Abstract
Medicinal plants are important elements of indigenous medical system that have persisted in developing countries. Many of the botanical chemo-preventions currently used as potent anticancer agents. However, some important anticancer agents are still extracted from plants because they cannot be synthesized chemically on a commercial scale due to their complex structures that often contain several chiral centers. The aim of this study was to test different extracts from the Moringa oleifera leaves (ML), its PLGA-CS-PEG nanocomposites (MLn), as well as root core (Rc) and outer (Ro) parts for activity against hepatocarcinoma HepG2, breast MCF7, and colorectal HCT 116/ Caco-2 cells in vitro. Nano-composites were prepared and characterized. Then, the nanocomposites and the free counterparts were screened on different propagated cancer cell lines. The underlying cytotoxic impact was followed using apoptosis measurements. All extracts kill the different cancer cells with different ratios, but intriguingly, the root core extract could kill the majority of cancer cells (approximately 70-80%), while sparing normal BHK-21 cells with minimal inhibitory effect (approximately 30-40%). Apoptotic cell increment came to confirm the cytotoxic effects of these extracts on HCT 116 cells (Rc: 212% and Ro: 180%, respectively) and HepG2 cells (ML: 567.5% and MLn: 608%, respectively) compared to control (100%) mechanistically wise. Moringa oleifera nanocomposites may have potential for use as a natural source of anti-cancer compounds.
Keywords: Moringa oleifera, apoptosis, cancer, PLGA-CS-PEG nanoparticles
Introduction
Botanical-based medicines, reported in ancient developing nations, like Egypt and China. This medicinal trend provides essential pharmaceutical agents that have been prescribed for numerous disorders over years. In the developed nations, people interested in alternative or natural therapies to avoid the burden impacts and high expenses of chemotherapy so they turned back to nature for a safe and wide alimental therapies (Abd-Rabou, 2011). Many studies have investigated that some naturally occurring phytochemical agents, such as phenolic compounds (e.g. alkaloids and flavonoids), specifically those ingested in the human beings diet, trigger cancer-cell death machineries and can be used as chemopreventive candidates against certain cancerous cell types (Abd-Rabou, 2016; Ahmed et al., 2015; Gao et al., 2002; Goodman, 2000). Moringa oleifera is an important species of the Moringaceae, a monogeneric family. Its tree, the drumstick tree, was native at the sub-Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan. In addition, Moringa oleifera was medically used by the ancient Egyptians and Greeks; after that, it was globally cultivated for spreading its medicinal benefits around the world (Oliveira et al., 1999).
All Moringa parts are edible and have long been utilized by human beings (Fuglie, 1999). Moringa oleifera leaves are well-known to have numerous good biological activities, such as antioxidant, antiatherosclerotic, hypolipidaemic, and preventer of cardiac disorders (Chumark et al., 2008; Iqbal and Bhanger, 2006), immune activator (Faizi et al., 1994), and tumor suppressor (Murakami et al., 1998). Moreover, Moringa leaves have been utilized for treatments of malaria, hypertension, asthma, diabetes, and stomach problems and to expel retained placenta, while a decoction of the root is used to treat malaria. Furthermore, the root and leave extracts showed activity against Trypanosoma brucei (Mekonnen and Gessesse, 1998), the parasite responsible for trypanosomiasis. Research investigators found that cultured HepG2 cell line revealed high sensitivity to the hepatotoxicant Moringa galactosamine when compared with in vivo experiments upon liver cancer-induced models (Mekonnen et al., 2005; Gomez-Lechon et al., 1988).
The levels of anti-oxidants in different parts of Moringa have been recently identified by Tesfay et al. There were significant differences in sugar concentration and anti-oxidant distribution in different parts of Moringa. The sucrose concentration was the dominant carbohydrate produced in different parts, except glucose in plant roots. Raffinose was detected only in leaf, stem and root, whereas the highest anti-oxidant concentration was also observed in: Total anti-oxidant (TAO) (1.8 mg), leaf-ascorbic acid (AsA) (2.0 mg), and total phenols (TP) (64.1 µg); stem-TAO (1.2 mg); root-carotenoids (29.7 mg), and TP (57.3 µg). Although Moringa had substantial amount of total crude protein, leaf (76.1 mg) had the highest concentration (Tesfay et al., 2011).
Cancers are the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.2 million cancer related deaths (Fuglie, 1999). The number of new cases is expected to rise by about 70% over the next 2 decades. Among men, the 5 most common sites of cancer diagnosed were lung, prostate, colorectum, stomach, and liver cancer. Among women the 5 most common sites diagnosed were breast, colorectum, lung, cervix, and stomach cancer (WHO, 2015; De Martel et al., 2012).
Very recently, Al-Asmari et al., (2015) have investigated the remarkable effects of Moringa oleifera leaves and bark on breast MDA-MB-231 and colorectal HCT-8 cancerous cells. They found that Moringa oleifera extracts significantly exhibit apoptosis-mediated cell death and cell cycle arrest associated with remarkable changes in the cell phenotypic properties in both beast MDA-MB-231 and colorectal HCT-8 cancerous cell lines. In addition, the analyses of these extracts using GC-MS indicated considerable compounds with anti-cancer prosperities (Al-Asmari et al., 2015; Al-Sharif et al., 2013; Sui et al., 2005; Matsuda et al., 2007; Peng et al., 2008). On the other hand, Balamurugan et al., (2014) assessed the anti-tumor impact of the Moringa leaf and bark extracts on hepatic cancer cell line (HepG2) using MTT experiment. They found that the leaf crude extract has a significant anticancer effect against HepG2 cells compared to that of the bark extract of Moringa (Balamurugan et al., 2014).
