics and apoptosis, 0.2×106 cells were seeded in 6 well plate and exposed with 1-20 µl/ml NSO (MCF-7); 5-25 µl/ml NSO (MDA MB-231) for 48 h. Cells were harvested using trypsin and washed with PBS followed by permeabilization of cells with chilled 70% ethanol for 30 min at 40 °C. After rewashing with PBS, cell were incubated with PI (40 µg/ml) and RNase (100 µg/ml) and analyzed using Flow Cytometer (Becton-Dickinson) employing CellQuest Software.
Annexin V/PI staining
Annexin V/PI binding assay was used to detect loss of membrane asymmetry and exposure of phosphatidylserine at the cell surface, an early event leading to apoptotic cell death. Briefly, cells (0.2×106 /well) were plated in 6 well plate and exposed to varying doses of NSO for 48 h. After incubation with the drug, cells were harvested, washed with chilled PBS and stained with Annexin V-FITC/PI for 30 min at room temperature. Subsequently, analyzed using Flow Cytometer (Becton-Dickinson) employing CellQuest software (Shyam et al., 2017).
Measurement of Mitochondrial Membrane Potential (Δψm)
Mitochondrial Membrane Potential (Δψm) was quantified using JC-1 dye. Briefly, 0.2×106 cells of each type were plated in a 6 well plate and treated with 1-20 µl/ml NSO (MCF-7); 5-25 µl/ml NSO (MDA MB-231) for 48 h. Subsequently, cells were harvested in chilled PBS containing 1 µM JC-1 and the samples were incubated in the dark at 37 °C for 20 min followed by washing with chilled PBS. Cells were analyzed through flow cytometer (Becton-Dickinson) employing CellQuest software.
Determination of intracellular Reactive Oxygen Species (ROS) generation
Reactive Oxygen Species (ROS) generated upon exposure of NSO was measured using 2’,7’-Dichlorofluorescein Diacetate (DCFDA) staining. Briefly, 0.2×106 MCF-7/MDA MB-231 cells were plated in 6 well plate and exposed to assigned doses of NSO for 48 h. At the end of treatment period, cells were washed with chilled PBS followed by incubation with 10µM DCFDA at 37 °C for 30 min. Thereafter, fluorescence was measured using flow cytometer (Becton-Dickinson) employing CellQuest Software.
HPLC analysis
To check the chemical profile of NSO, we performed HPLC analysis and compared with the external standards of pure Nimbin and Azadirachtin. HPLC system (CBM-20A, Shimadzu Co, Ltd., Japan) equipped with two gradient pump system (LC-20AT, Shimadzu), UV-detector (SPD-10A, Shimadzu), an auto-sample injector (SIL-20A, Shimadzu) and a column oven (35°C CTO-20A, Shimadzu) was used for analysis. 20µl of each sample was injected, and the separation was made on a C18 column (Synergi 4u MA-RP 80A, 150 × 4.6 mm, 4 micron Phenomenex). Elution was done at 40 °C using mobile phase of acetonitrile:water (73:27) mixture at a flow rate of 1.0 ml/min and UV detection at 210 nm (AK Ghimeray, 2009).
Statistical analysis
All the results are expressed as mean ± SD from one of the three similar set of experiments. The level of significance was determined using one way ANOVA. A statistically significant difference was defined as P < 0.05.
Results
NSO inhibits proliferation of MCF-7 and MDA MB-231 cells
Sulforhodamine-B (SRB) assay was employed for cytotoxicity determination. Results clearly depicted the antineoplastic action of NSO in both MCF-7 as well as MDA MB-231 cells (Figure 1 A). NSO (5-30 μl/ml) resulted in significant inhibition of MCF-7 cell growth, approximately 86, 51, 38, 22, 13 and 11% cell viability was obtained in 5, 10, 15, 20, 25 and 30 μl/ml NSO doses respectively. Similarly, MDA MB-231 cells also showed a significant dose-dependent reduction of cell viability after treatment with 10-30 µl/ml NSO. About 88, 81, 66, 44 and 23% viable cells were found at 10, 15, 20, 25 and 30 μl/ml NSO doses respectively. Interestingly, no significant changes in the viability of non-tumorigenic HMECs MCF-10A was found (Figure 1A).
