Abstract
Background and aim:
Glioblastoma (GBM) is one of the most common and aggressive brain tumors with a median survival of 12-14 months. The aim of present study was to evaluate the gene expression profile of stem cell markers Nanog and CD24 in GBM and to determine its relationship to outcome in terms of treatment response and overall survival.
Material and methods:
This was a retrospective as well as retrospective study which included 51 histologically confirmed cases of GBM. Expression of CD24, and Nanog was evaluated by RT-PCR. Control tissue included debrided brain tissue from open head injury cases. All cases of GBM underwent total surgical resection and subsequently chemotherapy. Immediate treatment response was evaluated at 3 months using Response Evaluation Criteria In Solid Tumors (RECIST) guidelines and overall survival was measured at 36 months.
Result:
As compared to control gene, expression of CD24 and Nanog was seen to be unregulated to 24.5% and 31.7% respectively. However, the difference in mean expression of cases and controls was not statistically significant. Correlation between expressions of these two markers was also not statistically significant. On univariate cox regression analysis, cases with >2 fold expression of CD24 and Nanog had significantly poor survival as compared to those with <2 fold expression. On multivariate analysis > 2 fold CD24 expression had a statistically significant correlation with poor survival.
Conclusion:
An overexpression of CD24 by more than two fold was associated with poor overall survival in GBM. Poor survival may be related to increased “stemness” of tumour cells. Targeted therapy inclusive of drugs targeting stem cells directly or indirectly may be a promising therapeutic option.
Keywords: Glioblastoma, Nanog, CD24, survival
Introduction
Glioblastoma (GBM) is one of the most common and aggressive brain tumours. Despite the advances in diagnosis and treatment modalities, GBM has a median survival of 14.6 months (Stupp et al., 2005). Even though little is known about the genetic mechanisms involved in its pathogenesis, the discovery of cancer stem cells (CSC) has shown that the presence of CSC is correlated with the aggressiveness of gliomas (Deng et al., 2011). The progenitor cell hypothesis of cancer development suggests that only a small sub-population of cells within a tumor (the CSCs) has stem cell-like properties and the ability to initiate new tumors (Clarke et al., 2006). CD24+cells isolated from human nasopharyngeal carcinoma cell lines express stem cell genes (Sox2, Oct4, Nanog, Bmi-1, and Rex-1), and show activation of the Wnt/β-catenin signaling pathway. CD24+ cells possess typical CSC characteristics that include enhanced cell proliferation, increased colony and sphere formation, maintenance of cell differentiation potential in prolonged culture, and enhanced resistance to chemotherapeutic drugs. CD24+ cells further show an increased invasion ability in vitro, which correlates with enhanced expression of matrix metalloproteinase 2 and 9, as demonstrated in our previous study. (Soni et al., 2016). Nanog is a stem cell transcription factor, enhanced by CD 24 expressing stem cells which is essential for embryonic development, reprogramming normal adult cells, malignant transformation and progression. In the present study, we have analyzed the expression profile of stem cell markers Nanog and CD24 in 51 cases of GBM and correlated the expression with prognosis.
Materials and Methods
Patients and tissue samples
The present study was approved by the Institutional review board and the stem cell ethics committee of the institute. Written informed consent was obtained from all the patients. Fresh glioma specimens were obtained in 51 histopathologically confirmed cases of GBM. Naïve cases which had surgical near total excision and had not undergone prior therapy in the form of neoadjuvant chemo and/or radiotherapy were included in the study. Post-surgery cases were treated with chemoradiation. Patient data was assessed in terms of age, gender, karnofsky performance score, surgery date, site of tumour, treatment given and follow up. Clinical history was retrieved from medical records and follow up was done mainly by telephonic correspondence or by follow up visits to OPD. To estimate patient’s survival all patients were followed till death. Overall survival was defined as the time interval between initial surgery and the day of patient’s death.