Due to the global ongoing interest in the nanotechnology with potential applications in health and drug delivery for cancer therapy (Shoo et al., 2007; Park et al., 2008), poly D-L-lactide-co-glycolide (PLGA) decorated with chitosan (CS) and polyethylene glycol (PEG), PLGA-CS-PEG, provides unique physicochemical characteristics resulting from the nano-size effect (Sumer and Gao, 2008; Parveen and Sahoo, 2011). The nanoparticles of FDA approved biodegradable polymer, PLGA, is widely used for the delivery of various natural treatments to the target site. However, rapid opsonization by phagocytes is a major challenge for achieving effective drug targeting by PLGA nano-formulation (Gref et al., 1995). Therefore, surface coating by biodegradable and biocompatible polymers with low toxicity such as CS and PEG were used to curb the phagocytic effects and to enhance the longevity of the nanoparticles (Hu et al., 2008; Illum, 1998; Parveen et al., 2010). Intriguingly, the chemical modification of CS with PEG not only improves the biocompatibility of CS (Zhang et al., 2002), but also reduces the adsorption of circulating plasma proteins onto the material surface (Amiji, 1997). This important effect of nanotechnology used in the study to improve the effectiveness of Moringa extracts against cancer cell lines. Moringa oleifera nanocomposites may have potential for use as a natural source of anti-cancer compounds against different cell lines, while sparing normal cells with minimal inhibitory effect.
The aim of this study was to assess the potential anti-cancer effects of Moringa oleifera leaves (ML) as well as root core (Rc) and outer (Ro) parts, grown in Egypt, against liver, breast, and colorectal cancer cells, as well as normal kidney cells. Hence, the novelty of our research is that we have tested the leaves extract-encapsulated PLGA-CS-PEG nanoparticles (MLn) and its free counterpart (ML) in addition to the plant root parts against these cancer types; in addition to, their impacts on sensitive and resistant colorectal cancerous cells.
Materials and Methods
Plant material
The present work was carried out at the Cell Culture Unit of Hormones Department at the National Research Centre “Egypt”. The ethanolic extracts of Moringa oleifera leaves (ML), as well as root core (Rc) and outer (Ro) parts were cordially obtained from the Egyptian Scientific Society of Moringa (ESSM), National Research Centre, Dokki, Giza, Egypt.
Preparation of Moringa leaves extract-loaded PLGA-CS-PEG nanocomposites
A slightly modified method for nano-preparation was used (Parveen and Sahoo, 2011). Briefly, poly D, L-lactide-co-glycolide (PLGA) nanoparticles were prepared by oil-in-water (O/W) single emulsion solvent evaporation method. Briefly, 100 mg of PLGA polymer was dissolved in 3 ml of chloroform to form a primary emulsion which was further emulsified in an aqueous polyvinyl alcohol (PVA) solution (12 ml, 2% w/v) to form an O/W emulsion using a microtip probe sonicator (VC 505, Vibracell Sonics, Newton, USA) set at 55W of energy output for 2 min over an ice bath. For the preparation of polyethylene glycol/ chitosan-blended PLGA (PLGA-CS-PEG) nano-void, 10, 20, or 30% w/w of CS solution in 1% glacial acetic acid and 5, 10, or 15% w/w of 2 kD PEG were added to the aqueous PVA solution prior to emulsification with the PLGA polymer to get PLGA-CS-PEG nano-void. The emulsion was stirred 8 h for the evaporation of the organic solvent. Excess amount of PVA was removed next day by ultracentrifugation at 50, 602×g at 4 °C for 20 min (Sorvall Ultraspeed Centrifuge, USA) followed by washing twice with double distilled water.
For therapeutic applications, Moringa leaves extract (ML)-encapsulated PLGA-CS-PEG nanocomposites (MLn) were prepared in a similar way by adding a certain concentration of ML prior to emulsification. The amount of ML extract was then measured spectrophotometrically (BMG Labtech, Germany) to calculate the entrapment efficiency.
Particle size analysis and zeta potential measurement
Particle size and zeta potential of the PLGA-CS-PEG nanocomposites were determined by photon correlation spectroscopy (PCS) using a Zeta Sizer (Nano ZS, Malvern Instruments, UK), with a red laser of wavelength λo=633 nm (He–Ne, 4.0 Mw). For size measurements, 1 mg of the nanocomposites was dissolved in 1 ml of water which was further diluted 10 times with water and measured for a minimum of 120 s. Similarly for zeta potential measurements, the samples were placed in an electrophoretic cell, where a potential of ±150 mV was established. Nanocomposites were maintained at a constant temperature of 25.0±0.1 °C.
Transmission electron microscopic (TEM)
Particle morphology and size of the nanocomposites were examined by transmission electron microscopy (TEM, Philips CM-10, FEI Inc., Hillsboro, OR, USA). 100 μg/ml of the nano-suspensions were dropped into Formvar-coated copper grids, and after complete drying, the samples were stained using 2% w/v uranyl acetate (Electron Microscopy Services, Ft. Washington, PA). Image capture and analysis was done using Digital Micrograph and Soft Imaging Viewer Software.
In-vitro studies
Cell culture, maintenance, and sub-culture
Human hepatoma HepG2, breast cancer MCF7, colorectal cancer HCT 116 and Caco-2, and normal kidney BHK-21 cell lines were purchased from American Type Culture Collection (ATCC, USA) and cultured using Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute (RPMI-1640) medium, and Hanks’ Modified Eagle (Hanks’ MEM) medium, supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and 1% L-glutamine obtained from Life Technologies, Gibco (Grand Island, NY). Cells were cultured in 5% CO2 at 37oC and then treated with 0.25% (w/v) trypsin/EDTA to affect cell release from the culture flask. After washing the cells with phosphate buffered saline (PBS), cells were suspended in the media.
Drug Screening and cytotoxicity
The anti-cancer activity and the mitochondrial-based cytotoxicity (Van Meerloo et al., 2011) of Moringa nano-leaves extract and its free counterpart and roots core and outer parts were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using HepG2, MCF7, HCT 116, Caco-2, and BHK-21 cell lines. Briefly, the cells were cultured in 96-well plates at a density of 5×103 cells/well. All treatments with different concentrations (0, 20, 40, 60, 80, 100 µM) were added in the media over those cells. Culture media with nano-void and without were added as controls for the treatment-loaded nanocomposites and their free counterparts. After a day incubation, MTT (Sigma) dissolved in PBS was added to each well at a final concentration of 5 mg/ml, and the samples were incubated at 37°C for 4 h. Water-insoluble dark blue formazan crystals that formed during MTT cleavage in actively metabolizing cells were then dissolved in dimethyl sulfoxide (DMSO). Absorbance was measured at 455 nm, using a microplate reader. The cell viability (%) was calculated and compared with the controls.
