Abstract
Studies show that approximately 20% of all breast cancer patients have a breast tumor that tests positive for Human Epidermal Growth Factor Receptor 2, otherwise known as the HER2 gene. As such, treatments for breast cancer usually include drugs that target HER2. The drug Trastuzumab is a recombinant antibody that has been approved by the FDA for the treatment of these HER2 positive breast cancers. However, researchers have found that mutations in associated genes, PIK3CA and KRAS, can cause the tumor to become resistant to Trastuzumab. The purpose of this article is to evaluate the sensitivity of the cancer cell lines to the drug Trastuzumab and investigate how this sensitivity is compromised by the PIK3CA, KRAS and BRAF gene mutations. Trastuzumab responsiveness was evaluated in breast cancer cell lines by treating the lines with an optimal concentration of the drug followed by a proliferation assay of the cell lines in the presence of monoclonal antibodies. We determined the optimum concentration of Trastuzumab to be 7 μg/well. The BRAF and KRAS mutated cell line, MDA-MB-231, showed the least sensitivity after being treated with trastuzumab when compared to the sensitivity of the PIK3CA mutated cell lines, MCF-7 and MDA-MB-361, and the KRAS/ BRAF/ PIK3CA cell line, MDA-MB-453. Clinical observations show that mutations in BRAF and KRAS genes in breast cancer cells do lower the responsiveness of Trastuzumab drug treatments.
Keywords: Trastuzumab, HER2 monoclonal antibody, BRAF, KRAS, PIK3CA
Introduction
Breast cancer is the leading cause of cancer-related death in nonsmoking women and is also the most common form of cancer in women worldwide. In 2016, approximately a quarter million new cases of breast cancer were diagnosed in women with an estimated forty thousand deaths expected from those cases (Siegel et al., 2016). Yet, the rates of survival in patients initially diagnosed with metastatic breast cancer have improved by six months in the past twenty years due to the advancing methods of targeted therapies supplementing standard chemotherapy (Mendes et al., 2015). Furthermore, treatment has evolved to focus on more specific tumor targets. Tumor antigens used in breast cancer immunotherapy are over expressed or mutated in these target cells. The antigens targeted for breast cancer treatment are involved in the growth and the signaling for differentiation: epidermal growth factor receptors EGFR or ERBB1, ERBB2 or HER2, and ERBB3. However, chemotherapy, either combined or as a monotherapy of HER2-targeted monoclonal antibody therapy is thought to be nonreceptive in tumor cells with the PIK3CA gene mutations (De Stefano and Carlomagno, 2014). The data from clinical trials confirm that the efficacy of mAbs in the treatment of breast cancer has significantly improved the disease-free survival and overall survival in patients with PIK3CA WT breast cancer with an improvement in response rates (Perez et al., 2007; Majewski et al., 2015). The E545K mutation results in an amino acid substitution within a highly conserved helical domain; and the mutant PIK3CA proteins have increased catalytic activity resulting in enhanced downstream signaling and oncogenic transformation in vitro (Kang et al., 2005). PIK3CA mutations are identified in 23% of HER2-positive breast tumors and is among the most commonly mutated oncogene in breast cancer (Pogue-Geile et al., 2015).