RT-PCR
Fresh glioma specimen and non neoplastic brain tissue were collected in trizol reagent and frozen for mRNA isolation at -80 °C. Total RNA extraction was done by trizol method and concentration was determined using Nanodrop (Thermofischer Scientific, USA). RNA samples with an OD of more than 250 were considered for experimentation. RT-PCR was performed in CFX-96 thermal cycler (BIORAD, USA) with the following primers designed by the genomics expression programme and DNA STAR (GENOMICS EXPRESSION VERSION 1.100 C2000 manufactured by MWG Euroffins) using SYBR green mixture. Primer sequences were as follows: CD 24, forward, 5’-TGCTCCTACCCACGCAG-3’ reverse, 5’-GGCCAAACCCAGAGTTG-3’; NANOG, forward, 5’-ATGCCTGTGATTTGTGGGCC-3’, reverse, 5’-GCCAGTTGTTTTTCTGCCAC-3’ and β actin, forward, 5’-GCGGGAAATCGTGCGTGACATT-3’, reverse, 5’-GATGGAGTTGAAGGTAGTTTCGTG-3’.
All gene specific mRNA expression values were normalized to β actin expression levels. Relative quantification of the expression of these genes was evaluated using the ΔΔCt method. The fold change in the relative gene expression was determined by calculating the 2-ΔΔCt.
Statistical analysis: Statistical analysis was carried out using the IBM-Statistical Package for Social Sciences (SPSS) version 16. Data was summarised as Mean ± SE (standard error of the mean). Groups were compared by Student’s t test. Overall survival between groups was compared by Kaplan-Meier method using Log rank test. Cox-regression was performed to assess independent predictors of overall survival. Correlation between the genes was done by Pearson’s correlation. A two-tailed p value less than 0.05 (p < 0.05) was considered statistically significant.
Results
The study group comprised of 51 cases of GBM. The mean age of patients was 41.73 years (range 10-76 years). Twenty nine (56.9%) patients were above 40 years; while twenty two (43.1%) patients were less than 40 years of age. There were 66.7% males and 33.3% females. Most common site for the tumor was frontal (37.3%) followed by temporal (33.3%), parietal and occipital lobes respectively. All the cases underwent near total surgical resection. Post-surgery they were treated with radiation alone or with chemotherapy. Patient characteristics and demographics are shown in Table 1.
Table 1.
Demographic and Clinico-Pathological Characteristics of GBM Patients
| Characteristics | Number of patients (n=51) (%) |
|---|---|
| Age (yrs) | |
| ≤40 yrs | 22 (43.1) |
| >40 yrs | 29 (56.9) |
| Sex | |
| Female | 17 (33.3) |
| Male | 34 (66.7) |
| Karnofsky Performance Score | |
| ≤50 | 17 (33.3) |
| >50 | 34 (66.7) |
| Tumor site | |
| Frontal | 19 (37.3) |
| Temporal | 17 (33.3) |
| Parietal | 13 (25.5) |
| Occipital | 02 (3.9) |
| Hemisphere | |
| Left | 21 (41.2) |
| Right | 30 (58.8) |
| Treatment | |
| Radiation | 31 (60.8) |
| Chemo-radiation | 20 (39.2) |
| Response | |
| Complete response | 10 (19.6) |
| Partial response | 13 (25.5) |
| Stable disease | 14 (27.5) |
| Progressive disease | 14 (27.5) |
Immediate response to therapy was assessed at 3 months using RECIST guidelines. 10 (19.6%) patients had complete remission (CR), 13 (25.5%) had partial remission (PR), 14 (27.5%) had stable disease (SD) and 14 (27.5%) had progressive disease (PD). For statistical evaluation, CR and PR were classified as responders while SD and PD as non-responders. When gene expression was studied by RT-PCR, expression of CD24 was 24.5% and Nanog 31.7% up regulated (Figure 1). The mean fold expression of CD24 and NANOG was also upregulated however the difference of none of the stem cell markers as compared to normal brain parenchyma was statistically significant (Figure 2). No significant correlation was found between stem cell markers when statistical evaluation was done using the Pearsons correlation test. The mean expression of these markers was higher in non-responders as compared to responders. But, the statistical significance was reached for CD24 only (Table 2). Patients with < 2 fold expression of CD24 and Nanog, had longer survival than patients with > 2 fold expression (Figure 3). On multivariate analysis level of CD24 expression and response to treatment were two statistically significant predictors of overall survival.
Figure 1.