Figure 1.
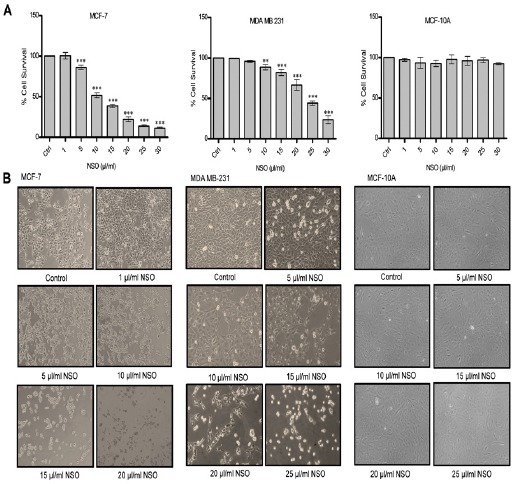
Effect of Neem Seed Oil (NSO) on the Growth of MCF-7, MDA MB-231 and MCF-10A Cells. (A) Evaluation of cytotoxicity using Sulforhodamine B assay (SRB). Data shown are the mean ± SD of one of the three similar experiments each performed in triplicate. * p < 0.05; ** p < 0.01; *** p < 0.001. (B) Morphological analysis using Nikon Eclipsed phase contrast microscope. All pictures are typical of three independent experiments each performed under identical condition.
Cell Morphology
The NSO induced alteration in the morphology of MCF-7 and MDA MB-231 cells after 48 h of treatment were observed and photographed using Nikon inverted phase contrast microscope (Figure 1B). Typical features of cell death like cell shrinkage, detachment of cells from substratum was observed upon NSO treatment in a dose dependent manner. MCF-7 cells treated at the dose of 10, 15, 20 μl/ml NSO displayed considerable cell death compared with control cells. Moreover, MDA MB-231 cells also exhibited the similar extent of cell death at the doses of 15, 20, 25 μl/ml, however, at 5 and 10 μl/ml doses, no significant difference compared to control was observed. No considerable changes were observed in MCF-10A cells exposed to 5-25µl/ml NSO.
Analysis of nuclear morphology
Nuclear morphological analysis was performed using nuclear staining dyes, acridine orange (AO) and propidium iodide (PI). AO generates green fluorescence after binding with nucleic acids and is permeable to both live and dead cells while PI enters only in dead cells and produces red fluorescence. Control cells of both the types showed normal nuclear morphology with uniform green fluorescence while MCF-7 cells treated with 10-20 µl/ml NSO and MDA MB-231 cells treated with 10-25 µl/ml NSO depicted damaged nucleus with both green and red fluorescence (Figure 2A).
Figure 2.
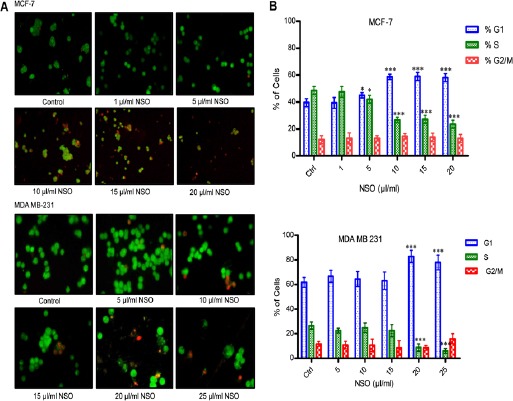
Cell Cycle and Nuclear Morphological Analysis. (A) Nuclear morphological analysis using Nikon eclipsed fluorescence microscope. All pictures are typical of three independent experiments each performed under identical condition. (B) Analysis of cell cycle using Fluorescence Activated Cell Sorter (FACS). Data shown are the mean ± SD of one of the three similar experiments each performed in triplicate. * p < 0.05; ** p < 0.01; *** p < 0.001.