Trastuzumab is a humanized IgG (1) kappa monoclonal antibody that is directed against the extracellular domain of HER2 and is indicated for the treatment of HER2-positive metastatic breast cancer (Singh et al., 2014). It has been proven that ER- and HER2-related genes have predictive signature for the degree of benefit from trastuzumab (Pogue-Geile et al., 2013). HER2, a transmembrane receptor with tyrosine kinase on chromosome 17, displays cancer causing characteristics upon amplification or over expression. This character of the gene’s function is responsible for an estimated 20% of invasive breast cancers testing HER2 positive (Iqbal and Iqbal, 2014). Trastuzumab is known to induce a significant antibody-dependent cell-mediated cytotoxicity (ADCC) response in HER-2-amplified MDA-MB-231, and HER-2 non-amplified cell lines MCF-7 and MDA-MB-361 with low levels of detectable HER-2, whereas trastuzumab was not found to induce ADCC in MDA-MB-468, which has very low levels of HER-2 (Subik et al., 2010; Voutsas et al., 2013). The mechanisms of action for trastuzumab have been proposed including: PI3K/Akt and MAPK signaling inhibition, antibody-dependent cell-mediated cytotoxicity exerted by the immune system, prevention of HER2 cleavage by matrix metalloproteinases, and angiogenesis inhibition (De et al., 2013; Griner et al., 2013; Barok et al., 2014). Yet, the exact in vivo mechanism of action of trastuzumab remains a mystery, given the direct effect it has on the ERBB2 signaling pathway and the indirect contributions to the immune system by eliciting ADCC (Yan et al., 2008). Nonetheless, the use of trastuzumab in treatment does lead to an improved prognosis in breast cancer patients with HER-2 positive tumors (Joensuu et al., 2014; Vu et al., 2014; Kawajiri et al., 2015; Yu et al., 2015). Not all patients respond to treatment, and therefore do not benefit from the therapy (Berns et al., 2016; Li et al., 2016; Nunes et al., 2016; Ozkavruk Eliyatkin et al., 2016). The possible cause of this failure may be due to alterations in one or more of the several molecular pathways regulating HER2 function resulting in the trastuzumab resistance. The EGFR stimulation pathway involving K-RAS and BRAF genes may also be points of interest when considering the failure of trastuzumab as a treatment. Mutations in these genes are important to take note of, especially during the treatment of patients with other cancers, such as in melanoma and colorectal cancer.
The effect these genes have on the development and progression of breast cancer has been investigated using in vitro studies (Boriack-Sjodin et al., 1995) and in the clinical setting (Esteva et al., 2010; Banerji et al., 2012; Shah et al., 2012; Kim et al., 2013). These studies focused on patients with triple negative breast tumors. From the information gathered during these studies, further elucidation is needed to determine if mutations in PIK3CA, KRAS, and BRAF affects the therapeutic effect of trastuzumab. As a result, the purpose of this investigation is to evaluate the frequencies of mutations in PIK3CA, KRAS and BRAF genes in breast cancer cell lines and determine whether the given mutations are truly critical to the therapeutic effectiveness of trastuzumab.
Materials and Methods
Cell Lines and Reagents
MDA-MB-231 (BRAF, c.1391G>T, p.G464V; KRAS, c.38G>A p.G13D), MCF-7 (PIK3CA, c.1633G>A, p.E545K), MDA-MB-361 (PIK3CA, c.1633G>A p.E545K) and MDA-MB-453 (BRAF, KRAS and PIK3CA wild type) were purchased from the National Centre for Cell Science (NCCS, Pune, India). The cell lines were routinely cultured in 75-mL tissue culture flasks (Nunc Thermofisher Scientific) in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin (all from Sigma, USA) at 37°C and 5% CO2, according to the protocol provided by NCCS. All cell lines were tested for Mycoplasma detection by the Sigma LookOut Mycoplasma PCR Detection Kit (MP0035).
Monoclonal Antibodies
Trastuzumab (CANMAb; Biocon, India) was purchased from the local hospital pharmacy and dissolved in sterile water at a stock concentration of 20 mg/mL. The cells were treated in triplicate with Trastuzumab at concentrations of 0.5–10 g per well, to determine the optimal concentrations for the proliferation assay.
Cell Proliferation and Antibody Optimization
A MTS cell proliferation assay was performed in triplicate over a period of 72 hours using a CellTiter 96 Aqueous nonradioactive cell-proliferation assay kit (Promega) to determine the resistance and sensitivity of the breast cancer lines to the monoclonal antibodies. Cells were seeded at a density of 5 × 103 cells/mL in a total volume of 100 μL medium into 96-well plates and maintained at 5% CO2 humidified atmosphere at 37°C for 24 hours. Trastuzumab was added to the cells in triplicate in concentrations of 0.5–10 μg to determine the optimal concentrations for the proliferation assay. A negative control experiment was carried out in parallel with 10 μg of monoclonal mouse IgG1 or IgG2A isotype control antibody on the plate. Following incubation of cells for 72 hour in a CO2 incubator at 5% CO2, 37°C, a 20 μl of MTS/PMS solution was added to each well and incubated for further 2 hours. Absorbance was measured immediately in a plate reader at 490 nm. Proliferation was recorded as a percentage of that obtained for the isotype control antibody-treated cells (100%).
Statistical Analysis
All statistics were performed through Graphpad Prism 6 software. For the statistical analysis of the cell culture studies the T-test was applied with a p value of < 0.05 considered to be significant.
