Abstract
Background:
Human tissue-type plasminogen activator (t-PA) is a key protease of the trypsin family. It catalyzes the activation of zymogen plasminogen to the fibrin-degrading proteinase, plasmin, leading to digestion of fibrin clots. The recombinant enzyme produced by recombinant technology issued to dissolve blood clots in treatment of various human diseases such as coronary artery thrombosis, pulmonary embolism, acute ischemic stroke (AIS). Pichia pastoris expression system is a unique system for the production of high level of recombinant proteins. GS115 and KM71H are two kinds of Pichia pastoris strains whilst production of recombinant proteins in these strains is not predictable. The aim of the study was evaluation of t-PA expression in KM71H strains.
Methods:
In this study, the cDNA of the t-PA gene was amplified by PCR, sequenced and cloned into Pichia pastoris KM71H host strain using pPICZalphaA expression vector that allows methanol-induced expression and secretion of the protein.
Results:
Dot blotting results confirmed the presence oft-PA in the cell supernatant. Western blotting test revealed the approximate size of 70 KDa for recombinant t-PA. Quantitative ELISA experiment showed 810 µg/L of t-PA in the supernatant samples. Zymography analysis confirmed the proteolytic activity and biological function of the expressed recombinant t-PA.
Conclusions:
Correspondingly, Pichia pastoris KM71H is an appropriate strain for production of active recombinant protein.
Keywords: Tissue plasminogen activator, Pichia pastoris, Fibrin, recombinant proteins
Introduction
Human tissue-type Plasminogen Activator (t-PA) is a kind of plasminogen activator which breaks down thrombin into blood clots (Pinheiro et al., 2013). This protein is contained of 527 amino acids with molecular weight of ~70,000 Dalton. Having five structural domains and 17 disulphide bands, the protein is assumed as a complex macromolecule (Ali et al., 2014). Extensive studies have shown the highly successful treatment of thrombotic occlusion and stroke associated with myocardial infarction in patients accompanied by recombinant t-PA (Broderick et al., 2013). Due to high cost of the recombinant t-PA, in developing countries streptokinase is used instead, while t-PA acts more specific and bind stronger to fibrin clots, and also isn’t immunogenic for human (Mutch et al., 2010; Gebbink, 2011). Since t-PA is a human protein with a complex structure, its production in prokaryotic hosts have encountered various challenges including low production yield, inclusion body formation, misfolding and lack of the activity (Majidzadeh-a et al., 2010; Gupta et al., 2017). Also hyperglycosylation, poor export and inappropriate folding, restrict the production of active t-PA in some eukaryotic hosts such as Saccharomyces cerevisiae and insect cells (Darby et al., 2012; Vhanmarathi et al., 2015). Recently, methylotrophic yeasts, particularly Pichia pastoris, have been one of the best candidates for production of mammalian recombinant proteins in the biopharmaceutical industry due to their ability to accomplish many eukaryotic posttranslational modifications (Lombardi et al., 2010; he Zhu et al., 2011; Liu et al., 2015; Yu et al., 2015). GS115 and KM71H are two strains of P. pastoris which are available for recombinant protein production (Steinle et al., 2010; Ahmad et al., 2014). Since AOX1 gene has been disrupted in KM71H, the growth of this strain in the presence of methanol is slower than GS115 (Lindenmuth and McDonald, 2011; Pedro et al., 2015). In this present study, functional full-length human t-PA was expressed in KM71H host strain.
Materials and Methods
Isolation of t-PA from the melanoma cell line
The melanoma cell line was generously provided to us by Dr. Khalaj, Pasture Institute of Iran, Tehran, toward the end of 2008. It had been achieved originally from pulmonary, metastatic melanoma cells from a patient and was maintained and exchanged among investigators because it secreted large amounts of plasminogen activator activity.
Strains and Vectors
E. coli TOP10F´ and Pichia pastoris KM71H (arg4 aox1: ARG4, MutS, Arg+) were purchased from Invitrogen Corporation (Carlsbad, California, USA). These strains were used for propagation of the expression vector and expression of the recombinant protein respectively.
