Abstract
Background:
Medullary thyroid cancer (MTC) is an endocrine tumor featuring parafollicular or C-cell differentiation, with calcitonin as a specific biomarker in MTC diagnosis. Germline mutations in the RET proto-oncogene are considered responsible for its familial occurrence and somatic mutations can cause sporadic lesions. MicroRNAs can act as oncogenes or tumor suppressors by inhibiting the expression of target genes.. The aim of this study was to investigate relationships between plasma levels of calcitonin and miRNA323 expression in MTC patients with or without RET mutation.
Methods:
In this cross-sectional study, MTC lesions (based on pathological confirmation) were investigated. Genomic DNA was extracted and Exons 10 and 11 of RET were genotyped using PCR-sequencing. Division was into two groups of 43 cases each with or without mutation. Plasma levels of calcitonin were determined in both.
Results:
miRNA323 was measured using real-time-PCR. After performing normality tests, independent T-tests and Mann Whitney tests were used for the statistical comparison of parametric and nonparametric data, respectively. Plasma levels of calcitonin were significantly higher in MTC cases without a RET mutation compared to those with a mutation.
Conclusion:
There was no significant difference between the two groups regarding the expression of miRNA323 so that this parameter could not be used as a bio-index germ line mutations in MTCs. However, determination of calcitonin levels in plasma might be helpful in this regard.
Keywords: Medullary thyroid carcinoma, RET proto-oncogene, Calcitonin, microRNA-323, FFPE
Introduction
Medullary thyroid carcinoma (MTC) is an endocrine tumor with the differentiation of parafollicular or C-cells that comprises 5-10% of primary malignancies of thyroid (Figlioli et al., 2013; Hedayati et al., 2015), 25% of MTC occurring as hereditary, and 75% as sporadic type (Carlson et al., 1994). Activating mutations in RET proto-oncogene is responsible for the medullary thyroid carcinoma. The familial type of the disease, including Familial medullary thyroid carcinoma (FMTC), and genetic syndromes known as multiple endocrine neoplasia type 2A and B. MEN2A is the prevalent type of the syndrome that is characterized by 95% MTC, 50% pheochromocytoma and hyperparathyroidism while MEN2B is characterized by 90% MTC, 45% pheochromocytoma, 100% ganglioneuromatosis, 65% Marfanoid habitus and eye disorders (Punales et al., 2004). FMTC is defined initially as the presence of solitary MTC in a family and is diagnosed based on the absence of pheochromocytoma or hyperparathyroidism in at least two generations of a family (Punales et al., 2004; Wells et al., 2013).
RET proto-oncogene is located on 10q11.21 chromosomal region and consists of 20 exons (Takahashi et al., 1985; Ceccherini et al., 1993). This gene encodes a membrane tyrosine kinase receptor (Ceccherini et al., 1993). Germ line mutations in RET proto-oncogene is responsible for MTC while somatic mutations in this gene cause sporadic MTC (Scurini et al., 1998). More than 90% of MEN2 syndromes and FMTC families possess missense mutations in one of the conserved cysteine residues in codons 609, 611, 618, and 620 (exon 10) or codon 630 or 634 (in codon 11) in cysteine rich extracellular domain (Santoro et al., 1995; Marsh et al., 1996; Kitamura et al., 1997).
RET germ line mutation detection possess all characteristics of an ideal genetic test for MTC and provides an effective and convenient strategy for the management of affected patients and their families (Yeganeh et al., 2015).
Calcitonin is secreted by C-cells of thyroid gland (Parfitt, 1993). This hormone plays a role in calcium and phosphorous metabolism(Melvin et al., 1971). Calcitonin is a specific biomarker for the diagnosis of C-cell hyperplasia or MTC in which the serum calcitonin levels increase with the severity of diseases (Kihara et al., 2016).
Many other diseases such as chronic renal failure, hyperparathyroidism, neuroendocrine neoplasm, pulmonary and prostate tumors and autoimmune diseases may cause elevation of serum calcitonin (Tashjian et al., 1970; Karanikas et al., 2004) Most SMTC (sporadic MTC) patients exhibit elevated serum calcitonin levels (Dralle et al., 1992; Weber et al., 2001; Scollo et al., 2003; Pacini et al., 2010) and measurement of serum calcitonin is an important follow up parameter following surgery in MTC patients since these biomarkers indicate the probable presence and residual volume of the tumor/disease (Stepanas et al., 1979; Nodules, 2006; Pacini et al., 2008).