Apoptosis
Annexin V stained flipped phospholipids on the HCT 116 and HepG2 cell membranes and used in determination of apoptosis after treatment with Rc, Ro, ML, MLn at 24 h of drug incubation. Annexin V apoptosis detection kit purchased from (ThermoFisher Scientific). The Annexin V-based apoptosis of the treated and untreated HCT 116 and HepG2 cancer cells with the IC50s of these formulations were analyzed in triplicate using the fluorescence module of the BMG LABTECH, Germany.
Statistical analysis
Comparisons between nanocomposites and their free counterparts versus controls were made using a two-tailed Student’s t test, and values of P < 0.05 were considered statistically significant.
Results
Particle size and zeta potential measurements
Three different nanocomposites of Moringa leaves (MLn) were prepared by varying the CS and PEG concentrations decorated the PLGA nanoparticles. The MLn (formulation 1; F1) prepared by the modified method mentioned in the methodology part has nano-size (152 ± 6 nm; Figure 1a), nano-stability with zeta potential (-9.73 ± 1.5 mV and polydispersity index (PDI)= 0.105 ± 0.023; Figure 2b), and well-fitted correlation data (Figure 1b and 2a) as illustrated in (Table 1).
Figure 1.
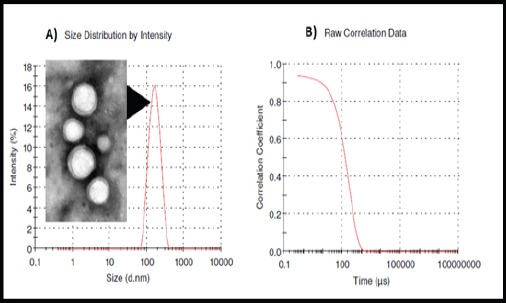
Size Distribution by Intensity and TEM Imaging (a) and raw correlation data (b) of Moringa leaf extract-loaded PLGA PLGA-based nanoparticles (MLn). Size= 152 nm and PDI= 0.105.
Figure 2.
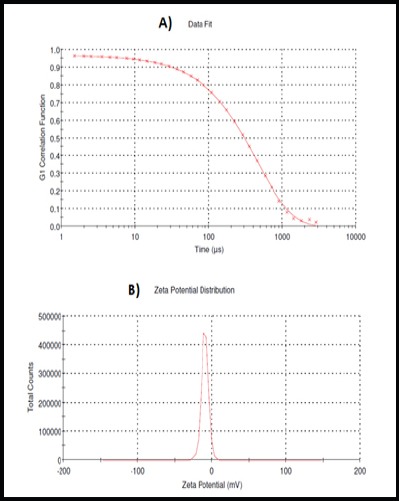
Data Fitting (a) and Zeta Potential (b) of Moringa leaf extract-loaded PLGA-basednanoparticles (MLn). Zeta potential= -9.73 mV.
Table 1.
The Effect of CS: PEG Ratios on the Particle Size and Zeta Potential of Mln-Based PLGA-CS-PEG Nanoparticles (Mean ± S.E., N = 3)
| Formulation | CS (%) | PEG (%) | Nano-Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|
| F1 | 10 | 5 | 152 ± 6 | 0.105 ± 0.023 | -9.73 ± 1.5 |
| F2 | 20 | 10 | 266.5 ± 18.4 | 0.698 ± 0.340 | 12.48 ± 3.19 |
| F3 | 30 | 15 | 376.3 ± 12.8 | 1.00 ± 0.012 | 30.70 ± 4.62 |
Notes: MLn; Moringa leaves extract nanoparticles; PLGA, poly D-L-lactide-co-glycolide; CS, Chitosan; PEG, polyethylene glycol; F1, formula 1 (10% CS + 5% PEG); F2, formula 2 (20% CS + 10% PEG); and F3, formula 3 (30% CS + 15% PEG); PDI, polydispersity index; S.E., standard error
Moringa Rc remarkably induces colon cancer cell death, while sparing normal cells with minimal effect
The cytotoxicity of Moringa leaves extract-loaded PLGA-CS-PEG nanocomposites (MLn) and its free counterpart, as well as the two parts of root extracts was tested over colon cancer HCT 116 and Caco-2 cell lines at different concentrations 0, 20, 40, 60, 80, 100 µM) using MTT assay. Data illustrated in (Figure 3) show the percentage of viabilities of HCT 116 and Caco-2 cells after 24 h incubation with treatments versus controls. The results revealed dose dependent decreases (P < 0.05) in cell viabilities of sensitive colorectal cancer HCT 116 cells to 100 µM MLn reaching 71.2% compared to ML reaching 76.3% HCT 116 cell viability. On the contrary, Caco-2 cells revealed resistant to 100 µM MLn reaching 96.4% compared to ML reaching 75.8% Caco-2 cell viability (Table 2).
Figure 3.
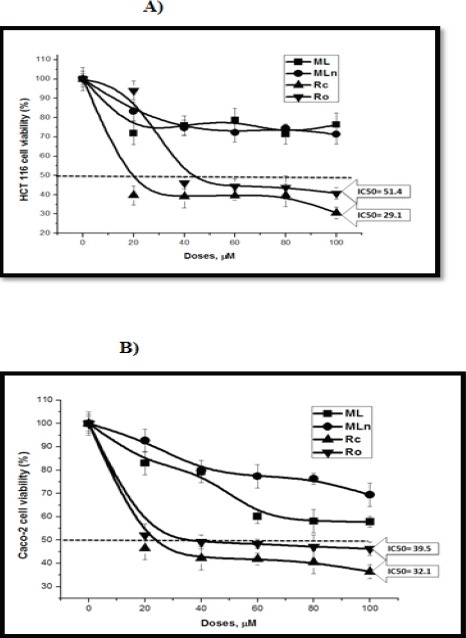
The Cytotoxic Effects of Moringa Extracts on HCT 116 and Caco-2 Colon Cancer Cell Lines. Moringa root core (Rc) and outer crust (Ro) extracts significantly induce colon cancer HCT 116 (a) and Caco-2 (b) cell death compared to Moringa leaf extract-loaded PLGA nanoparticles (MLn) and its free counterpart.