NSO alters cell cycle kinetics
Figure 2B, shows the cell cycle kinetics of MCF-7 and MDA MB-231 cells treated with NSO. The results clearly demonstrate G1 phase arrest of MCF-7 cells at the dose of 5 μl/ml (p < 0.05), 10, 15 and 20 μl/ml (p < 0.001). Similarly, in MDA MB-231 cells, NSO induces G1 phase arrest at the dose of 20 and 25 μl/ml (p < 0.001). However, no significant alteration as compared to control was observed at G2/M phase in both the cells.
NSO induces apoptosis in HBCCs
The NSO mediated induction of apoptosis was evaluated using Annexin V/PI staining. The result showed 4.62, 9.62, 19.78, 29.75% apoptosis at the dose of 5, 10, 15, 20 μl/ml NSO respectively in MCF-7 cells, which is significantly very high compared to only 0.13% in control. Additionally, apoptosis was also observed in MDA MB-231 cells exposed to NSO. Approximately, 8.83, 11.56, 20.87, 32.38 % apoptotic cells were obtained at the dose of 10, 15, 20, 25 μl/ml NSO respectively in MDA MB-231 cells compared to 5.59% in control (Figure 3A and B). The results suggest apoptosis-mediated cell death induced by NSO in HBCCs.
Figure 3.
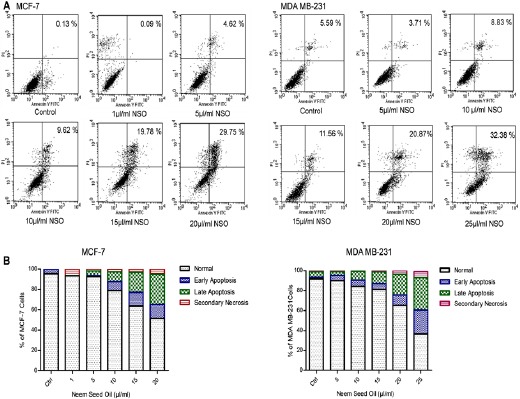
NSO Induces Apoptosis in MCF-7 and MDA MB-231 HBCCs. (A) Analysis of apoptosis using Annexin V/PI staining. Briefly, after 48 h of treatment with NSO, cells were stained with Annexin V/PI and analyzed through flow cytometer. (B) Comparative graphical representation of flow data. Data shown are one of the three similar experiments each performed under identical condition.
NSO modulates generation of Reactive Oxygen Species (ROS) and Mitochondrial Membrane Potential (MMP)
Generation of ROS was evaluated using DCFDA staining (Figure 4A). Significantly higher ROS generation was observed at a dose of 5 μg/ml (p < 0.001) in MCF-7 cells, however, at higher doses, ROS generation was decreased. In contrast, MDA MB-231 cells displayed dose-dependent increase in ROS generation, which was significantly higher at 25 μg/ml NSO (p < 0.001). NSO induced alteration in Mitochondrial Membrane Potential (Δψm) was evaluated using JC-1 dye (Figure 4B). The result illustrates that NSO decreases MMP significantly in a dose-dependent manner in both the cell types (p < 0.001).
Figure 4.
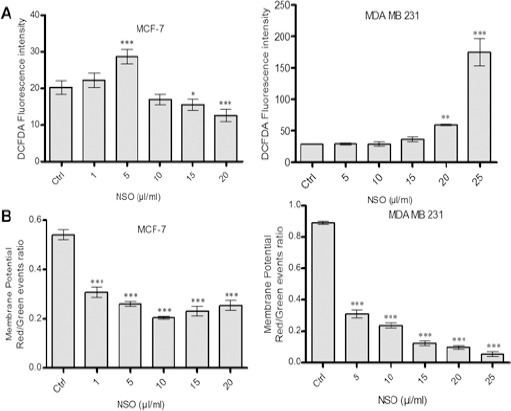
NSO Induces Alteration in Reactive Oxygen Species (ROS) Generation and Mitochondrial Membrane Potential (MMP) in HBCCs. (A) Analysis of ROS generation by DCFDA staining. (B) Detection of MMP by JC-1 staining using Flow cytometry. Data shown are the mean ± SD of one of the three similar experiments each performed in triplicate. * p < 0.05; ** p < 0.01; *** p < 0.001.