E. coli TOP10F´ was cultured in Luria-Bertani agar (1% Tryptone, 0. 5% Yeast Extract, 0. 5% NaCl, pH 7. 0) (Merck, Germany). YPD medium (1% yeast extract, 2% peptone, 0. 2% glucose) and YPDS plates (1% yeast extract, 2% peptone, 2% dextrose, 1 M sorbitol, 2% agar) were employed for the growth of Pichia pastoris KM71H. Expression was carried out in Buffered Glycerol-complex Medium (BMGY) and Buffered Methanol-complex Medium (BMMY) (1% yeast extract, 2% peptone, 100 mM potassium phosphate, pH 6. 0, 1. 34% YNB 4 x 10-5% biotin, 1% glycerol for BMGY or 0. 5% methanol for BMMY). pPICZalphaA shuttle vector (Invitrogen, Carlsbad, California, USA) was engaged for integration of the desire gene in genomic DNA of Pichia pastoris.
Primer design and cloning
According to multiple cloning site of pPICZalphaA shuttle vector, XhoI sequence was added to 5´-end of t-PA gene amplifying forward and reverses primers. Owing to the presence of kex2 signal cleavage site in the middle of XhoI restriction sites in MCS of the shuttle vector (Figure 1), the same sequence was added to 5´-end of the forward primer. Given the facts, the forward primer (CTCGAGAA AAGAGAGGCTGAAGCTTCTTACCAAGTGATC) andreverseprimer (CTCGAGTCGGTCGCATGTTGTCACGAATC) were designed. Genomic DNA of CHO 1-15 (ATCC- CRL 9606) cell line (transfected by full length cDNA of human t-PA) was subjected to amplify t-PA gene using the primers. The PCR product was purified through PCR purification kit (Bioneer, Korea) and was ligated into the pTZ57R/T cloning vector (Thermo Scientific, Rochester, USA). E. coli TOP10F´ was transformed with the ligation product according to manufacture instruction. The presence of t-PA gene in the vector was confirmed by PCR and sequencing (Keyvani et al., 2016). The confirmed pTZ57R/T-t-PA was digested with XhoI and the released t-PA sequence was ligated to XhoI digested pPICZalphaA (Majidzadeh et al., 2014). The propagation of pPICZalphaA-t-PA was done by transforming E. coli TOP10F´and culturing on low salt LB medium supplemented with 25 mg/L zeocin. The proper orientation of the insert in pPICZalphaA-t-PA was evaluated by a digestion reaction using SacI endonuclease enzyme.
Figure 1.
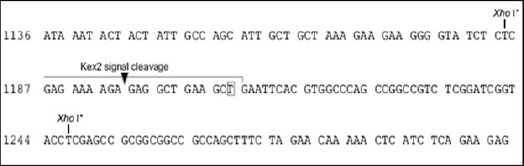
The Parts of the pPICZalphaA Shuttle Vector Multiple Cloning Sites. As seen, the Kex2 signal cleavage site presented in the middle of XhoI restriction sites.
Pichia pastoris Transformation
In order to improve the integration of the pPICZalphaA-t-PA into genomic DNA of Pichia pastoris, a single colony of Pichia pastoris KM71H host strain was cultured in YPD medium at 30oC overnight. Preparation of the competent cells from the overnight culture was performed according to the work instruction of Pichia Expression Kit (Easy Select Pichia Expression Kit, Invitrogen), hence the competent cells were electroporated with 5µg of linearized pPICZalphaA-t-PA with PmeI (Thermo Scientific, Rochester, USA) at 37 0C for 16 h) using a Bio-Rad Gene Pulser instrument (1500 V, 25 IF, 200X). The transformed cells were transferred into YPDS medium supplemented with 100 µg/mL of zeocin and incubated at 30oC for 10 days. Genomic DNA of a single recombinant colony was extracted as described by Yuzbashev et al (Yuzbashev et al., 2010). The presence of the t-PA gene in the recombinant colony was confirmed accompanied by specific primers of t-PA gene through PCR. Real-time PCR-based absolute quantification method was used to estimate copy number of inserted t-PA gene into the transformed P. pastoris (Dhanasekaran et al., 2010). Met2, a single copy gene in P. pastoris genome was cloned into the pTZ57R/T plasmid and used as a reference gene to normalize the assay. After serial diluting of pTZ57R/T-t-PA and pTZ57R/T-Met2 plasmids, their copy numbers were calculated. The t-PA and Met2 SYBR green real-time PCR were conducted on dilutions of the relevant standard plasmids in duplicate. Standard curves were obtained by plotting the threshold cycle (Ct) on the Y-axis and the natural log of concentration (copies/μL) on the X-axis. Also the SYBR Green real time PCR assays were performed on genomic DNA of the transformed P. Pastoris in duplicate. Finally based on the data, copy number of t-PA gene in genomic DNA of the transformed yeast was calculated.