Table 2.
The Inhibitory Effect at the Highest Dose (100µM) of ML and MLn on Various Cell Lines
| Cell lines | Dose (100µM) | |
|---|---|---|
| ML | MLn | |
| Caco-2 | 57.8369 | 69.40428 |
| HCT 116 | 76.37475 | 71.2831 |
| HepG2 | 67.96117 | 66.01942 |
| MCF7 | 74.34944 | 78.06691 |
| BHK | 85.9423 | 71.72744 |
Notes: ML, Moringa leaves extract; MLn, Moringa leaves extract nanoparticles
The results of the cytotoxic effects of Moringa root core and outer parts revealed dose dependent decreases in cell viabilities of HCT 116, Caco-2, and BHK-21 cell lines, with IC50 values (ML: 29.14, 32.16, and >100 µM; MLn: 51.4, 39.5, and 49.7 µM at 24 h, respectively) (Table 3, Figures 3 and 7). When comparing colorectal cancer cells with normal cells, the inhibitory effects of Rc were approximately 3 times over their effects on normal BHK cells, while there were no remarkable differences (P > 0.05) between cancer and normal cells when cells treated with Ro (Table 2).
Table 3.
The Inhibitory Effect (IC50) of Moringa Rc and Ro on Various Cell Lines
| Cell lines | IC50 (µM) | |
|---|---|---|
| Rc | Ro | |
| Caco-2 | 32.16 | 39.51147 |
| HCT 116 | 29.14 | 51.4279 |
| HepG2 | 29.1 | 53.9 |
| MCF7 | 46.15 | > 100 |
| BHK | > 100 | 49.75148 |
| Caco-2 vs BHK-21 | 3.10 ** | 1.25 |
| HCT 116 vs BHK-21 | 3.43 ** | 0.96 |
| HepG2 vs BHK-21 | 3.43 ** | 0.92 |
| MCF7 vs BHK-21 | 2.16 * | 0.49 |
Notes: Rc, Moringa root core; Ro, Moringa root outer; IC50, The half maximal inhibitory concentration
the fold change in the cytotoxic effects of Rc on the cancer cell lines (Caco-2, HCT 116, HepG2) were over 3 times of its effect on the normal BHK-21 one
the fold change in the cytotoxic effect of Rc on the breast cancer MCF7 cells was over 2 times of its effect on the normal BHK-21 one.
Figure 4.
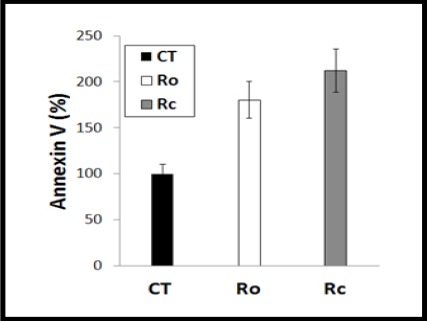
Apoptosis Measurement Using Annexin V. To study the underlying mechanism of colon cancer HCT 116 cell death, Moringa root core (Rc) and outer crust (Ro) extracts were tested upon these cells, showing a significant induction of apoptosis-mediated cell death by both root parts compared to the untreated cells (n=3).
Figure 5.
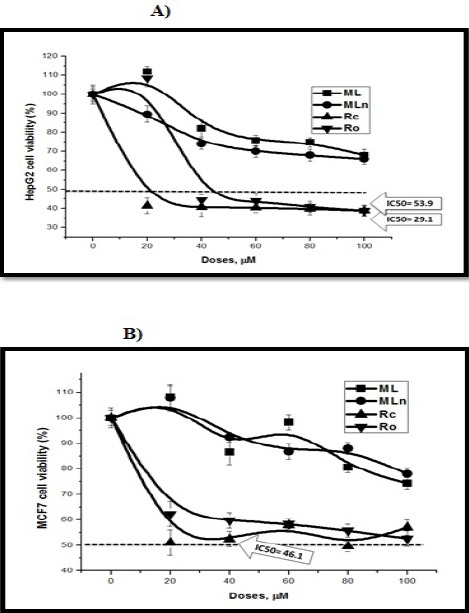
The Cytotoxic Effects of Moringa Extracts on HepG2 and MCF7 Cancer Cell Lines. Moringa root core (Rc) extract significantly induces liver cancer HepG2 (a) and breast cancer MCF7 (b) cytotoxicity compared to Moringa leaf extract-loaded PLGA nanoparticles (MLn) and its free counterpart. While, Moringa root outer crust (Ro) extract significantly induces liver cancer HepG2 (a) cytotoxicity compared to breast cancer MCF7 (b).
Figure 6.
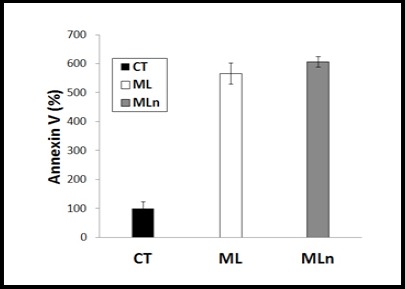
Apoptosis Measurements of ML and MLn Using Annexin V. To study the underlying mechanism of liver cancer HepG2 cell death, Moringa leaves extract-encapsulated nanoparticles (MLn) and its free counterpart (ML) were tested upon HepG2 cell line, showing a significant induction of apoptosis-mediated cell death by both formulations, with a non-significant higher effect of MLn over ML compared to the untreated cells (n=3).
