Abstract
Herbal-derived medicines have introduced as sources of novel drugs due to minimum systemic side effects. Silibinin as a flavonoid compound has showed with effective chemotherapeutic effects on different cancers. Here, we investigated the impact of combination therapy of silibinin, with paclitaxel and cisplatin in inhibition of proliferation and induction of apoptosis in MCF-7 cells. Cell proliferation was assessed by MTT assay and the percentage of apoptotic cells was measured using flowcytometric assay. Understand of molecular mechanism of this combination related to apoptotic pathway were evaluated by Real Time RT-PCR assays. The IC50 values for silibinin, paclitaxel and cisplatin were 160 ± 22.2 µM, 33.7 ± 4.2 nM and 3.2 ± 0.5 µM, respectively. Paclitaxel and cisplatin induced higher percentage of apoptosis in MCF-7 (P < 0.05). Treatment of cell line with combination of silibinin and paclitaxel or cisplatin showed enhanced early apoptosis 56% and 61%, respectively (P < 0.05). Gene expression patterns demonstrated a significant decrease in anti-apoptotic Bcl-2 with increase in pro-apoptotic Bax, P53, BRCA1 and ATM mRNA levels. Taken together combination therapy of breast cancer cells by applying paclitaxel or cisplatin with silibinin synergistically increases the anti-proliferative effect of single agents.
Keywords: Silibinin, paclitaxel, cisplatin, MCF-7, combination therapy
Introduction
Breast cancer is common leading causes of cancer-related death worldwide (Siegel et al., 2012). According to 2014 epidemiological studies in US, approximately 40,000 females died from breast cancer. In the past decade, many improvements in cancer treatment have been reported (Armat et al., 2016; Mohammadian et al., 2016). Routine screening, early diagnosis, novel treatment alternatives have raised survival rates (DeSantis et al., 2014). Treatment choices are including surgery, radiation therapy and chemotherapy. Chemotherapy is the most predominant strategy for treatment of solid tumors particularly in metastatic cases (Sharifi et al., 2014). Anti-mitotic chemotherapeutic agents are generally applied as the first-line treatments for remedy of breast cancer. These agents which induce apoptosis by changing microtubule stability are divided into two groups of microtubule-stabilizing agents (e.g., taxanes such as docetaxel and paclitaxel) and microtubule depolymerizing agents (e.g. vinca alkaloids). Paclitaxel binds to B-tubulin and induces apoptosis by stabilizing microtubules and inducing cell-cycle arrest (Fabbri et al., 2006). Adverse side effects induced by paclitaxel were considered with focus on well-known drug toxicities, such as gastrointestinal toxicity, hypersensitivity, neurotoxicity and myelo suppression(Frederiks et al., 2015). Combination therapy using different chemotherapeutic agents can be desirable option for overcoming this problem (Kanekiyo et al., 2016). Cisplatin interferes with DNA replication, which kills the fastest proliferating cells. One important issue regarding chemotherapeutic agents is the emergence of chemo resistance to the drug regimen which in turn reduces the efficacy of chemotherapy(Sadava and Kane, 2013). Drug designed to take action against single molecular target usually cannot attack multigenic disease such as cancer. It is now obvious that growth and progression of tumors rely on more than one signaling pathway which manages growth, survival, invasion and metastasis. Furthermore, various cell signaling pathways may control mentioned step in tumor-genesis. Thus combination of chemotherapeutic drugs could inhibit multiple signaling pathways that are requiring for cancer treatment. Furthermore, Combination therapy using active anticancer pharmaceutical ingredients can be particularly worthwhile because potential synergies determined by these screens can be rapidly move into preclinical and clinical development (Lesterhuis et al., 2013). Combination therapy using various chemotherapeutic agents (usually binary and ternary drug regimens) can be a desirable option for overcoming chemo-resistance. Multiple chemotherapeutic agents can compete with each other for the same transporter, molecular target, or have adverse effects on the cell cycle. Studies suggest that cancer cells are more sensitive to multiple drugs in comparison with single agents (Mohseni et al., 2016). Combination therapy protocols rely fundamentally on experimental data produced from in vitro and in vivo studies. Combination therapy not only improves the efficacy of drugs but also lowers the doses of chemotherapeutic agent which can lead to a decrease in the side-effects and increase in the quality of life of the patients (Wilson et al., 2016). Cisplatin and paclitaxel are administrated as the major chemotherapy of breast. In this study, we investigated the impact of combination therapy with silibinin, cisplatin and paclitaxel on cell proliferation and induction of apoptosis in MCF-7 breast cancer.