Expression of the recombinant protein inKM71H strain
A single colony was cultured in 25 mL BMGY medium containing 100µg/mL zeocin, at 30 0C overnight. After that, 200 mL of BMGY medium containing 100 µg/mL zeocin was prepared by inoculating 25 mL of the preculture. The culture medium was incubated in shaking incubator for 16 h at 30oC until an OD600= 20. To induce expression, the cells were harvested by centrifuging at 2000 x g for 5 minutes and were resuspended in 400 mL BMMY medium containing 100 µg/mL zeocin. To maintain induction, 0.5% methanol was added every 24 h. For evaluating the growth, the samples were collected at time points 24, 48, 72, 96, 120, and 144 hours, and OD600 of them were measured in these time points. Then, growth curve of recombinant Pichia pastoris KM71H in expression medium was depicted using Microsoft Excel 2010.
Dot Blotting and Western Blotting analysis
The Supernatants of cultures were subjected to evaluation of protein expression using dot and western blotting. In dot blot analysis, the supernatants of 4 to 6 days were transferred to a nitrocellulose membrane (Schleicher and Schuell, Inc., Keene, NH) through blotting instrument (Multiphore IINova Blot Unit, Amersham Pharmacia Biotech, Buckinghamshire, UK). Anti t-PA rabbit Polyclonal antibody (10 unit/mL PBS) (Abcam, USA) and Goat anti-rabbit IgG-HRP (10 unit/15 mL PBS) (Santa Cruz, USA) were used as first and second antibody respectively. Western blotting experiment was performed with the explained antibodies agree to standard protocol demonstrated by Sambrook et al (Sambrook and Russell, 2001).
Quantitative ELISA
The secreted recombinant proteins were employed for quantity determination. Accordingly, 1:1000 ratio of the supernatant (related to 6 day) was subjected to immunoassay by using Human t-PA ELISA (Bender Med Systems, Austria). For design of standard curve, 1:2 serial dilution of reconstituted standard Human t-PA (supplemented with kit) was prepared with the initial concentration of 2000 pg/mL. The assay was performed according to the kit instruction (to draw a standard curve) and in duplicate. Optical absorption measurement for each sample was performed at 450 and 630 nm as the primary and the reference wave length respectively using ELISA reader (Human, Germany).
Zymography test
Biological activity of the recombinant t-PA was assessed by the zymography test as explained previously (Majidzadeh-a et al., 2010). Briefly, 11% resolving polyacrylamide gel was copolymerized with plasminogen (Chromogenix, Italy) and gelatin (Sigma, USA). The 4% staking gel was prepared according to the standard protocols. The samples, in non-reducing condition were electrophoresed in the gel at a constant current of 8 mA (at 4oC). To remove residual SDS, the gel was washed in 2.5% (w/v) triton X-100 for 1h at room temperature. Then the gel was incubated in 0.1 M glycine/NaOH (pH 8.3) for 5 h at 37oC. The final process was done by staining with Coomassie Brilliant Blue G (Acros, USA).
Results
Molecular cloning and transformation
The coding region of t-PA gene showed amplified band of the predicted size, 1600bp (data not shown). After cloning of PCR product in T-vector, examination of the desired DNA in T-vector was performed with sequencing process. Sequence analysis with CLC sequence viewer software (version 6) didn’t show mutation in the coding region of t-PA sequence in the pTZ57R/T-t-PA. Restriction analysis of pPICZalphaA-t-PA with SacI enzyme revealed the existence of 2992 bp and 2208 bp bands in agarose gel electrophoresis (Figure 2). This proved the correct orientation of t-PA sequence in-frame to the alpha factor secretion signal, downstream of the alcohol oxidase I promoter in pPICZalphaA. Integration of the expression cassette into the host strain resulted in creation of the recombinant Pichia pastoris KM71H which was resistant to zeocin and could grow on YPDS medium supplemented with zeocin antibiotic. PCR analysis of genomic DNA of the recombinant colony revealed the existence of the t-PA (data not shown). According to real time PCR-based absolute quantification method, the copy numbers of t-PA gene in genomic DNA of recombinant KM71H strains were calculated 1 copy.
Figure 2.