Figure 7.
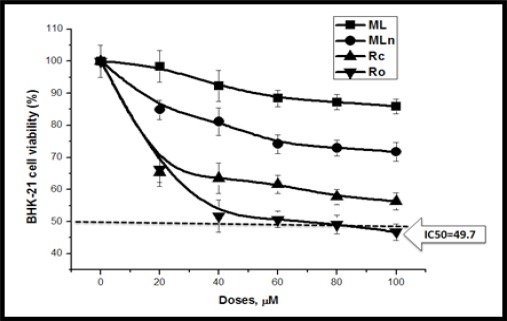
The Cytotoxic Effects of Moringa Extracts on BHK-21 Normal Cell Line. Moringa parts induce slight BHK-21 cytotoxicity compared to other cancer cell lines, while Moringa root outer crust (Ro) extract induces BHK-21 cytotoxicity with IC50= 49.7.
The underlying apoptotic mechanism of Moringa Rc and Ro on HCT 116 cells
Annexin V-based apoptosis in HCT 116 cell line was tested up on 29.14 µM (the IC50 of Rc) and 51.4 µM (the IC50 of Ro) against untreated control cells after 24 h incubation as illustrated in (Figure 4). It was appeared that the underlying mechanism of Rc and Ro was mainly dependent on triggering apoptosis (212% and 180%, respectively) compared to control (100%). Therefore, the analyses for apoptosis measurement using Annexin V showed a significant induction of apoptosis-mediated cell death by both root parts, especially the Moringa Rc, compared to the untreated cells.
Moringa nanocomposites kill liver and breast cancer cells
The cytotoxicity of Moringa leaves extract-loaded PLGA-CS-PEG nanoparticles (MLn) and its free counterpart, as well as the two parts of root extracts was tested over liver cancer HepG2 cell line and breast cancer MCF7 cell line at different concentrations (0, 20, 40, 60, 80, 100 µM) using MTT assay. Data illustrated in (Figure 5) show the percentage of viabilities of HepG2 and MCF7 cells after 24 h incubation with treatments versus controls. The results revealed dose dependent decreases (P < 0.05) in cell viabilities of HepG2 cells reaching 66% at 100 µM MLn compared to ML reaching 67.9% HepG2 cell viability. On the contrary, MCF7 cells revealed a bit resistant to 100 µM MLn reaching 87% compared to ML reaching 74.3% MCF7 cell viability (Table 2).
The results of the cytotoxic effects of Moringa root core and outer parts revealed dose dependent decreases in cell viabilities of HepG2, MCF7, and BHK-21 cell lines, with IC50 values (ML: 29.1, 46.15, and >100 µM; MLn: 53.9, >100, and 49.7 µM at 24 h, respectively) (Table 3). When comparing liver and breast cancer cells with normal BHK-21 cells, the inhibitory effects of Rc were approximately 2-3 times over their effects on normal BHK cells, while there were bit differences between cancer and normal cells when treated with Ro (Table 2).
The underlying apoptotic mechanism of MLn and ML on HepG2 cells
Annexin V-based apoptosis in HepG2 cell line was investigated upon 50 µM of ML and MLn against untreated control cells after 24 h incubation as illustrated in (Figure 6). The underlying mechanism of ML and MLn are mainly dependent on triggering apoptosis with increment percentages (567.5% and 608%, respectively) compared to control (100%). Therefore, the analyses for apoptosis measurement using Annexin V showed a significant induction of apoptosis-mediated cell death by both free and nano-ML formulations, especially the MLn, compared to the control cells.
Discussion
The main obstacle of chemotherapy is that it cannot spare normal cells without damaging, due to it is blinded compounds have no ability to differentiate between tumor and normal cells. This often impacts the efficacy of the therapy, making it impossible to cure patients suffered from cancer. One of the requisite of cancer therapy is cancer cell eradication by cell cycle arrest and/or induction of apoptosis with minimal toxicity up on normal cells (Srivastava and Gupta, 2006; Tian et al., 2006). Moringa oleifera, a common vegetable plant in various countries like Egypt, has many therapeutic compounds with beneficial healthcare features, including anti-cancer (Abd-Rabou et al., 2016) and anti-oxidant properties (Abdull Razis et al., 2014). This plant extracts activate anti-tumor potential by interfering with oncogenesis, cancer cell growth, and progression (Tiloke et al., 2013).
It was reported that Moringa extract had a very elevated antioxidant potentiality with 77% inhibition of free radical production (Khalafalla et al., 2010) and the damageable effect of reactive oxygen species that might help attenuate oxidative stress (Khalafalla et al., 2010; Halliwell and Gutteridge, 1999). This is owing to the presence of flavonoids and phenolics in high levels in the plant extracts. These botanical products have potent medicinal applications, as a group of natural antioxidants with valuable beneficial effects on human beings’ healthcare (Meyer et al., 2003).
In the current study, we indicated that increasing treatment concentrations with Moringa leaves extract and its nanocomposition, decreased the HepG2 cell viabilities by 67.9% and 66%, suggesting that prolonged interaction with this natural product augments the deleterious impact on HepG2 cells. This is in agreement with the study of Mekonnen (2005) who confirmed that this botanical treatment leads to increment of LDH leakage which approved the cytotoxic results, and GSH levels depleted significantly compared to control. On the other hand, authors indicated that the cell death percentage of mononuclear leukemic and HepG2 cells treated with Moringa extract is approximately 75% compared to the control. To test the specific effect of this extract up on AML cells, the same concentrations of Moringa extract were also added up on normal mononuclear cells from healthy volunteers, and they resulted that there was no significant difference in cell vaibilities when comparing those treated with those untreated normal cells (Khalafalla et al., 2010; El-Shemy et al., 2007).