Materials and Methods
Silibinin, cisplatin and paclitaxel were procured from Gedeon Richter Ltd. (Budapest, Hungary). RPMI-1640 Medium and penicillin–streptomycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Fetal Bovine Serum (FBS) and 3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide (MTT) was provided from Invitrogen (Auckland, New Zealand). Primers were provided by MWG Biotech (Ebersberg, Germany). Annexin V-fluorescein isothiocyanate (FITC), propidium iodide (PI) were supported from (E-bioscience, USA) and RNA isolation kit (RNX-Plus) was obtained from CinnaGen Co. (Tehran, Iran). Power SYBR® Green PCR Master Mix (5ml) was purchased from Applied Biosystems (Warrington, UK).
Single and combined treatment of MCF-7 cells
In single drug treatment, MCF-7 cells (5,000/well) were harvested in 96-well plates and then were treated with increasing serial concentrations of silibinin, cisplatin and paclitaxel alone for 24 and to study the drug efficacy in induction of apoptosis. Consequently, the IC50 values of cisplatin and paclitaxel were determined for MCF-7 cells. In combination treatments, we applied variable concentrations of one agent along with IC50 value of another agent to determine the effects of cisplatin/paclitaxel along with silibinin on cell death.
Cell viability assay
To measure cell proliferation MTT assay was applied as follow; after passing the incubation time of treatment of MCF-7 cells with silibinin, cisplatin and paclitaxel, the medium was substituted with (200 µL) of fresh media containing 50 µL of MTT solution (2 mg/ml in PBS) and the cells were treated for an additional 4 h at 37 °C. Then media/MTT mixture was removed and 200 μL of DMSO with 25 μL of Sorenson’s glycine buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5) was added to each well. After 15 minutes of shaking in the plate thermo-shaker, the absorbance of each well was measured applying microplate reader (Biotek, ELx 800- USA) at 570 nm. MTT solution with DMSO (without the cells and medium) was applied as a blank control (Tupal et al., 2016).
Determination of IC50 of silibinin, cisplatin and paclitaxel against MCF-7 cells
Plots of percent cytotoxicity index (%CI= [1- (ODtreated/ODcontrol)] x100) vs. drug concentrations were planned from each test. IC50 of silibinin, cisplatin and paclitaxel were calculated based on the slope and intercept.
Analysis of drug interactions by combination Index
Combination index (CI) is a quantitative representation of pharmacological interaction between two drugs(Mostafavi-Pour et al., 2017). For this purpose, breast cancer cells were incubated with silibinin, paclitaxel and cisplatin alone and in combination. Breast cancer cells were treated with increasing doses of silibinin (1-400 µM) for 24 h and after washing with PBS, cultured with (1-10 µM) cisplatin for 6 h. Then, paclitaxel (1-200 nM) was added for 24 h. Cell growth inhibition was detected applying MTT assay, as previously described. CI value of 1 shows an additive effect; while a CI < 1 or > 1 exhibit synergism or antagonism, respectively.
Analysis of apoptosis
Breast cancer cells were harvested at a population of 5 × 105 cells / well in 6 well plates and incubated with silibinin, cisplatin and paclitaxel for 24 h. After washing with PBS, cancer cells cultured with silibinin, cisplatin and paclitaxel for 24 h. Next, they were washed twice with phosphate buffered saline and were stained with Annexin V and PI in the binding buffer for 30 min in the dark and then investigated through flow cytometry (BD FACSCalibur) (Sabzichi et al., 2016a).