Micro-RNAs are a large subgroup of non-coding 18-25 nucleotide RNAs that are evolutionary conserved. These molecules control post transcriptional gene expression through inhibition of mRNA translation or induction of its degradation (Hwang and Mendell, 2006). Micro RNAs can act as oncogene or tumor suppressor gene (He et al., 2005; Negrini et al., 2009). Therefore, micro-RNAs can be used as biomarkers for diagnosis, prediction, and even treatment of disease (Ruan et al., 2009; Cho, 2010). Cancer cells undergo several genetic changes that could affect micro-RNA expression through direct or indirect pathways. Genomic rearrangement, different micro-RNA gene expression, disturbances in epigenetic micro-RNA regulation and gene mutation are examples of these changes (Schaefer et al., 2010).
The aim of this study was to investigate the relationship between levels of plasma calcitonin and the expression of miRNA323 in individuals suffering from MTC with or without mutation in RET proto-oncogene. Since functional studies on miR-323 and its relationship with calcitonin have not been demonstrated, investigating and conducting this study can propose a biochemical marker and molecular index for the prevention and treated of MTC disease.
Materials and Methods
In this cross-sectional study, the studied population consisted of 86 MTC patients diagnosed based on pathologic evidences and undergone surgical operation in training hospitals and medical centers that were enrolled to the study on their own consent. Informed consent was obtained from all individual participants included in the study. A total of individuals were examined for common mutations of RET proto-oncogene. Following peripheral blood sampling, plasma were separated and stored at -20°C. Genomic DNA was then extracted by standard saturated salt/proteinase K method.
Primers (F5´GCGCCCCAGGAGGCTGATGC3´); (R5´CGTGGTGGTCCCGCCGCC3´) and (F5´ CCTCTGCGGTGCCAAGCCTC3´); (R5´CACCGGAAGAGGAGTAGCTG3´) were used for replication of exon 10 and exon 11, respectively.
PCR reaction was performed in 35 µl volume under following condition: Taq DNA Polymerase (0.5U), dNTP (142.85µm), Tris-Hcl pH=9 (5.75Mm), KCl (17.14 Mm), and 1µl of each forward and reverse primers (10pmol/µl), DNA sample 100 ng/µl, and 32 µl of sterile distilled water. The reaction was performed in automatic thermo-cycler (peqSTAR 96X HP, Peqlab Co, Germany).
The condition for exons studied consisted of 30 thermal cycles with the temperature of 94°C for 10 minutes for initial denaturation, 94°C for 45 seconds for the second denaturation, 60°C for 45 seconds for the annealing of primers, 72°C for 30 seconds for extension and 72°C for 10 minutes for final extension.
PCR products were evaluated using 8% polyacrylamide gel and silver nitrate staining, appropriate samples were sequenced for RET mutation detection. Sequencing results were analyzed using Chromas software through performing Blast and comparing with reference sequence (gene bank).
Two groups were selected according to the RET gene mutation: RET positive and RET negative groups. Plasma calcitonin levels were measured in both group using ELIZA according to the kit procedure (human calcitonin Elisa kit, Catalog number: CSB-Eo5131h). Subsequently, 10 individuals were randomly selected from each group for determination of miR-323 expression. miR-323 expression measurement was performed from formalin fixed paraffin embedded (FFPE) tumoral tissue blocks. Hematoxylin and eosin staining (Hand E) were done on the samples. Tissue blocks and their stained slides were placed along with each other and areas more than 70% cell tumors were selected and cut into 8-10µm thick sections. Total RNA was extracted from FFPE tumoral tissue samples according to kit procedure (miRNeasy FFPE Qiagen Germany, cat number: 217504). Purity of extracted RNA was determined by spectrophotometry using NanodropND-1000.
cDNA syn specific primers (miR-323-a-3p and U6) from Pars Genome Company were used for cDNA synthesis. Prior to synthesis of first strand of cDNA poly A tail was added to RNA. The synthesis of first strand of cDNA was performed according to the cDNA synthesis kit procedure (Pars Genome Co, cat number 448702).
miR-323 expression was performed by real time PCR according to the kit procedure (Pars Genome Co, cat number: 448702). Whole PCR process consisted of one cycle of denaturation in 95 ºC for 5 minutes, 95 ºC for 5 seconds and 40 cycles in 65ºC for 20 seconds and 70 ºC for 30 seconds that was performed by Rotor gene-6000. All samples were run in pairs and the mean Ct values were evaluated.