Our results of mitochondrial-based cytotoxicity also revealed a mitochondrial dysfunction over dose increment, which confirmed by Mekonnen (2005) who found that mitochondrial and glycolytic ATP levels in HepG2 cells are decreased up on Moringa extract treatment. Depletion of cellular ATP may have attributed to HepG2 cell injury and death by the included natural products of Moringa leaves, especially at the highest doses. On the contrary, it was reported that the aqueous extract of Moringa stenopetala leaves raises ATP levels at the highest dose, could be explained by the presence of high carbohydrate levels in the extract that may enroll in HepG2 cellular glycolysis and thereafter the ATP production (Kerai and Timbrell, 1997). All the previous experiments are consistent with that the Moringa extracts are cytotoxic to the cancer cells resulting in cell death and loss of ATP as well as GSH. However, it is obscure from the data whether this is a result or a cause of the cancer cell death. Intriguingly, the significant depletion of both ATP and GSH only seems to occur at concentrations of extract that cause LDH leakage and cell viability decrease, owing to loss of the cancer cell membrane integrity and uptake of trypan blue (Guevara et al., 1999; Mekonnen and Gebreyesus, 2000; and Timbrell, 1998).
The intriguing inhibitory effects of Moringa leaves and root extracts on breast MCF7 and colorectal HCT 116/ Caco-2 cells is owing to some natural compounds that are found in Moringa oleifera extracts, including eugenol (Al-Sharif et al., 2013), D-allose (Sui et al., 2005), and isopropyl isothiocynate (Matsuda et al., 2007). The first phenolic natural compound targets E2F1/survivin for effectively killing breast and colorectal cancers. These compounds possess anticancer effect against cancer cell lines. Very recently, Al-Asmari and his companions (Al-Asmari et al., 2015) investigated the anti-cancer effects of Moringa leaves and bark extracts against breast cancer MDA-MB-231 and colorectal cancer HCT-8 cells. In this study, the inhibitory effects of Rc were approximately 3 times over their effects on normal BHK cells when comparing colorectal cancer cells with normal cells, while there were no remarkable differences between cancer and normal cells when cells treated with Ro. Therefore, Moringa Rc remarkably induces colon cancer cell death, while sparing normal cells with minimal effect. This finding is supported by the previous study indicated that D-allose in Moringa extract inhibits the cancer cells proliferation and arrest G1 cell cycle phase through specific thioredoxin interacting protein (TXNIP) induction and subsequent p27kip1 protein stabilization, while sparing normal cells without cytotoxic effects (Yamaguchi et al., 2008). The presence of the organosulphur compound, isothiocynate, in Moringa bark extract attributes to potentiality of its anti-tumor activity. In addition, the existence of palmitic acid in all Moringa extracts selectively targets human leukemic cells (Harada et al., 2001). On the other side, previous studies also showed that eugenol has a strong anti-tumor activity against melanoma (Pisano et al., 2007), osteosarcoma (Shin et al., 2007), leukemia (Yoo et al., 2005), as well as gastric (Manikandan et al., 2011), skin (Pal et al., 2010), and prostate (Ghosh et al., 2009) cancers.
When scientists tested these compounds for killing breast cancer MDA-MB-231 and colon cancer HCT-8 cells, a significant decrease was observed. The effects of the leaves and bark extracts on cell survival were significantly effective within minor variation in the number of viable cells. This variation in the number of viable cells could be due to the differential cellular uptake mechanism in this particular set of experiment. They further found that the cell survival is significantly reduced in the presence of these extracts as compared with the corresponding control. The decrease in cell number can be attributed to the chemical compounds found in the extracts as discussed above, which promote the inhibition of cancer survival protein expression (Al-Sharif et al., 2013). All of the pervious findings approved that our results are in good agreement with previous work demonstrating Moringa extracts possessed anti-cancer characteristics (Jung, 2014).
In the present study, apoptosis in HCT 116 cells was investigated up on 29.14 µM (IC50 of Rc) and 51.4 µM (IC50 of Ro) against untreated control cells after 24 h. The underlying mechanism of Rc and Ro are mainly dependent on triggering apoptosis (212% and 180%, respectively) compared to control (100%). Similarly, apoptosis in HepG2 cells was investigated up on 50 µM of ML and MLn against untreated control cells after 24 h. The underlying mechanism of ML and MLn are mainly dependent on triggering apoptosis with increment percentages (567.5% and 608%, respectively) compared to control (100%). Therefore, the analyses for apoptosis detection showed a significant induction of apoptosis-mediated cell death by Moringa leaves and roots both, especially the MLn and the Rc, compared to the control cells. Our findings are close to Lacroix (2006) who reported the role of eugenol in MDA-MB-231 cancer cell-mediated apoptosis by increasing apoptotic Bax protein expression (Ghosh et al., 2009). Following the same mechanism of apoptosis caused by eugenol, Al-Sharif et al., (2013) recently found the possible mechanism of action of eugenol by down regulating the E2F1 protein which shows promising outcomes in breast cancer.
Our results are also in accordance with the reports (Al-Asmari et al., 2015; Berkovich et al., 2013) obtained on leaves extract, a perceptible increase in total numbers of apoptotic cells was detected when the cells from breast and colorectal cancers were treated with leaves or bark extracts of Moringa oleifera. The rate of increased apoptosis is gradual and also dose dependent. Therefore, the increased apoptotic events in the presence of Moringa extracts could be the manifestation of down regulation of E2F1 and up-regulation of Bax proteins. The overall machinery of reduced cell viability and active participation in normal cellular activity could be attributed to the “shutting down” of various cancer survival pathways including the NF-Kβ signaling cascade by down regulating the important component p65 of NF-kβ by the active compounds of Moringa extracts (Berkovich et al., 2013).