HPLC analysis of NSO
HPLC analysis was performed to identify the various components present in NSO and was compared with the azadirachtin and nimbin standards by matching their retention times (RT) and spectra. Results suggest that NSO contains azadirachtin and nimbin with retention time 2.2 and 4.1 min respectively (Figure 5A-D). Additionally, NSO was spiked with the standards which further confirmed the presence of azadirachtin and nimbin in NSO.
Figure 5.
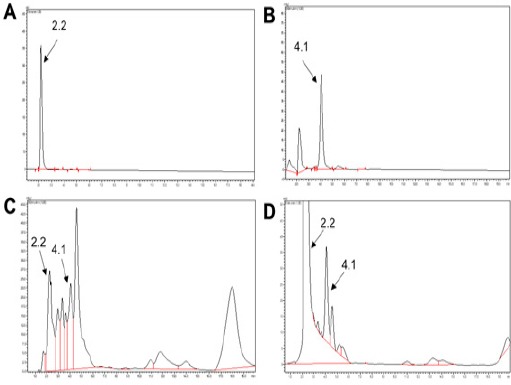
HPLC Analysis of Neem Seed Oil (NSO). (A) Azadirachtin standard with retention time 2.2 min. (B) Nimbin standard with retention time 4.1 min. (C) NSO showing Azadirachtin and Nimbin. (D) Spike chromatogram of NSO with Azadirachtin and Nimbin
Discussion
In spite of the improvement in the diagnosis and treatment of breast cancer, it remains a major health issue for women (Torre et al., 2015). Existing therapies are limited due to substantial adverse effects, therefore, search for novel agents with less or no side effects are warranted in this area. Recently, great interest has raised in developing natural molecules as potential anticancer agents (Aggarwal and Shishodia, 2006). Various parts of the neem tree (Azadirachta indica) have been shown to inhibit the growth of cancer cells through various mechanisms (Hao et al., 2014). Numerous bioactive compounds are also isolated from neem plant, of which azadirachtin and nimbolide are most widely studied. P. Elumalai et al., (2012) have demonstrated that nimbolide induces apoptosis in MCF-7 and MDA MB-231 human breast cancer cells via extrinsic and intrinsic pathways. In this present study, we have taken NSO as it contains azadirachtin and a range of limonoids, stimulating cancer cell death by different cell-death mechanisms. Focus was made on the antiproliferative potential of NSO on MCF-7 and MDA MB-231 human breast cancer cell lines. Uncontrolled cell growth and proliferation leads to cancer, therefore, inhibition of tumor cell growth is a common characteristic of chemotherapeutic agents (Hao et al., 2014). Our results demonstrate that NSO significantly decreases the viability of both estrogen receptor ER+ve/-ve MCF-7/MDA MB-231 cells (Figure 1A and B) without affecting non-tumorigenic HMECs MCF-10A cells, suggesting safety of NSO towards normal cells. It has been found that extracts of neem inhibit the growth of tumor cells by disrupting the cell cycle progression (Priyadarsini et al., 2010). Neem Leaf Extract (NLE) was found to prevent the growth of prostate cancer (Hanahan and Folkman, 1996). Also, Neem Seed Oil (NSO) has been shown to inhibit the growth of HeLa cervical cancer cells (Ricci et al., 2008).
To further analyze the effect of NSO in cell cycle progression, we performed cell cycle analysis using flow cytometry. The results revealed that NSO arrests both MCF-7 and MDA MB-231 cells at the G0/G1 phase of cell cycle (Figure 2B). Other investigators have also reported similar findings. Priyadarsini and coworkers have shown that azadirachtin decreases the levels of cyclin D1, cyclin B and increases the expression CKI p21, which ultimately led to arrest of cell cycle at G0/G1 phase in human cervical cancer (Priyadarsini et al., 2010). In addition to cell inhibition via cell cycle arrest, neem components exert anticancer effect via induction of apoptosis (Hao et al., 2014). For instance, nimbolide induces apoptosis in cervical cancer, breast cancer, colon cancer, prostate cancer, lymphoma, leukemia and hepatocarcinoma (Roy et al., 2007; Harish Kumar et al., 2009; Priyadarsini et al., 2010; Mahapatra et al., 2011; Elumalai et al., 2012; Srivastava et al., 2012). Similarly, azadirachtin induces apoptosis in HeLa cells (Priyadarsini et al., 2010). In the present study, apoptosis was analyzed by Annexin V/PI staining, where it was found that NSO significantly induces apoptosis in MCF-7 and MDA MB-231 cells (Figure 3A and B). Further, AO/PI staining depicted morphological features representative of apoptotic cell death such as alteration in nuclear morphology and detachment of cells from the substratum.