Data was analyzed statistically using Med-Calc software. Data normality was tested by kolmogorov-smirnov test prior to the comparison of calcitonin levels between two groups. Since the data was non parametric, Mann Whitney test was used for the comparison of groups. To compare the expression of miRNA323 between groups independent T-test was performed after testing normality by Shapiro-Wilk test.
Results
The studied population consisted of 86 individual affected with MTC, which was divided into two groups with (W) or without (WO) mutation in RET proto-oncogene, according to the RET proto-oncogene mutation.
A total of 43 patients were included in the group with mutation (W) in exon 10 and 11 (23 male, 20 female; with the mean age of 35 years ranging 12-65. Other 43 patients were included in the group without mutation (WO) in exon 10 and 11 group (13 male, 30 female; the mean age of 43 years ranging 19-71). Following determination of plasma calcitonin, comparison was made between 42 members from W group (22 male, 20 female; average age 35) and 40 members from WO group (12 male, 28 female; average age 42.7).
Plasma levels of calcitonin in individuals of WO was higher than those in W group and the difference was statistically significant (P-value=0.0014). There was no significant difference in plasma calcitonin levels between male and female MTC patients within both W ((P-value=0.48) and WO groups (P-value=0.59).
Male patients had the same levels of calcitonin in plasma in both groups (P-value=0.12) but females in WO group had significantly higher plasma levels of calcitonin compared to those in W group (P-value=0.003).
There was no significant relationship, analyzed by Spearman’s Rho, between age and serum calcitonin in both W and WO groups. To determine the expression of miR-323, 10 individuals from W group (5 male, 5 female; mean age of 40.3 with the range of 29-56) and 10 from WO group (5 male, 5 female; mean age of 30.1 with the range of 22-40) were randomly selected. No significant difference was observed in miR-323 expression between W and WO group (P-value=0.13) (Figure 2). The expression of miR-323 did not differ between male and female within both W (P-value=0.17) and WO groups (P-value=0.42). Male patients in both groups showed the same expression of miR-323 (P-value=0.98) but the expression was greater in females of W group compared to those in WO (P value=0.02). There was no significant relationship between serum calcitonin levels and miR-323 in W group (P value=0.2) as well as in WO (P value=0.6).
Figure 1.
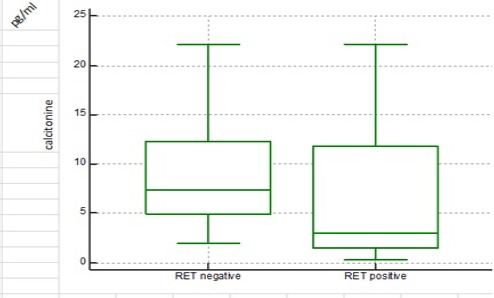
Serum Calcitonin Levels in Medullary Thyroid Carcinoma Patients without Mutation in RET Proto-Oncogene (WO Group) with the Median of (7.35 pg/ml) was Significantly Higher Than Those Patients with Mutation (W Group) with the Median of (3.07)
Figure 2.
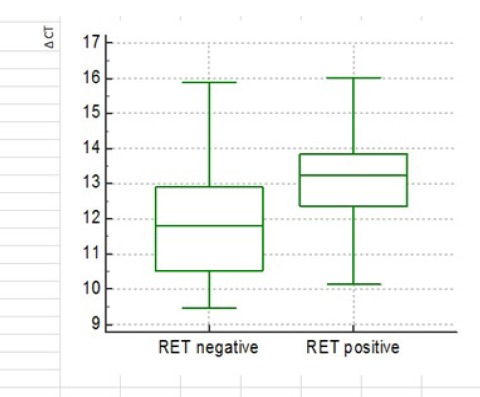
No Significant Difference in Tumor Tissue Expression of miR-323 between MTC Patient with (W) and without (WO) Mutation in RET Proto-Oncogene (P-value=0.13)
Table 1.
According to the Statistical Formula for Calculating Sample Size of Two Group, the Minimum Sample Size of 39 People in Each Group, Considering the 10% Chance of Failing Increased to 43 People in Each Sample Group.
| N | |||||||
|---|---|---|---|---|---|---|---|
| ((Z2)*(s2)/d2)*DE | Z | Z2 | s | s2 | d | d2 | DE |
| 39 | 1.96 | 3.8416 | 23 | 529 | 12.5 | 156.25 | 3 |
| α=0.05 |
Table 2.