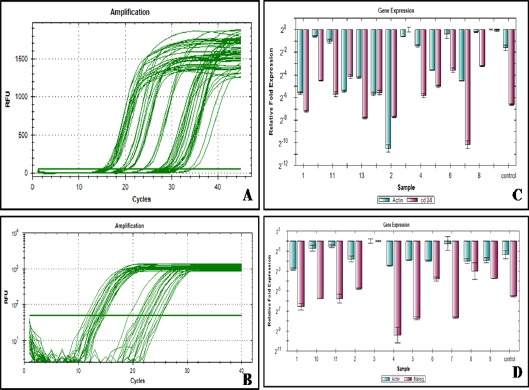
Amplification Plot of CD24 and β Actine (A), Amplification Plot of Nanog and β actine (B) CD24 gene expression of the individual cases (C) Nanog gene expression of the individual cases (D)
Figure 2.
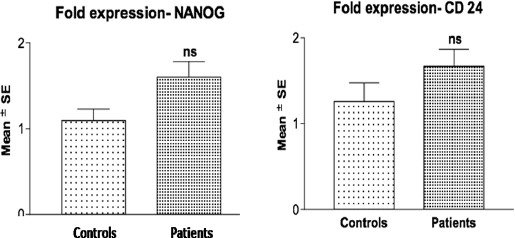
Comparative Mean Fold Stem Cell Marker Expression of Controls Vs. GBM Patients Using Student’s T-Test Nsp>0.05- Controls Vs. Patients (Ns = Not Significant)
Table 2.
Correlation of Expression of Markers with Treatment Response
| Mean expression (Mean Fold increase) | Responders (n=23) | Non- responders (n=28) | P- value |
|---|---|---|---|
| CD24 | 1.23 | 2.02 | 0.049 |
| Nanog | 1.59 | 1.61 | 0.9 |
Figure 3.
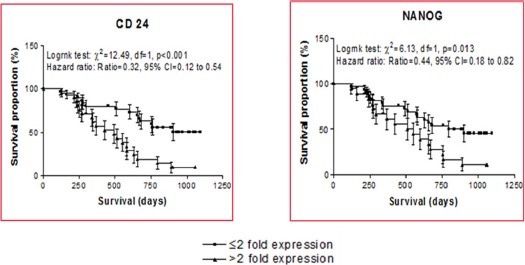
Correlation of CD24 and Nanog Expression with Overall Survival
Discussion
Glioblastoma is the most common brain tumor accounting for approximately 12-15% of all the intracranial neoplasms and 60-75% of astrocytic tumors. The world health organization defines glioblastoma as grade IV glioma based on nuclear atypia, cellular pleomorphism, mitotic activity, vascular thrombosis, microvascular proliferation and necrosis on histopathology. It is primarily a tumor of adults and affects children in less than 1% cases (Kleihues, 2008). Despite recent advancements in various treatment modalities the prognosis is still dismal with an average survival period of 14.6 months. Subset variations in survival have been observed in GBM. Histopathological parameters like presence of oligodendroglial component and molecular genetic changes like IDH1 mutation, MGMT gene methylation, are some of the factors that affect prognosis.