It has been reported that various intrinsic and external factors compromising balance between the anti-apoptotic and pro-apoptotic mechanisms, dysfunction of mitochondrial membrane potential (MMP), caspase activation and generation of reactive oxygen species (ROS) are the significant factors determining apoptosis in tumor cells (D’Autreaux and Toledano, 2007; Wong, 2011). Since the proliferation of cancer cells is rapid and uncontrolled, therefore, it becomes important to understand the mechanism of cell death in cancer prevention and therapy. Thus, to check the involvement of mitochondrial events in the induction of apoptosis JC-1 staining assay was performed and the result illustrates that 1-25 µl/ml NSO significantly decreases MMP in MCF-7 cells. Similar results were obtained when MDA MB-231 cells were exposed with 5-30 µl/ml NSO (Figure 4B). Generation of ROS in controlled manner plays an important role in cell signaling, whereas when unstrained, it has been found to enhance carcinogenesis. However, there is a threshold of ROS in all cells, beyond which leads to cell death. This knowledge has been utilized as a therapeutic strategy for selective killing of cancer cells. Cancer cells normally function at a high level of intrinsic oxidative stress hence, they are more prone to oxidative stress induced apoptosis. Therefore, we next analyzed the generation of ROS using DCFDA staining. The result showed that 5 µl/ml NSO significantly increases ROS in MCF-7 cells after 24 h. However, this effect was observed in MDA MB-231 cells at 20 and 25 µl/ml NSO (Figure 4A), suggesting that ROS generation as an early event in NSO induced apoptosis, which further leads to cell death. Other researchers have also shown the similar results. In cervical cancer, azadirachtin and nimbolide have been found to increase the ROS generation and reduction in MMP with consequent release of cytochrome c (Priyadarsini et al., 2010). Moreover, to check the different components present in NSO, we performed HPLC analysis, and our results revealed that NSO contains azadirachtin and nimbin (Figure 5A-D) which might be responsible for NSO mediated induction of apoptosis. Taken together these results elucidates that NSO induces apoptosis in human breast cancer cells via alteration in mitochondrial membrane potential and G0/G1 phase arrest (Figure 6). Thus, the study suggests that NSO may be used as a potential anticancer agent against breast cancer. However, further in-depth studies are required to identify the specific factors involved in NSO mediated apoptosis.
Figure 6.
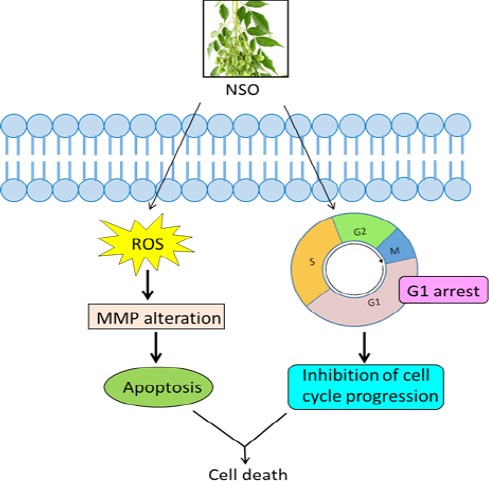
Schematic Diagram Showing NSO Action in HBCCs. NSO Arrests MCF-7 and MDA MB-231 Cells in G0/G1 Phase, Alters MMP and Induces ROS Dependent Apoptosis.
Acknowledgements
Director, CSIR-CDRI is thanked for permitting to carry out the experiments. Mr. A. L. Vishwakarma is thanked for the experiments with flow cytometry. Shweta Kaushik gratefully acknowledges the fellowship received from UGC, New Delhi. The work has been supported by the funds from MLP-0750, CSIR-Central Drug Research Institute, Lucknow, India.