Results
Optimization of Antibody Concentrations
Cells were seeded at a density of 5 × 103 cells/mL with a total volume of 100 μL medium into each well. The cell lines were then treated with amounts of trastuzumab varying from 0.5 μg to 10 μg. A trastuzumab concentration of 7 μg per well was found to be the optimal treatment concentration to provide the maximum inhibition of all breast cancer cell lines (Figure 1)
Figure 1.
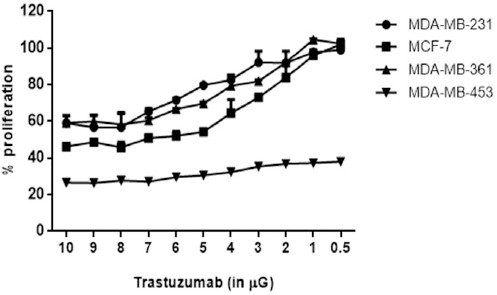
Proliferation Assay of Breast Cancer Cells treated with Trastuzumab
Correlation Between PIK3CA Status and Responsiveness to Trastuzumab Treatment
The isotope control had 100% proliferation after being treated with trastuzumab. The proliferation rate of the PIK3CA mutated cells lines, MCF-7 and MDA-MB-361, were 57.86% and 62.36% respectively relative to the isotype control. MDA-MB-231, the BRAF and KRAS mutations cell line, had a proliferation rate of 84.36% (p=<0.001). The cell line with the least amount of the proliferation rate was MDA-MB-453 (PIK3CA, BRAF and KRAS WT) with a proliferation rate of 31.7% (p = 0.002). (Figure 2) Therefore, it can be determined from the established data that the cell lines with the least sensitivity to the trastuzumab treatment was MDA-MB-231, then MDA-MB-361, and MCF-7. The cell line most sensitive to trastuzumab is MDA-MB-453, the PIK3CA, BRAF and KRAS wild type.
Figure 2.
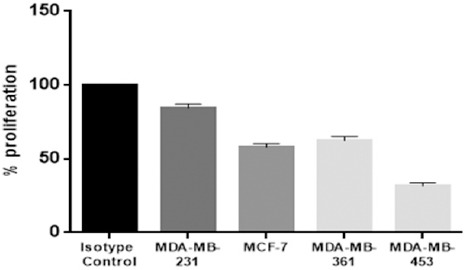
Response of Different Breast Cancer Cell Lines to 7 μg Trastzumab Treatment
Discussion
In this study, several breast cancer cell lines with BRAF, KRAS and PIK3CA mutations were used to evaluate the in vitro effect of trastuzumab. MDA-MB-231 cell lines with BRAF and KRAS mutations showed resistance to the anti-HER2 mAb trastuzumab. They had the highest proliferation rate compared to the other cell lines with PIK3CA mutations (MCF-7 and MDA-MB-361) and PIK3CA wild type cells (MDA-MB453). Trastuzumab responded intermediately in cell lines with the PIK3CA mutation. The rate of proliferation decreased inversely to an increase in the Trastuzumab dosage which indicates dose-response activity. Yet, in comparison when the antibody was used against the PIK3CA wildtype cell line MDA-MB-453, it showed the lowest proliferation rate with the highest response to trastuzumab. The relative benefit of the addition of HER-2 targeted therapy is proposed to be similar in first-line docetaxel, trastuzumab, pertuzumab, first or second-line chemotherapy, and lapatinib in advanced cancer. Yet, PIK3CA mutations are associated with a shorter, progression-free survival. Interestingly, PIK3CA-mutant and wild-type tumors results in a similar overall response rate and progression-free survival when treated with trastuzumab-emtansine or second or third-line treatment. Therefore, the status of PIK3CA could be clinically useful for treatment selection in an advanced cancer.