RNA isolation and semi-quantitative RT-PCR analysis
Total RNA was extracted using the RNA isolation kit (RNX-Plus TM CinnaGen Co) 24 h after treatment with different concentrations of silibinin, paclitaxel and cisplatin alone and in combination. Total extracted RNA was calculated by optical density measurement (A260/A280 ratio) with Nano Drop 1,000 Spectrophotometer (Wilmington, DE, USA) and qualified by agarose gel electrophoresis. cDNA production was accomplished applying revertaa-l (RT reagents kit). Samples were then kept at 70° C. The RT-PCR control was prepared without addition RNA. Real-time RT-PCR was done in a total capacity of (25 µL) using the iQ5 Optical System (Bio-Rad Laboratories, Inc., CA-USA). Each well enclosed (1 µL) of cDNA, (5.75 µM) of each primer and (12.5 µL) of 2X Power SYBR Green PCR Master Mix. The internal control was the constitutively expressed housekeeping human glyceraldehyde 3-phosphate dehydrogenase (GAPDH)(Sabzichi et al., 2016b). Primers were planned by published Gene Bank sequences employing Beacon Designer TM 5.01 software. Primers for human Bcl-2 were as follows: F: 5’-CATCAGGAAGGCTAGAGTTACC-3’ and R: 5’-CAGACATTCGGAGACCACAC-3’. The primers for human GAPDH were as follows: F 5’-AAGCTCATTTCCTGGTATGACAACG-3’ and R: 5’-TCTTCCTCTTGTGCTCTTGCTGG-3’. Bax: Forward: 5’-GATGCGTCCACCAAGAAG-3’ reverse: 5’-AGTTGAAGTTGCCGTCAG-3’ P53: 5’-TGAGGACCTGGTCCTCTGAC-3’; and reverse, 5’-AGAGGAATCCCAAAGTTCCA-3’. Samples were assayed in triplicate on the 7,500 Real Time PCR System (Applied Biosystems). All reactions were done in triplicate in the presence of a negative control. Explanation of the statistics was quantitated via ΔΔCt technique with efficiency correction by Pfaffl performance and the CT (Cycle threshold) standards were uniform with respect to GAPDH expression.
Statistical analysis
All techniques were continued as three independent tests. Data from all tests are signified as means. Statistical analyses were done using SPSS software. Group means of treatments, at several doses or time points, were evaluated with mean ± SD values for non-treated control groups applying one-way ANOVA. The differences were considered as substantial if P < 0.05.
Results
Cytotoxic effects of silibinin, cisplatin and paclitaxel on MCF-7 cells
The cytotoxicity of silibinin, cisplatin and paclitaxel was measured by both MTT assay. To define the optimal concentration and IC50 value of each chemotherapeutic agent, MCF-7 cells were incubated with increasing concentrations of silibinin, cisplatin and paclitaxel for 24 h. incubation of the cells with 160 µM silibinin decreased the viability of the cells to (50 ± 6 %) after 24 h (P<0.05) (Figure 1A). Paclitaxel (33.4 nM) also decreased the cell viability to (48 ± 4 %) after 24 h (P<0.05) (Figure 1B). Treatment breast cancer cells with 3.2 µM cisplatin inhibited proliferation of cells up to 50%. These findings indicate that paclitaxel induces a slightly higher apoptotic response, in MCF-7 cells, in comparison with cisplatin (Figure 1C).
Figure 1.
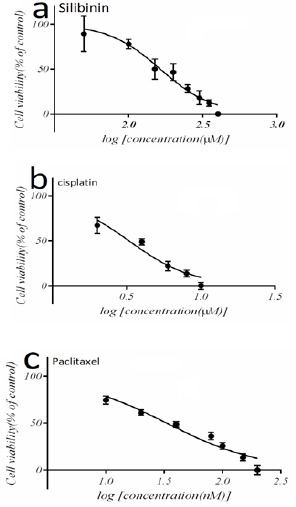
Inhibition Effect of Silibinin, Cisplatin and Paclitaxel on Growth of MCF-7 (Breast Cancer Cell Line). Cells were treated with different concentrations of Silibinin, cisplatin and paclitaxel. IC50 determined for Silibinin, cisplatin and paclitaxel by MTT assay (a) (b) and (c). The results was considered as the mean ± standard deviation (n=3)*p<0.05, **p<0.01.
Combination therapy reduces the IC50 values of silibinin, cisplatin and paclitaxel
We also studied whether or not combination therapy enhances the antitumor effects of silibinin, cisplatin and paclitaxel. A combination of silibinin, (150 µM) along with paclitaxel at 10 nM for 24 h, elevated the combination index up to 0.75 (Table 1). Incubation of the cells with paclitaxel (30 nM), along with IC50 value of silibinin, showed that the CI increased to 0.88. Combination treatment of the cells reduced IC50 values from (3.2 µM) to (1.8 µM) for cisplatin and from (33 nM) to (26 nM) for paclitaxel (P<0.05) (Table 2).
Table 1.
Synergistic Effects of Combination Treatment of Cancer Cells with Silibinin, Cisplatin and Paclitaxel Drugs. Combination index (CI) curves calculated for combinations with two dietary products and cytotoxic drugs in MCF-7 cells.
| Silibinin dose (μM) | Paclitaxel dose (nM) | Combination Index |
|---|---|---|
| 150 | 10 | 0.75 |
| 150 | 20 | 0.79 |
| 150 | 30 | 0.88 |
| 150 | 40 | 0.71 |
Table 2.