References
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- AK Ghimeray CJ, Ghimire BK, Cho DH. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A Juss grown in foothills of Nepal. Afr J Biotechnol. 2009;8:3084–91. [Google Scholar]
- Arakaki J, Suzui M, Morioka T, et al. Antioxidative and modifying effects of a tropical plant Azadirachta indica (Neem) on azoxymethane-induced preneoplastic lesions in the rat colon. Asian Pac J Cancer Prev. 2006;7:467–71. [PubMed] [Google Scholar]
- Babykutty S, S PP, J NR, et al. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-kappaB in colon cancer cells. Mol Carcinog. 2012;51:475–90. doi: 10.1002/mc.20812. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U, Biswas K, Sengupta A, et al. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 2004;75:2867–78. doi: 10.1016/j.lfs.2004.04.050. [DOI] [PubMed] [Google Scholar]
- Bose A, Haque E, Baral R. Neem leaf preparation induces apoptosis of tumor cells by releasing cytotoxic cytokines from human peripheral blood mononuclear cells. Phytother Res. 2007;21:914–20. doi: 10.1002/ptr.2185. [DOI] [PubMed] [Google Scholar]
- Charles V, Charles SX. The use and efficacy of Azadirachta indica ADR (‘Neem’) and Curcuma longa (‘Turmeric’) in scabies A pilot study. Trop Geogr Med. 1992;44:178–81. [PubMed] [Google Scholar]
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Elumalai P, Gunadharini DN, Senthilkumar K, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215:131–42. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hao F, Kumar S, Yadav N, et al. Neem components as potential agents for cancer prevention and treatment. Biochim Biophys Acta. 2014;1846:247–57. doi: 10.1016/j.bbcan.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, et al. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27:246–52. doi: 10.1007/s10637-008-9170-z. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Shyam H, Sharma R, et al. Genistein synergizes centchroman action in human breast cancer cells. Indian J Pharmacol. 2016;48:637–42. doi: 10.4103/0253-7613.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar A, Sharma N, Sachdeva R. Effect of supplementation of tulsi Ocimum sanctum) and neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Ethno-Med. 2009;3:5–9. [Google Scholar]
- Mahapatra S, Karnes RJ, Holmes MW, et al. Novel molecular targets of Azadirachta indica associated with inhibition of tumor growth in prostate cancer. AAPS J. 2011;13:365–77. doi: 10.1208/s12248-011-9279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Prasad M, Sah NK. Anticancer biology of Azadirachta indica L (neem): a mini review. Cancer Biol Ther. 2011;12:467–76. doi: 10.4161/cbt.12.6.16850. [DOI] [PubMed] [Google Scholar]
- Prashant GM, Chandu GN, Murulikrishna KS, et al. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: an in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- Priyadarsini RV, Murugan RS, Sripriya P, et al. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic Res. 2010;44:624–34. doi: 10.3109/10715761003692503. [DOI] [PubMed] [Google Scholar]
- Ricci F, Berardi V, Risuleo G. Differential cytotoxicity of MEX: a component of Neem oil whose action is exerted at the cell membrane level. Molecules. 2008;14:122–32. doi: 10.3390/molecules14010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MK, Kobori M, Takenaka M, et al. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica) Phytother Res. 2007;21:245–50. doi: 10.1002/ptr.2058. [DOI] [PubMed] [Google Scholar]
- Shyam H, Singh N, Kaushik S, et al. Centchroman induces redox-dependent apoptosis and cell-cycle arrest in human endometrial cancer cells. Apoptosis. 2017;22:570–84. doi: 10.1007/s10495-017-1346-6. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Yadav N, Lella R, et al. Neem oil limonoids induces p53-independent apoptosis and autophagy. Carcinogenesis. 2012;33:2199–207. doi: 10.1093/carcin/bgs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149–6. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wilkins HR, Doucet K, Duke V, et al. Estrogen prevents sustained COLO-205 human colon cancer cell growth by inducing apoptosis, decreasing c-myb protein, and decreasing transcription of the anti-apoptotic protein bcl-2. Tumour Biol. 2010;31:16–22. doi: 10.1007/s13277-009-0003-2. [DOI] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]