Mechanistically wise, the Moringa extracts delivered into cancer cells using the PLGA nanocapsule labeled with CS and PEG which have a critical role in the protection against rapid phagocytosis to enhance the longevity of the nanoparticles. The surface coating by biodegradable and biocompatible polymers (CS and PEG), with low cytotoxicity, allows a controlled and a sustained release of the extracts causing cancer cell death-mediated apoptosis (Abd-Rabou and Ahmed, 2017). The GC-MS analysis of the Moringa oleifera extracts can confirm the mode of action of the studied extracts, where it revealed many known anti-cancer agents, called isopropyl isothiocynate, eugenol, hexadeconoic acid ethyl ester, and D-allose, all of which possess long chain hydrocarbons, sugar moiety and an aromatic ring. This proposes that the anti-cancer properties of Moringa oleifera could be ascribed to the bioactive agents present in its extracts (Al-Asmari et al., 2015).
In conclusion, the impact of Moringa extracts on different cancer cell lines was investigated showing that Moringa root core (Rc) effectively kills all types of cancer HepG2, MCF7, HCT 116, and Caco-2 cell lines, sparing normal cells with minimal effects. On the other hand, Moringa root outer crust (Ro) effectively kills HepG2, HCT 116, and Caco-2 cell lines, while slightly inhibits breast cancer MCF7 cells.
In summary, Moringa ML, MLn, Rc, and Ro extracts act as anti-cancer agent by decreasing cell proliferation and exhibiting apoptosis-mediated cell death in liver HepG2, colon HCT 116 and Caco-2, and breast MCF7 cancer cell lines, while Rc extract succeeded to spare healthy BHK-21 cell line with minimal cytotoxic effect. Additionally, low cell survival and high apoptosis were detected upon treatment with the extracts of MLn/ML and Rc/Ro compared to control cells. Finally, our findings provide a growing evidence supporting the promising role of Moringa extracts as anti-cancer candidates and open a new vista for molecular analysis of their actions on the predominant signaling mechanisms responsible for cancer development. Therefore, Moringa extracts may represent a valuable therapeutic approach for aggressive breast, liver, and colorectal carcinomas.
Conflict of interest
There is no conflict of interest.
Acknowledgements
We are thankful to the project “Recent approaches in the utilization of Moringa oleifera and Moringa peregrina as a good nutritional, medicinal and industrial plant in Egypt” (ID. 5979), Science and Technology Development Fund (STDF) Academy of Scientific Research of the Ministry of Scientific Research, for partial financial support.
References
- Abd-Rabou AA. Calcium, a cell cycle commander, drives colon cancer cell diffpoptosis. Ind J Clin Biochem. 2016;32:9–18. doi: 10.1007/s12291-016-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Rabou AA, Zoheir K, Ahmed HH. Potential impact of curcumin and taurine on human hepatoma cells using huh-7 cell line. Clin Biochem. 2012;45:1519–21. doi: 10.1016/j.clinbiochem.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Abd-Rabou AA, Ahmed HH. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv Med Sci. 2017;62:357–67. doi: 10.1016/j.advms.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Abd-Rabou AA, Zoheir KMA, Kishta MS, Shalby AB, Ezzo MI. Nano-micelle of Moringa oleifera seed oil triggers mitochondrial cancer cell apoptosis. Asian Pac J Cancer Prev. 2016;17:4929–33. doi: 10.22034/APJCP.2016.17.11.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdull Razis AF, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev. 2014;15:8571–6. doi: 10.7314/apjcp.2014.15.20.8571. [DOI] [PubMed] [Google Scholar]
- Ahmed HH, Abd-Rabou AA, Hassan AZ, Kotob SE. Phytochemical analysis and anti-cancer investigation of Boswellia serrata bioactive constituents in vitro. Asian Pac J Cancer Prev. 2015;16:7179–88. doi: 10.7314/apjcp.2015.16.16.7179. [DOI] [PubMed] [Google Scholar]
- Al-Asmari AK, Albalawi SM, Athar MT, et al. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS One. 2015;10:e0135814. doi: 10.1371/journal.pone.0135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Albalawi SM, Athar MT, et al. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS One. 2015;19:e0135814. doi: 10.1371/journal.pone.0135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sharif I, Remmal A, Aboussekhra A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer. 2013;13:600. doi: 10.1186/1471-2407-13-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiji MM. Synthesis of anionic poly (ethylene glycol) derivative for chitosan surface modification in blood-contacting applications. Carbohydr Polym. 1997;32:193–9. [Google Scholar]
- Balamurugan V, Balakrishnan V, Robinson JP, Ramakrishnan M. Anticancer and apoptosis-inducing effects of Moringa concanensis using hepG2 cell lines. Bangladesh J Pharm. 2014;9:4. [Google Scholar]
- Berkovich L, Earon G, Ron I, et al. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumark P, Khunawat P, Sanvarinda Y, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam Leaves. J Ethnopharmacol. 2008;116:439–46. doi: 10.1016/j.jep.2007.12.010. [DOI] [PubMed] [Google Scholar]
- De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Dilworth C, Timbrell JA. An investigation into the sensitivity of heat shock proteins as markers of cellular damage: a comparative study of hydrazine and cadmium chloride in primary rat hepatocyte cultures. Biomarkers. 1998;3:177–90. doi: 10.1080/135475098231200. [DOI] [PubMed] [Google Scholar]
- El-Shemy HA, Aboul-Enein AM, Aboul-Enein KMI, Fujita K. Willow leaves extracts contains anti-tumor agents effective against three cells types. PLoS One. 2007;2:178. doi: 10.1371/journal.pone.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi S, Siddiqui B, Saleem R, Saddiqui S, Aftab K. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod. 1994;57:1256–61. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- Fuglie LJ. The miracle tree Moringa oleifera: natural nutrition for the tropics church world service, dakar. Revised in 2001 and published as the miracle tree. The Multiple Attributes of Moringa. 1999;68:172. [Google Scholar]