There is a need for greater understanding of molecular markers involved in proliferation and survival of tumor cells. With the advent of cancer stem cell theory, proposed initially in relation to acute myeloid leukemias and later extended to many solid organ malignancies (Binello and Germano, 2011), glioma stem cells (GSCs) have been identified to have the characteristic properties of self-renewal, multipotency and induction of tumorigenesis. These GSCs have been shown to be associated with the recurrence and metastasis of high grade gliomas (Guo et al., 2011). To understand the mechanism involved with GSC, the first question was to identify these cells. Initial studies showed that GSCs expressed CD133 on its surface. However, with further studies CD133 negative GSCs were also identified (Beier et al., 2007). Other stem cell markers were then studied and a new concept of using a panel of markers rather than single marker evolved. CD24 gene, first discovered in 1990 is located on chromosome 6q21 (Horiguchi et al., 2010). The CD24 protein is a heavily glycosylated mucin like cell surface protein, attached to cell membranes by a GPI anchor (Poncet et al., 1996). CD24 acts a ligand for P-Selectin and the cell adhesion molecule L1-CAM. It helps in adhesion of neutrophils and monocytes to activated endothelial cells. Thus, CD24 expressing tumor cells can disseminate more easily and efficiently and have been found to have important role in cancer metastasis (Lee et al., 2010; Kristiansen et al, 2010). It has been also found that CD24 is regulated by Ral GTPases, highlighting its role as transcriptional target of Ral signaling pathway (Wang et al., 2010, Su et al., 2009). In recent studies, expression of CD24 and its prognostic role has been studied in many tumors, like Non-Small cell lung carcinoma, breast cancer, intrahepatic cholangiocarcinoma, hepatocellular carcinoma, oesophageal squamous cell carcinoma, urothelial carcinoma, prostate and ovarian carcinoma (Deng et al., 2012). In these malignancies, over-expression of CD24 was correlated with a poor prognosis because of increased lymph node and distant metastasis. GBM being essentially a tumor with low metastatic potential, expression of CD24 might be responsible for high tumour invasion into surrounding brain parenchyma, hence an incomplete resection. This hypothesis comes from the fact that in the above mentioned cancers, CD24 overexpression was related not only to lymph node metastasis but also high tumor proliferation status, high invasiveness and activation of WNT/beta catenin pathway (Yang et al, 2009). Role of CD24 in pineal parenchymal tumours has been studied by Feve-Montange et al., (2006), who proposed that CD24 expression might be one of the candidates for grading pineal parenchymal tumors. Senner et al., (1999) demonstrated overexpression of CD24 in human GBM cells. Lo et al., (2009) further showed that CD24 gene plays an important role in invasive property of GBM cells. Deng et al., (2012) did one of the pioneers and largest study on the prognostic significance of CD24 and concluded that overexpression of CD24 is correlated with advanced clinico- pathological parameters and poor prognosis in patients with glioma. Our study also confirms that CD24 overexpression correlates with poor response to treatment and poor prognosis. We further observed that more than 2-fold level of expression of CD 24 also correlates with poorer overall survival on multivariate analysis.
Nanog on the other hand, plays a critical role in cell differentiation during embryonic development. Expression of Nanog is limited only to the pluripotent cells and its expression is down regulated with the onset of differentiation. Many studies revealed overexpression of Nanog in various cancers like carcinoma cervix, breast, kidney and osteosarcoma cell lines (Niu et al., 2011). Chiou et al., (2008) and Jeter et al., (2009) demonstrated that overexpression of Nanog correlates with the tumour progression in oral squamous cell carcinoma and prostate carcinoma respectively. However, it is still not clear whether Nanog is critical for GBM prognosis. Our findings corroborate with the results of Niu et al., (2011) who observed overexpression of Nanog in glioma tissue as compared with normal brain tissue. Our results also supported the findings of Ben-Porath., et al (2008) who demonstrated that Nanog is overexpressed in poorly differentiated tumors such as glioblastoma as compared to well differentiated tumours. One of the main mechanisms of action of GSCs is the chemo-resistance to the standard therapy. The chemo-resistance of GSCs can be either because of more efficient DNA repair mechanism in GSC or due to the presence of Notch, Hedgehogo/Gli and Sir T1 pathways (Clement et al., 2007). As Nanog plays an important role in HEDGEHOG-GLI signaling pathway which is required for GBM growth and GBM stem cell renewal (Clement et al., 2007) it may also act as therapeutic target. Regarding DNA repair mechanism, glioma stem cells exhibits significantly higher expression of 0-6methylguanine-DNA-Methyltransferon (MGMT) and are more resistant to Temozolamide (TMZ) treatment as expected (Liu et al., 2006). Poor treatment response by stem cells is not only by their inherent property but also by interaction of these GSC with the surrounding vascular, hypoxic and immune niche.