Cells were Exposed to Combination Treatment (1:1:1:1) with Increasing Doses of Silibinin, Cisplatin and Paclitaxel. CI values were obtained by linear regression. Trend lines indicate CI values at any given effect.
| Silibinin dose (μM) | Cisplatin dose (μM) | Combination Index |
|---|---|---|
| 150 | 1 | 0.69 |
| 150 | 2 | 0.71 |
| 150 | 4 | 0.66 |
| 150 | 5 | 0.62 |
Combined effect of silibinin, cisplatin and paclitaxel on Apoptotic induction
The percentage of apoptotic cells was calculated by flow cytometric analysis of the cells that were coincidentally stained with Annexin V and PI. In addition, flow cytometric analysis demonstrated that treatment with silibinin, cisplatin induced marked increase in apoptotic cell population compared with each agent alone (Figure 2). For example, cisplatin increased early apoptotic cells up to 35.4% in MCF-7 while in combination treatment with silibinin population of early apoptosis increased up to 61.8%. Furthermore, paclitaxel increased population of early apoptotic cells from 25.7% alone while in combination with silibinin elevated to 56.8% in MCF-7 cells after 24h incubation.
Figure 2.
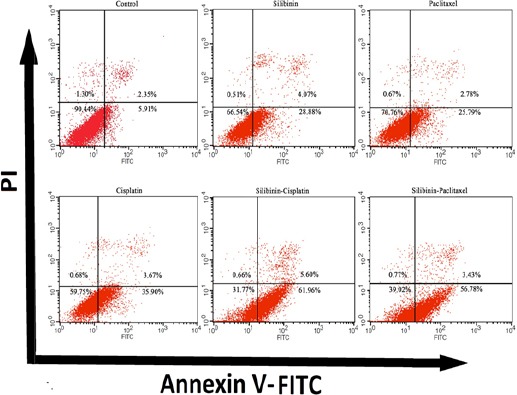
The Combination Therapy Induces Early Phase of Apoptosis in Cancer Cells. Cells were treated with Silibinin, cisplatin and paclitaxel in IC50 concentration including 160 µM, 3.2 µM and 33.4 nM, respectively. Flow cytometry following staining with Annexin V-FITC and PI were done on MCF-7. Data are calculated as mean ± SEM of three dependent experiments (n = 3).
Combined effect of silibinin, cisplatin and paclitaxel on the expression of genes involved in apoptotic pathway
Key pro-apoptotic and anti-apoptotic genes involved in induction of apoptosis were investigated after incubation of the cells with combination of silibinin (160 µM), cisplatin (3.2 µM) and paclitaxel (30 nM) Applying quantitative Real-Time PCR. Our data revealed that the combination treatment significantly decreased the expression levels of Bcl-2. Bax, P53, BRCA1 and ATM mRNA level was increased after treatment with silibinin (160µM) cisplatin (3.2 µM) and paclitaxel (30 nM) in combination (Figure 3). These results revealed that silibinin can control expression of fundamental genes implicated in apoptosis.
Figure 3.
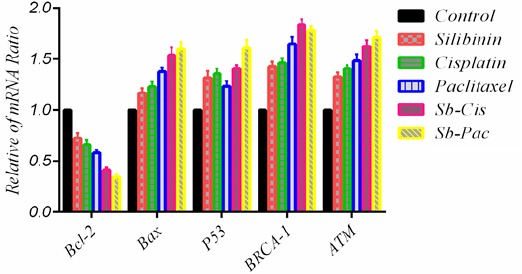
Effect of Combination of Silibinin, Cisplatin and Paclitaxel in Mrna Expression Pattern of, Bcl-2, Bax, P53, BRCA1 and ATM Genes. The results was calculated as the mean ± standard deviation (n=3).