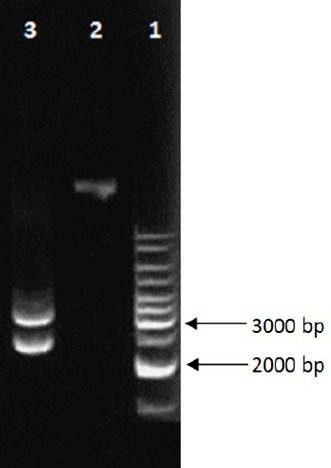
The Results Related to Restriction Map Preparation from Plasmid Constructs via SacI Enzyme. Line 1: 1 kb DNA marker; lines 2: corresponding to undigested plasmid; lines 3: corresponding to digested plasmid. The presence of 2992 bp and 2208 bp bands after digestion of recombinant construct proved the correct orientation of t-PA in pPICZalphaA.
Recombinant protein expression analysis
The confirmed recombinant colonies were subjected for recombinant protein production in BMMY medium. Growth curve of cells, transformed by pPICZalphaA-t-PA under induction of 0.5% methanol, showed progressive increase in growth of the recombinant Pichia pastoris in the expression medium. Maximum growth was obtained at 144 hours with OD600=35. 5. Dot blotting test revealed the expression of the recombinant t-PA in the culture supernatants. As seen in Figure 3A, the level of the recombinant protein production at different time points was not equal while the highest level of the protein expression happened at 144 h. Western blot analysis of the recombinant t-PA showed the approximate size of 70 KDa (Figure 3B). Using quantitative ELISA, A450 of the standard Human t-PA dilution series and the test samples were calculated. According to concentrations of the standard Human t-PA dilution series against of A450, standard curve was depicted with R2 = 0.9985 (Figure 4). Based on the standard curve, the quantity amounts of the extracellular recombinant t-PA was determined 810.79 µg/L.
Figure 3.
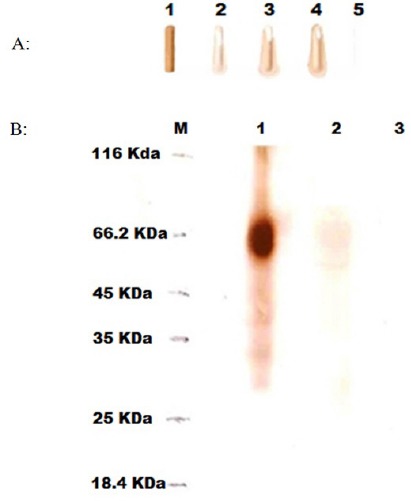
Dot Blotting (A) and western blotting (B) analysis results. A: The lane 1: Positive control (t-PA standard, Actylase); lane 2-4 related to supernatants of time points 96 (4 days), 120 (5 days), and 144 (6 days) respectively; lane 5: Negative control (The supernatant of Pichia pastoris KM71H without t-PA gene). B: M: Protein marker; lane 1: Positive control (t-PA standard, Actylase); lane 2: the supernatant of recombinant Pichia pastoris KM71H; lane 3: Negative control (The supernatant of Pichia pastoris KM71H without t-PA gene).
Figure 4.
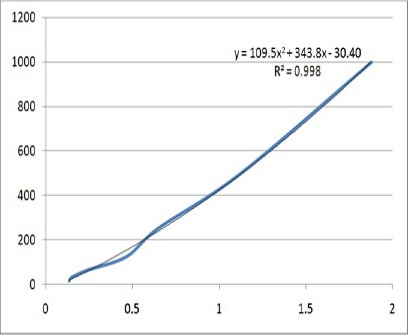
The Standard Curve Related to Concentrations of Standard Human t-PA against of A450. According to This Curve, the Quantity of Extracellular t-PA Recombinant Protein (1/1,000) Calculated 810.79 µg/L.
Zymography
According to Figure 5, the supernatants of the expression medium which had been applied in native condition (without boiling), showed white band in dark blue background due to the production of plasmin with cleavage of plasminogen by the recombinant t-PA. At the presence of plasmin, the gelatin is digested and white band is created. But the boiled supernatant sample did not generate white band. The results confirmed biological and proteolytic activity of the recombinant t-PA which had been expressed by the transformed Pichia pastoris KM71H.
Figure 5.