Serum Calcitonin Levels in Medullary Thyroid Carcinoma Patients without Mutation in RET Proto-Oncogene (WO Group) with the Median of (7.35 pg/ml) was Significantly Higher Than Those Patients with Mutation (W Group) with the Median of (3.07)
| RET negative | RET positive | |
|---|---|---|
| Sample size | 40 | 42 |
| Lowest value | 2.01 | 0.34 |
| Highest value | 22.1 | 22.1 |
| Median | 7.35 | 3.07 |
| 95% CI for the median | 6.0000 to 10.0322 | 2.0100 to 7.5528 |
| Interquartile range | 4.9000 to 12.3000 | 1.5000 to 11.8000 |
| Mann-Whitney test (independent samples) | ||
| Average rank of first group | 50.125 | |
| Average rank of second group | 33.2857 | |
| Mann-Whitney U | 495 | |
| Test statistic Z (corrected for ties) | 3.201 | |
| Two-tailed probability | P = 0.0014 | |
Discussion
In the present study the plasma levels of calcitonin and miR-323expression in tumor tissue of individuals suffering from MTC with or without mutation in RET proto-oncogene were investigated. The results indicated that plasma calcitonin levels were significantly higher in MTC patients without mutation in RET proto-oncogene compared to those patients with mutation (P-value=0.0014). This result is in agreement with previous finding that in most cases of sporadic medullary thyroid carcinoma (SMTC) serum calcitonin remains elevated (Weber et al., 1992; Dralle et al., 1992).
In the current study, no significant difference was observed in the expression of miR-323 between 10 randomly selected individuals from W and WO groups (P-value=0.13). Limited research has been done on the role of micro-RNA in MTC (Nikiforova et al., 2008; Abraham et al., 2011; Mian et al., 2012). In the present study, miR-323 was considered for investigation since it has been reported as highly expressed micro-RNAs in MTC and review articles support its role in thyroid malignancies while functional studies on this micro-RNA was still lacking(Russo et al., 1997). Other micro-RNAs such as 375, 224, 9*, 129, 10a, 21, 370 have been reported to be dysregulated in MTC indicating the principle role of micro-RNAs in the biology of MTC and their potential as prognosis biomarkers (Nikiforova et al., 2008; Abraham et al., 2011; Mian et al., 2012). Cancer micro-RNAs may be used as biomarkers for diagnosis, prediction or even treatment purposes (Ruan et al., 2009; Cho, 2010).
Although plasma calcitonin concentration is routinely measured in patients with nodular thyroid as a screening procedure(Weber et al., 2009; Kloos et al., 2009) but many other conditions such as chronic renal failure, sepsis, pulmonary or gastrointestinal neuroendocrine tumors, hypergastrinemia, mastocytosis, thyroid autoimmune diseases, and pseudohypoparathyroidism type Ia can cause elevated serum calcitonin (Hegedüs, 2004). Some evidences suggest that calcitonin screening can lead to early diagnosis of MTC (Elisei et al., 2004; Cheung et al., 2008; Costante et al., 2009b). while other studies show that in 20-25% of cases it results in diagnosis of MTC in later stages. In addition, there is no evidence on reduced occurrence of advanced tumors due to calcitonin screening (Costante et al., 2009a; Sama et al., 2016)
Investigating germ line RET mutations possess all characteristics of an ideal genetic test for cancer and provides an effective and convenient strategy for the management of involved people (Yeganeh et al., 2015). With genetic identifying of people having mutation in RET and lacking clinical signs, early diagnosis of the disease could be possible (Marsh et al., 1996).
Regarding the differences in plasma calcitonin concentration between MTC patients with or without mutation in RET proto-oncogene, it might be possible that mutation in these patients affects plasma calcitonin concentration and according to the results of this study, investigating this mutation can potentially influence the process of diagnosis and prognosis of the disease. Additionally, the expression of micro-RNAs are influenced by method of DNA extraction, sample preparation and storage, age, diet, exercise, race, medicines and chemicals which might contribute to differences in miR-323 expression between current and previous studies (Becker and Lockwood, 2013). More functional studies are warranted to investigate the relationship between the expression of RET and RAS mutations and the expression of micro RNAs.
Compliance with Ethical Standards.
Authors’ contributions
The conception and design of the study or acquisition of data: Mehdi Hedayati, Seyed Asadolah Amini.
Acquisition of data
Samira Ehyaei, Marjan Zarif Yeganeh, Sara Sheikholeslami.
Pathological study and confirmation
Mahsa Ahadi.
Analysis and interpretation of data
Marjan Zarif Yeganeh, Sara Sheikholeslami.