Discussion
Finding and developing novel and advanced strategies to increase the efficacy of chemotherapy is an urgent need. Recently herbal-derived medicines such as silibinin have been investigated as a novel source of anti-cancer drugs (Pirouzpanah et al., 2015). Silibinin has introduced with more properties for anticancer application, chemo-preventive efficacy and nontoxic roles in humans (Singh and Agarwal, 2006). In vitro and animal studies exhibit that the chemoprotective role of silibinin is defined by cell proliferation, angiogenesis and metastasis in several cancers (Gupta et al., 2010). Aim of this study was to detect that silibinin had a potential inhibitory effect on MCF-7 cells in reducing cell growth and increasing apoptosis when incubated as adjuvant along with cisplatin and paclitaxel. The central finding of present study is that silibinin strongly synergizes the therapeutic effect of cisplatin in advanced human MCF-7 cells, via an induction of apoptotic cell death. Combination index (CI) was increased from 0.88 to 0.77 when MCF-7 cells incubated silibinin (150 µM) along with paclitaxel. This phenomenon indicate that silibinin have a potential synergism properties besides of paclitaxel. This finding is significant because paclitaxel as well as other chemotherapy drugs cause high cytotoxicity to normal tissues during treatment. Their adverse health effects, such as immunosuppression and cardiomyopathy, which severely increases in a dose-dependent manner, as well as development of primary or secondary drug resistance in tumor cells, limit their clinical success in cancer chemotherapy (Raina and Agarwal, 2007; Wang et al., 2014). In the present study, at concentrations range of 160 μmole, silibinin inhibited the cell growth and decreased the CI in the face of cisplatin from 71 to 62. Several studies showed that silibinin induced apoptosis via decreased Bcl-2 family levels, and increased pro-apoptotic molecules in different carcinoma cells models (Kauntz et al., 2011; Hagelgans et al., 2014). How silibinin triggered cell death and initiation of signal pathway of apoptosis is not clear. Ghasemi et al., (2013) showed that silibinin had a robust cytotoxic outcome on liver cell line (HepG2 and Hep3B) and triggered the cells undergo apoptosis. In a similar study, silibinin had an inhibitory influence on the growth of MCF-7 cells against UV radiation (Noh et al., 2011). Flowcytometry results showed silibinin could induce cell apoptosis successfully in a dose-dependent manner. Total early and late population of apoptotic cells reach to 33% when incubated with silibinin in concentration 160 µM. Another study with similar results showed that silibinin exerts dose-dependent effects on colorectal cancer cells (Kumar et al., 2015). Consistent with this explanation, the data of our study show evidently that silibinin caused 35% early apoptosis was associated with a noticeable increase up to 61% apoptotic cells in combination with cisplatin. Studies have shown that silibinin has apoptotic and anti-growth effects on different cell lines (Faramarzi et al., 2014). Wang et al revealed the anti-inflammatory and apoptosis-inducing effects of silibinin in brain cells in association with increased expression of Bcl-2 gene (anti-apoptotic) and decreased expression of Bak gene (apoptotic) after stroke (Wang et al., 2014).Tyagi et al., (2006) examined the effect of silibinin on the testicular cancer cells and proposed that the expression of P53 was increased as a result of treatment with silibinin. They showed that caspase 2 was activated and expression of Bid gene was increased. In another study, it was found that silibinin protects dermal and epidermal cells against UV radiation through increasing the expression of P53 gene (Gholami et al., 2017). In parallel study with similar results, Roy et al., (2007) in their study on prostate cancer cells reported the expression of P21after treatment with silibinin increased.This study addressed that silibinin inhibited proliferation, induced apoptosis and caused up regulated BRCA1, ATM, and p53 genes levels in human breast cancer MCF7 Cells.
In conclusion, we showed that silibinin can cause a remarkable increase in efficacy of cisplatin and paclitaxel in induction of apoptosis in MCF-7 breast cancer cell lines. This combination with silibinin leads to synergism effects in cancer cells against to single treatment or anticancer drug resistance. If clinical trials prove the in vivo efficacy of these combinations, they might be able to decrease dose-dependent side effects and possibly increase the overall survival of patients as well as their quality of life.