A variety of active HER2-targeted treatment choices are currently under clinical trials. Previously, clinical trials were carried out for the treatment of HER2+ advanced or metastatic breast cancer using PI3K pathway inhibitors with trastuzumab (Rexer and Arteaga, 2013). Intriguingly, the combination was well tolerated. Of the patients treated, there were 17% partial responses and 58% of patients with stable disease suggesting clinical activity (Saura et al., 2014). A phase 2 study of this combination is currently underway. Preclinical data demonstrated that the dual targeting of HER2 and PIK3 pathways are more effective than individual targeting of these pathways in HER2-positive breast cancer because the mutations in PIK3CA uncouples HER2 and PI3K signaling which renders resistance to HER2-targeted trastuzumab (Rexer et al., 2014). This could possibly explain the lower proliferative capacity of the PIK3CA mutated cell lines in our study, which exhibited definite resistance to trastuzumab when compared with the BRAF and KRAS mutated cell lines. It has been repeatedly recognized that HER2 mediates cross-talk between the PI3K-AKT-mTOR pathway. Alterations in the PI3K/AKT/mTOR pathway can take the form of HER2 amplification, loss of tumor suppressor PTEN, mutations in AKT, and other aberrations (Serra et al., 2011). Furthermore, the study emphasized the need for combined inhibition of HER2 and PIK3CA pathway to inactivate both pathway end points (Davis et al., 2014). This variability in the HER2 targeted mAb therapy in cell lines and their response to the HER2 inhibition in tumor cells, depending on the particular PIK3CA mutation, explains the discrepant growth inhibition. HER2 and the PI3K/PTEN/Akt/mTORC1 pathways, which are associated with breast cancer, are important for the classification and prognosis of breast cancer in patients. Both HER2 and PIK3CA are abnormally expressed or mutated in many cases of breast cancer. Therefore, targeting these genes through the drug therapy may prove to be effective therapeutically. Any novel or reported mutations, or change in expression of the components of these pathways provide essential information related to the targeted therapy of trastuzumab.
From our preclinical breast cancer cell line model, we observed differences in the responses to trastuzumab treatments depending on the particular PIK3CA mutations treated. MDA-MB-231 cells containing BRAF and KRAS mutations displayed resistance to treatment with trastuzumab whereas PIK3CA cell lines displayed immediate reaction to both treatments, compared to MDA-MB-453 cell lines which are PIK3CA wildtype. Nonetheless, further data is needed to fully unmask the role of PIK3CA mutations for trastuzumab-based treatment. An in vitro study reported that PIK3CA mutations may not be shown to be resistance markers to trastuzumab in NSBAP B-3. Patients (n = 672) who received any trastuzumab-based treatment, regardless of concomitant chemotherapy, showed a statistically significant longer overall survival and progression-free survival in those with PIK3CA mutations (Pernas Simon, 2014). However, the nonresistance should not be understood as contraindication to the clinical development of PI3K pathway inhibitors in HER2-positive breast cancer. Knockout experiments in breast cell lines with HER2-amplified genes indicated a PIK3CA mutation or PTEN loss which indicates the potential mechanism for trastuzumab resistance (Berns et al., 2007). These findings were supported in a recent systematic study in which HER2-positive cancer cells were treated with combining trastuzumab with a PIK3CA inhibitor. An additive effect was observed which suggests some signaling through multiple pathways on PI3K(PIK3CA, AKT, mTOR) (Cancer Genome Atlas, 2012). Further studies and clinical trials can continue to determine if PIK3CA mutations may be useful biomarkers used to predict responses to treatments. Recently, the CLEOPATRA clinical trial suggested that combinations of PI3K inhibitors were superior to lone treatments of trastuzumab. Also, there were less benefits for tumors with PIK3CA mutations in comparison to those containing the wild-type gene (Baselga et al., 2014). A clinical phase II trial (GeparOcto) is underway (NCT02311344) to analyze the molecular response in breast cancer HER2+/HR+ patients using pertuzumab-trastuzumab therapy alone or coupled with Letrozole, a non-steroidal aromatase inhibitor in relation to the PIK3CA mutations (Baselga et al., 2010). All of this research, we hope, will result in the development of an extremely personalized approach to the treatment of these breast cancers making use of trastuzumab therapies as well as additional targeted therapies. Due to these types of clinical trials and studies, we will soon be able to employ an arsenal of treatments for PIKA3CA mutated breast cancers improving the survival rates of breast cancer as a whole.