To summarize, GSC have emerged as one of the culprit in the poor survival in high grade gliomas. Our survival analysis shows a correlation of > 2fold expression of CD24 with a shorter survival with a mean survival of 16 months in high expression GBM vs 27months in cases expressing less than 2 fold amplification. Hence, stem-like cells may be targeted as therapeutic targets to overcome resistance to standard therapy by either blocking their function or by inducing their differentiation.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding Statement
This paper is a part of work funded by ICMR (Indian Council of Medical Research) for providing fellowship support for Senior Research Fellow Ms. Priyanka Soni
References
- Beier D, Hau P, Proeschold M, et al. CD133+and CD133- glioblastoma- derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–15. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binello E, Germano IM. Targeting glioma stem cells: A novel framework for brain tumors. Cancer Sci. 2011;102:1958–66. doi: 10.1111/j.1349-7006.2011.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Yu CC, Huang CY, et al. Positive correlations of OCT-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Canc Res. 2008;14:4085–95. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, deTribolet, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Gao G, Wang L, et al. CD24 Expression as a marker for predicting clinical outcome in human gliomas. J Biomed Biotechnol. 2012;2012:5171–72. doi: 10.1155/2012/517172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fèvre-Montange M, Champier J, Szathmari A, et al. Microarray analysis reveals differential gene expression patterns in tumors of the pineal region. J Neuropathol Exp Neurol. 2006;65:675–84. doi: 10.1097/01.jnen.0000225907.90052.e3. [DOI] [PubMed] [Google Scholar]
- Guo Y, Liu S, Wang P, et al. Expression profile of embryonic stem cell associated genes oct4, Sox 2 and Nanog in human gliomas. Histopathology. 2011;59:763–75. doi: 10.1111/j.1365-2559.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Toi M, Horiguchi S, et al. Predictive value of CD24 and CD44 for neoadjuvant chemotherapy response and prognosis in primary breast cancer patients. J Med Dent Sci. 2010;57:165–75. [PubMed] [Google Scholar]
- Jeter CR, Badeaux M, Choy G, et al. Functional evidence that the self-renewal gene Nanog regulates human tumour developement. Stem Cells. 2009;27:993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen G, Machado E, Bretz N, et al. Molecular and clinical dissection of CD24 antibody specificity by a comprhensive comparative analysis. Lab Invest. 2010;90:1102–16. doi: 10.1038/labinvest.2010.70. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choe G, Jheon S, et al. CD24, a novel cancer biomarker, predicting disease- free survival of non-small cell lung carcinomas: a retrospective study of prognostic factor analysis from the viewpoint of forthcoming (Seventh) New TNM classification. J Thorac Oncol. 2010;5:649–57. doi: 10.1097/JTO.0b013e3181d5e554. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemo-resistance of CD133+cancer stem cells in glioblastoma. Mol Cancer. 2006;2:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HW, Zhu H, Cao X, et al. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69:6790–8. doi: 10.1158/0008-5472.CAN-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P, Burger PC, Aldape, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavene WK, editors. In WHO classification of tumours of the central nervous system. 4th Edition. Lyon: IARC press; 2007. pp. 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu CS, Li DX, Liu YH, et al. Expression of NANOG in human gliomasand its relationship with undifferentiated glioma cells. Oncol Rep. 2011;26:593–601. doi: 10.3892/or.2011.1308. [DOI] [PubMed] [Google Scholar]
- Poncet C, Frances V, Gristina R, et al. CD24, a glycosylphos- phatidylinositol-anchored molecule, is transiently expressed during the development of human central nervous system and is a marker of human neural cell lineage tumors. Acta Neuropathol. 1996;91:400–8. doi: 10.1007/s004010050442. [DOI] [PubMed] [Google Scholar]
- Senner V, Sturm A, Baur I, et al. CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol. 1999;58:795–802. doi: 10.1097/00005072-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Soni P, Husain N, Chandra A, et al. Do phosphatase of regenerating liver-3, matrix metalloproteinases-2, matrix metalloproteinases-9, and epidermal growth factor receptor-1 predict response to therapy and survival in Glioblastoma? Indian J Pathol Microbiol. 2016;59:287–93. doi: 10.4103/0377-4929.188121. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Brent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolamide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Su D, Deng H, Zhao X, et al. Targeting CD24 for treatment of ovarian cancer by short hair pin RNA. Cytotherapy. 2009;11:642–52. doi: 10.1080/14653240902878308. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang X, Peng L, et al. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010;101:112–9. doi: 10.1111/j.1349-7006.2009.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XR, Xu Y, Yu B, et al. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15:5518–27. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]