Acknowledgements
This project was financially supported by a grant from Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Armat M, Bakhshaiesh TO, Sabzichi M, et al. The role of Six1 signaling in paclitaxel-dependent apoptosis in MCF-7 cell line. Bosn J Basic Med Sci. 2016;16:28. doi: 10.17305/bjbms.2016.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- Fabbri F, Carloni S, Brigliadori G, et al. Sequential events of apoptosis involving docetaxel, a microtubule-interfering agent: a cytometric study. BMC Mol Biol. 2006;7:6. doi: 10.1186/1471-2121-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faramarzi H, Forghani N, Forghani A. Effect of Silibinin on the induction of apoptosis and the inhibition of cell growth on the MCF-7 cell line. Eur Online J Nat Soc Sci. 2014;3:404. [Google Scholar]
- Frederiks C, Lam S, Guchelaar H, et al. Genetic polymorphisms and paclitaxel-or docetaxel-induced toxicities: a systematic review. Cancer Treat Rev. 2015;41:935–50. doi: 10.1016/j.ctrv.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Ghaffari SH, Momeny M, et al. Multitargeting and antimetastatic potentials of silibinin in human HepG-2 and PLC/PRF/5 hepatoma cells. Nutr Cancer. 2013;65:590–9. doi: 10.1080/01635581.2013.770043. [DOI] [PubMed] [Google Scholar]
- Gholami M, Moallem SA, Afshar M, et al. Gestational exposure to Silymarin increasessusceptibility of BALB/c mice fetuses to apoptosis. Avicenna J Med Biotechnol. 2017;9:66. [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Prasad S, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelgans A, Nacke B, Zamaraeva M, et al. Silibinin down-regulates expression of secreted phospholipase A2 enzymes in cancer cells. Anticancer Res. 2014;34:1723–9. [PubMed] [Google Scholar]
- Kanekiyo S, Takeda S, Nakajima M, et al. Efficacy and safety of biweekly docetaxel in combination with nedaplatin as second-line chemotherapy for unresectable or recurrent esophageal cancer. Anticancer Res. 2016;36:1923–7. [PubMed] [Google Scholar]
- Kauntz H, Bousserouel S, Gossé F, et al. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis. 2011;16:1042. doi: 10.1007/s10495-011-0631-z. [DOI] [PubMed] [Google Scholar]
- Kumar S, Raina K, Agarwal R. In ‘Multi-targeted approach to treatment of cancer’. Springer; 2015. Chemopreventive and anticancer efficacy of Silibinin against colorectal cancer; pp. 339–50. [Google Scholar]
- Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PloS One. 2013;8:e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadian J, Sabzichi M, Molavi O, et al. Combined treatment with stattic and docetaxel alters the Bax/Bcl-2 gene expression ratio in human prostate cancer cells. Asian Pac J Cancer Prev. 2016;17:5031–5. doi: 10.22034/APJCP.2016.17.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Samadi N, Ghanbari P, et al. Co-treatment by docetaxel and vinblastine breaks down P-glycoprotein mediated chemo-resistance. Iran J Basic Med Sci. 2016;19:300. [PMC free article] [PubMed] [Google Scholar]
- Mostafavi-Pour Z, Ramezani F, Keshavarzi F, et al. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol Lett. 2017;13:1965–73. doi: 10.3892/ol.2017.5619. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Noh E-M, Yi MS, Youn HJ, et al. Silibinin enhances ultraviolet B-induced apoptosis in mcf-7 human breast cancer cells. J Breast Cancer. 2011;14:8–13. doi: 10.4048/jbc.2011.14.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirouzpanah MB, Sabzichi M, Pirouzpanah S, et al. Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev. 2015;16:2087–92. doi: 10.7314/apjcp.2015.16.5.2087. [DOI] [PubMed] [Google Scholar]
- Raina K, Agarwal R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: efficacy and mechanisms. Acta Pharmacol Sin. 2007;28:1466–75. doi: 10.1111/j.1745-7254.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Roy S, Kaur M, Agarwal C, et al. P21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6:2696–707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- Sabzichi M, Mohammadian J, Yari Khosroushahi A, et al. Folate-targeted nanostructured lipid carriers (NLCs) enhance (Letrozol) efficacy in MCF-7 breast cancer cells. Asian Pac J Cancer Prev. 2016a;17:5185–8. doi: 10.22034/APJCP.2016.17.12.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzichi M, Samadi N, Mohammadian J, et al. Sustained release of melatonin: A novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf B. 2016b;145:64–71. doi: 10.1016/j.colsurfb.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Sadava D, Kane SE. Silibinin reverses drug resistance in human small-cell lung carcinoma cells. Cancer lett. 2013;339:102–6. doi: 10.1016/j.canlet.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi S, Barar J, Hejazi MS, et al. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15:8617–22. doi: 10.7314/apjcp.2014.15.20.8617. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics for hispanics/latinos 2012. CA Cancer J Clin. 2012;62:283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–42. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- Tupal A, Sabzichi M, Ramezani F, et al. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J Microencapsul. 2016;33:372–80. doi: 10.1080/02652048.2016.1200150. [DOI] [PubMed] [Google Scholar]
- Tyagi A, Singh RP, Agarwal C, et al. Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip1/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis. 2006;27:2269–80. doi: 10.1093/carcin/bgl098. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, Cai H, Jiang G, et al. Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition. Asian Pac J Cancer Prev. 2014;15:6791–8. doi: 10.7314/apjcp.2014.15.16.6791. [DOI] [PubMed] [Google Scholar]