- Gao X, Xu YX, Divine G, et al. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–81. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Ganapathy M, Alworth WL, Chan DC, Kumar AP. Combination of 2-methoxyestradiol (2-ME 2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2009;113:25–35. doi: 10.1016/j.jsbmb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Larrauri A, Donato T, et al. Predictive value of the in vitro test of hepatotoxicity of xenobiotics. In: Guillouzo A, editor. In liver cells and drugs. Paris: Colloque INSERM John Libbey Eurotext Ltd; 1988. pp. 371–7. [Google Scholar]
- Goodman GE. Lung cancer. 1: Prevention of lung cancer. Thorax. 2000;57:994. doi: 10.1136/thorax.57.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Domb A, Quellec P, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Delivery Rev. 1995;16:215–33. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara AP, Vargas C, Sakurai H, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440:181–8. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Third ed. Oxford Clarendon; 1999. pp. 200–783. [Google Scholar]
- Harada H, Yamashita U, Kurihara H, et al. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2001;22:2587–90. [PubMed] [Google Scholar]
- Hu FQ, Meng P, Dai YQ, et al. PEGylated chitosan-based polymer micelle as an intracellular delivery carrier for anti-tumor targeting therapy. Eur J Pharm. Biopharm. 2008;70:749–57. doi: 10.1016/j.ejpb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–31. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compos Anal. 2006;19:544–55. [Google Scholar]
- Jung IL. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS One. 2014;9:e95492. doi: 10.1371/journal.pone.0095492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerai MDJ, Timbrell JA. Effect of fructose on the biochemical toxicity of hydrazine in isolated rat hepatocytes. Toxicol. 1997;120:221–30. doi: 10.1016/s0300-483x(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Khalafalla MM, Abdellatef E, Dafalla HM, et al. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr J Biotechnol. 2010;9:8467–71. [Google Scholar]
- Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- Manikandan P, Vinothini G, Priyadarsini RV, Prathiba D, Nagini S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Invest New Drugs. 2011;29:110–7. doi: 10.1007/s10637-009-9345-2. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Ochi M, Nagatomo A, Yoshikawa M. Effects of allyl isothiocyanate from horseradish on several experimental gastric lesions in rats. Eur J Pharm. 2007;561:172–81. doi: 10.1016/j.ejphar.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Mekonnen A, Gebreyesus T. Chemical investigation of the leaves of Moringa stenopetala. Bull Chem Soc Ethiop. 2000;14:51–5. [Google Scholar]
- Mekonnen N, Houghton P, Timbrell J. The toxicity of extracts of plant parts of Moringa stenopetala in HEPG2 cells in vitro. Phytother Res. 2005;19:870–5. doi: 10.1002/ptr.1720. [DOI] [PubMed] [Google Scholar]
- Mekonnen Y, Gessesse A. Documentation on the uses of Moringa stenopetala and its possible antileshmanial and antifertility effects. Sinet Ethiop J Sci. 1998;21:287–95. [Google Scholar]
- Meyer KJ, Watkins CB, Pritts MP, Liu RH. Antioxidant and antiproliferative activities of strawberries. J Agric Food Chem. 2003;51:6887–92. doi: 10.1021/jf034506n. [DOI] [PubMed] [Google Scholar]
- Murakami A, Kitazonz Y, Jiwajinda S, Koshimizu K, Ohigashi H. Niaziminin, a thio carbamate from the leaves of Moringa oleifera holds a strict structural requirement for inhibition of tumor promoterinduced Epstein-Barr virus activation. Planta Med. 1998;64:319–23. doi: 10.1055/s-2006-957442. [DOI] [PubMed] [Google Scholar]
- Oliveira JTA, Silveira SB, Vasconcelos KM, Cavada BS, Moreira RA. Compositional and nutritional attributes of seeds from the multiple purpose tree Moringa oleifera Lamarck. J Sci Food Agric. 1999;79:815–20. [Google Scholar]
- Pal D, Banerjee S, Mukherjee S, et al. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J Dermat Sci. 2010;59:31–9. doi: 10.1016/j.jdermsci.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee S, Kim JH, et al. Polymeric nanomedicine for cancer therapy. Prog Polym Sci. 2008;33:113–37. [Google Scholar]
- Parveen S, Mitra M, Krishnakumar S, Sahoo SK. Enhanced antiproliferative activity of carboplatin-loaded chitosan–alginate nanoparticles in a retinoblastoma cell line. Acta Biomater. 2010;6:13120–31. doi: 10.1016/j.actbio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharm. 2011;670:372–83. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Peng Y, Bao-An C, De-Long L. Anticancer mechanisms and researches of isothiocyanates. Chinese J Nat Med. 2008;6:325–32. [Google Scholar]
- Pisano M, Pagnan G, Loi M, et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer. 2007;6:8. doi: 10.1186/1476-4598-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Park JH, Kim GC, et al. The mechanism of apoptosis induced by eugenol in human osteosarcoma cells. J Korean Assoc Oral Maxillofac Surg. 2007;33:20–7. [Google Scholar]
- Shoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanotech. 2007;3:20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346:447–53. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- Sui L, Dong Y, Watanabe Y, et al. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int J Oncol. 2005;27:907–12. [PubMed] [Google Scholar]
- Sumer B, Gao J. Therapeutic nanomedicine for cancer. Nanomed. 2008;3:137–40. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- Tesfay SZ, Bertling I, Odindo AO, Workneh TS, Mathaba N. Levels of anti-oxidants in different parts of moringa (Moringa oleifera) seedling. Afr J Agric Res. 2011;6:5123–32. [Google Scholar]
- Tian Z, Lin G, Zheng RX, et al. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J Gastroent. 2006;12:874–9. doi: 10.3748/wjg.v12.i6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiloke C, Phulukdaree A, Chuturgoon AA. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement Altern Med. 2013;13:226. doi: 10.1186/1472-6882-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- WHO cancer updated February 2015 fact sheet N°297. http://www.who.int/mediacentre/factsheets/fs297/en/
- Yamaguchi F, Takata M, Kamitori K, et al. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int J Oncol. 2008;32:377–85. [PubMed] [Google Scholar]
- Yoo CB, Han KT, Cho KS, et al. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005;225:41–52. doi: 10.1016/j.canlet.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li XH, Gong YD, Zhao NM, Zhang XF. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials. 2002;23:2641–8. doi: 10.1016/s0142-9612(01)00403-3. [DOI] [PubMed] [Google Scholar]