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Cortes J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Berns K, Sonnenblick A, Gennissen A, et al. Loss of ARID1A activates ANXA1, which serves as a predictive biomarker for trastuzumab resistance. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2996. vol? pages? [DOI] [PubMed] [Google Scholar]
- Boriack-Sjodin PA, Heck RW, Laipis PJ, et al. Structure determination of murine mitochondrial carbonic anhydrase V at 2.45-A resolution: implications for catalytic proton transfer and inhibitor design. Proc Natl Acad Sci U S A. 1995;92:10949–53. doi: 10.1073/pnas.92.24.10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NM, Sokolosky M, Stadelman K, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget. 2014;5:4603–50. doi: 10.18632/oncotarget.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: What is their clinical relevance? Cancer Treat Rev. 2013;39:925–34. doi: 10.1016/j.ctrv.2013.02.006. [DOI] [PubMed] [Google Scholar]
- De Stefano A, Carlomagno C. Beyond KRAS: Predictive factors of the efficacy of anti-EGFR monoclonal antibodies in the treatment of metastatic colorectal cancer. World J Gastroenterol. 2014;20:9732–43. doi: 10.3748/wjg.v20.i29.9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–56. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner SE, Wang KJ, Joshi JP, et al. Mechanisms of adipocytokine-mediated trastuzumab resistance in HER2-positive breast cancer cell lines. Curr Pharmacogenomics Person Med. 2013;11:31–41. doi: 10.2174/1875692111311010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Outcome of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab in the Finland Capecitabine Trial (FinXX) Acta Oncol. 2014;53:186–94. doi: 10.3109/0284186X.2013.820840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri H, Takashima T, Kashiwagi S, et al. Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Expert Rev Anticancer Ther. 2015;15:17–26. doi: 10.1586/14737140.2015.992418. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim J, Lee HD, et al. Spectrum of EGFR gene copy number changes and KRAS gene mutation status in Korean triple negative breast cancer patients. PLoS One. 2013;8:e79014. doi: 10.1371/journal.pone.0079014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhai L, Wang H, et al. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.8413. vol? pages? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33:1334–9. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes D, Alves C, Afonso N, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes J, Zhang H, Angelopoulos N, et al. ATG9A loss confers resistance to trastuzumab via c-Cbl mediated Her2 degradation. Oncotarget. 2016 doi: 10.18632/oncotarget.8504. vol? pages? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkavruk Eliyatkin N, Aktas S, Ozgur H, et al. The role of p95HER2 in trastuzumab resistance in breast cancer. J BUON. 2016;21:382–9. [PubMed] [Google Scholar]
- Perez E, Romond E, Suman V, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. ASCO Annual Meeting Proceedings. 2007:512. [Google Scholar]
- Pernas Simon S. Neoadjuvant therapy of early stage human epidermal growth factor receptor 2 positive breast cancer: latest evidence and clinical implications. Ther Adv Med Oncol. 2014;6:210–21. doi: 10.1177/1758834014535650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile KL, Kim C, Jeong JH, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst. 2013;105:1782–8. doi: 10.1093/jnci/djt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile KL, Song N, Jeong JH, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;33:1340–7. doi: 10.1200/JCO.2014.56.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexer BN, Arteaga CL. Optimal targeting of HER2-PI3K signaling in breast cancer: mechanistic insights and clinical implications. Cancer Res. 2013;73:3817–20. doi: 10.1158/0008-5472.CAN-13-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexer BN, Chanthaphaychith S, Dahlman K, et al. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+breast cancer cells. Breast Cancer Res. 2014;16:R9. doi: 10.1186/bcr3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura C, Bendell J, Jerusalem G, et al. Phase Ib study of Buparlisib plus Trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on Trastuzumab-based therapy. Clin Cancer Res. 2014;20:1935–45. doi: 10.1158/1078-0432.CCR-13-1070. [DOI] [PubMed] [Google Scholar]
- Serra V, Scaltriti M, Prudkin L, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–98. doi: 10.1038/bjc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subik K, Lee JF, Baxter L, et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- Voutsas IF, Mahaira LG, Fotopoulou K, et al. Gamma-irradiation induces HER-2/neu overexpression in breast cancer cell lines and sensitivity to treatment with trastuzumab. Int J Radiat Biol. 2013;89:319–25. doi: 10.3109/09553002.2013.765617. [DOI] [PubMed] [Google Scholar]
- Vu T, Sliwkowski MX, Claret FX. Personalized drug combinations to overcome trastuzumab resistance in HER2-positive breast cancer. Biochim Biophys Acta. 2014;1846:353–65. doi: 10.1016/j.bbcan.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J. 2008;14:178–83. doi: 10.1097/PPO.0b013e318172d71a. [DOI] [PubMed] [Google Scholar]
- Yu AF, Yadav NU, Lung BY, et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat. 2015;149:489–95. doi: 10.1007/s10549-014-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