Drafting of manuscript
Samira Ehyaei Mehdi Hedayati, Marjan Zarif Yeganeh
Critical revision: Mehdi Hedayati, Seyed Asadolah Amini.
Final approval of the version to be submitted
Marjan Zarif Yeganeh.
This work was supported by the Shahrekord University of Medical Science under Grant number # 1708.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethical approval code in Shahrekord University of Medical Science # 6-9-93) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors do not have any actual or potential conflict of interest of the work submitted. All authors agreed to submit the work to Journal of Cancer Research and Clinical Oncology, and the work has not been submitted to another journal.
Acknowledgments
The authors are grateful to all patients and their relatives who agreed to participate in this work. The research is supported by grants from the Department of clinical biochemistry, Faculty of Medical Sciences, Shahrekord University of Medical Science, Shahrekord, Iran, and Cellular and Molecular Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
References
- Abraham D, Jackson N, Gundara JS, et al. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin Cancer Res. 2011;17:4772–81. doi: 10.1158/1078-0432.CCR-11-0242. [DOI] [PubMed] [Google Scholar]
- Becker N, Lockwood CM. Pre-analytical variables in miRNA analysis. Clin Biochem. 2013;46:861–8. doi: 10.1016/j.clinbiochem.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Carlson KM, Dou S, Chi D, et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A. 1994;91:1579–83. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccherini I, Bocciardi R, Luo Y, et al. Exon structure and flanking intronic sequences of the human RET proto-oncogene. Biochem Biophys Res Commun. 1993;196:1288–95. doi: 10.1006/bbrc.1993.2392. [DOI] [PubMed] [Google Scholar]
- Cheung K, Roman SA, Wang TS, et al. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 2008;93:2173–80. doi: 10.1210/jc.2007-2496. [DOI] [PubMed] [Google Scholar]
- Cho WCS. MicroRNAs in cancer - from research to therapy. Biochim Biophys Acta. 2010;1805:209–17. doi: 10.1016/j.bbcan.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Costante G, Durante C, Francis Z, et al. Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. Nat Clin Pract End Met. 2009a;5:35–44. doi: 10.1038/ncpendmet1023. [DOI] [PubMed] [Google Scholar]
- Costante G, Durante C, Francis Z, et al. Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. N at Clin Pract Endocrinol Metab. 2009b;5:35–44. doi: 10.1038/ncpendmet1023. [DOI] [PubMed] [Google Scholar]
- Dralle H, Damm I, Scheumann GF, et al. Frequency and significance of cervicomediastinal lymph node metastases in medullary thyroid carcinoma: results of a compartment-oriented microdissection method. Henry Ford Hosp Med J. 1992;40:264–7. [PubMed] [Google Scholar]
- Elisei R, Bottici V, Luchetti F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163–8. doi: 10.1210/jc.2003-030550. [DOI] [PubMed] [Google Scholar]
- Figlioli G, Landi S, Romei C, et al. Medullary thyroid carcinoma (MTC) and RET proto-oncogene: mutation spectrum in the familial cases and a meta-analysis of studies on the sporadic form. Mutat Res. 2013;752:36–44. doi: 10.1016/j.mrrev.2012.09.002. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati M, Zarif Yeganeh M, Sheikholeslami S, et al. Medullary thyroid cancer screening using the RET proto oncogene genetic marker. Iran J Endocrinol Metab. 2015;17:157–70. [Google Scholar]
- Hegedüs L. The thyroid nodule. N Engl J Med. 2004;351:1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–80. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas G, Moameni A, Poetzi C, et al. Frequency and relevance of elevated calcitonin levels in patients with neoplastic and nonneoplastic thyroid disease and in healthy subjects. J Clin Endocrinol Metab. 2004;89:515–9. doi: 10.1210/jc.2003-030709. [DOI] [PubMed] [Google Scholar]
- Kihara M, Miyauchi A, Kudo T, et al. Reference values of serum calcitonin with calcium stimulation tests by electrochemiluminescence immunoassay before/after total thyroidectomy in Japanese patients with thyroid diseases other than medullary thyroid carcinoma. Endocr J. 2016;63:627–32. doi: 10.1507/endocrj.EJ16-0107. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Goodfellow PJ, Shimizu K, et al. Novel germline RET proto-oncogene mutations associated with medullary thyroid carcinoma (MTC): mutation analysis in Japanese patients with MTC. Oncogene. 1997;14:3103–6. doi: 10.1038/sj.onc.1201102. [DOI] [PubMed] [Google Scholar]
- Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Andrew SD, Eng C, et al. Germline and somatic mutations in an oncogene: RET mutations in inherited medullary thyroid carcinoma. Cancer Res. 1996;56:1241–3. [PubMed] [Google Scholar]
- Melvin KE, Miller HH, Tashjian AH., Jr Early diagnosis of medullary carcinoma of the thyroid gland by means of calcitonin assay. N Engl J Med. 1971;285:1115–20. doi: 10.1056/NEJM197111112852004. [DOI] [PubMed] [Google Scholar]
- Mian C, Pennelli G, Fassan M, et al. MicroRNA profiles in familial and sporadic medullary thyroid carcinoma: preliminary relationships with RET status and outcome. Thyroid. 2012;22:890–6. doi: 10.1089/thy.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini M, Nicoloso MS, Calin GA. MicroRNAs and cancer-new paradigms in molecular oncology. Curr Opin Cell Biol. 2009;21:470–9. doi: 10.1016/j.ceb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Tseng GC, Steward D, et al. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–8. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodules AATFoT. American association of clinical endocrinologists and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- Pacini F, Castagna MG, Cipri C, et al. Medullary thyroid carcinoma. Clin Oncol. 2010;22:475–85. doi: 10.1016/j.clon.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Pacini F, Schlumberger M, Dralle H, et al. European consensus on the management of patients with differentiated carcinoma of the thyroid from follicular epithelium. Vestn Khir Im I I Grek. 2008;167:52–6. [PubMed] [Google Scholar]
- Parfitt AM. In ‘Physiology and pharmacology of bone’. Berlin, Heidelberg: Springer Berlin Heidelberg; 1993. Calcium homeostasis; pp. 1–65. [Google Scholar]
- Punales MK, Rocha AP, Gross JL, et al. Medullary thyroid carcinoma: clinical and oncological features and treatment. Arq Bras Endocrinol Metabol. 2004;48:137–46. doi: 10.1590/s0004-27302004000100015. [DOI] [PubMed] [Google Scholar]
- Ruan K, Fang X, Ouyang G. MicroRNAs: Novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–26. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Russo D, Arturi F, Chiefari E, et al. A case of metastatic medullary thyroid carcinoma: Early identification before surgery of an RET proto-oncogene somatic mutation in fine-needle aspirate specimens. J Clin Endocrinol Metab. 1997;82:3378–82. doi: 10.1210/jcem.82.10.4278. [DOI] [PubMed] [Google Scholar]
- Sama MT, Rossetto Giaccherino R, Gallo M, et al. Clinical challenges with calcitonin-negative medullary thyroid carcinoma. J Cancer Res Clin Oncol. 2016;142:2023–9. doi: 10.1007/s00432-016-2169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–3. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Jung M, Kristiansen G, et al. MicroRNAs and cancer: Current state and future perspectives in urologic oncology. Urol Oncol. 2010;28:4–13. doi: 10.1016/j.urolonc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Scollo C, Baudin E, Travagli J-P, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070–5. doi: 10.1210/jc.2002-021713. [DOI] [PubMed] [Google Scholar]
- Scurini C, Quadro L, Fattoruso O, et al. Germline and somatic mutations of the RET proto-oncogene in apparently sporadic medullary thyroid carcinomas. Mol Cell Endocrinol. 1998;137:51–7. doi: 10.1016/s0303-7207(97)00234-7. [DOI] [PubMed] [Google Scholar]
- Stepanas AV, Samaan NA, Hill CS, Jr, et al. Medullary thyroid carcinoma: importance of serial serum calcitonin measurement. Cancer. 1979;43:825–37. doi: 10.1002/1097-0142(197903)43:3<825::aid-cncr2820430308>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–8. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Tashjian AHJ, Howland BG, Melvin KEW, et al. Immunoassay of human calcitonin. New Eng J Med. 1970;283:890–5. doi: 10.1056/NEJM197010222831702. [DOI] [PubMed] [Google Scholar]
- Weber T, Schilling T, Frank-Raue K, et al. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery. 2001;130:1044–9. doi: 10.1067/msy.2001.118380a. [DOI] [PubMed] [Google Scholar]
- Wells SA, Jr, Pacini F, Robinson BG, et al. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98:3149–64. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh MZ, Sheikholeslami S, Hedayati M. RET proto oncogene mutation detection and medullary thyroid carcinoma prevention. Asian Pac J Cancer Prev. 2015;16:2107–17. doi: 10.7314/apjcp.2015.16.6.2107. [DOI] [PubMed] [Google Scholar]