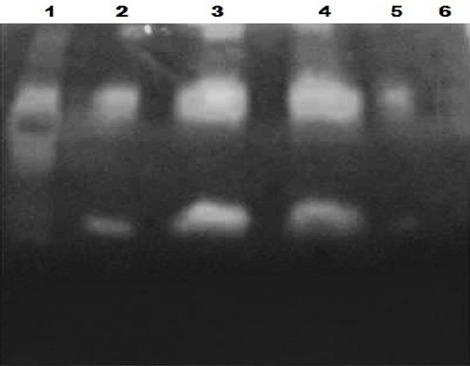
The Result of Zymography Test. The lane 1: Positive control (t-PA standard, Actylase); lane 2 and 3 related to unconcentrated and concentrated supernatants of time point 120 (5 days) respectively; lane 4 and 5 related to unconcentrated and concentrated supernatants of time point 144 (6 days) respectively; lane 6: related to boiled supernatant of time point 144 as negative control.
Discussion
Acute ischemic stroke is a leading cause of death worldwide and in developed countries stroke is one of the major cause for adult long-term disability. Human tissue plasminogen activator (t-PA) is one of the best candidates for recombinant production of proteins for treatment of some diseases such strokes, also t-PA may play a significant role in the metastatic process (Wardlaw et al., 2012). Because of the complexity of human t-PA (a glycosylated protein containing 527 amino acids and 17 disulfide bands), prokaryotic systems are incapable to produce biologically active form of the protein. Owing to eukaryotic source of the protein, in recent years, the production of recombinant forms of t-PA protein have been reported in various eukaryotic host cells. Researcher expressed t-PA in filamentous fungus, Aspergillus nidulans. Muller et al., (2015) used Drosophila melanogaster cell line as a host for production of t-PA (Müller et al., 2015). Hou et al., (2012) reported production of this protein in Saccharomyces cerevisiae (Hou et al., 2012). Soleimani et al., (2006) evaluated Leishmania tarentolae, a nonpathogenic protozoan for expression of recombinant t-PA (Soleimani et al., 2007).
CHO cells (Chinese-hamster ovary) are one of the other eukaryotic expression systems using for production of recombinant t-PA. But high cost and the likelihood of contamination of cell-culture media with viruses and prions are some disadvantages of these systems. Currently recombinant t-PA drug is produced commercially in CHO cell line (Davami et al., 2011). Regardless of the above mentioned situations, some limitations (such as hyperglycosylation and heterogenicity in the produced recombinant t-PA protein), have been described to produce the recombinant t-PA in the eukaryotic hosts and efforts are continuing to search for better hosts with fewer drawbacks (Majidzadeh-a et al., 2010). Not very long ago, Pichia pastoris expression system has been introduced to produce recombinant proteins. Pichia pastoris has been used effectively to produce proteins across a wide range of functional types, including antigens, enzymes and engineered antibody fragments, such as P-Glycoprotein, human angiogenin, L1 protein from different types of HPV, xylanase, rHSA fusion protein, rIL-3and many more (Gasser et al., 2013). Comparing to other eukaryotic expression systems, this system has more advantages such as 10- to 100-fold higher heterologous protein expression levels. Unlike Saccharomyces cerevisiae, Pichia pastoris doesn’t hyperglycosylate heterologous recombinant protein and doesn’t recruit core oligosaccharides containing terminal α 1, 3 glycan linkages to glycosylate the recombinant protein. It has been suggested that α 1, 3 glycan linkages in glycosylated proteins cause hyper-antigenic properties, so these proteins are not appropriate for therapeutic uses. Consequently, Pichia pastoris was successfully used to produce biologically active recombinant-t-PA protein. Full length t-PA gene was amplified with well-designed primers and purified PCR product was cloned into pPICZalphaA expression vector. Native α-factor secretion signal sequence incorporated into vector permits extracellular expression of recombinant t-PA protein. The expression cassette pPICZalphaA-t-PA was linearized and electroporated into Pichia pastoris KM71H strain to achieve genome integration and consistency of t-PA gene. Isolated colonies grown on YPDS medium supplemented with Zeocin were screened for positive transformants. Majidzadeh et al. (2010) employed Pichia pastoris GS115 host strain for expression of human t-PA. They obtained high-level of the recombinant t-PA (1650 IU/mL) as the secretory form in supernatant of the transformed host. They reported 10 mg/L of produced recombinant t-PA in GS115 strain using densitometric technique (Majidzadeh-a et al., 2010). In this study, the amount production of the recombinant t-PA in culture supernatant of the KM71H strain was determined 0.81 mg/L with Human t-PA ELISA (Bender Med Systems, Austria). This variation may correlate with the difference in copy number of integrated t-PA gene in genomic DNA of the strains or slower growth of KM71H than GS115 strain.
One of the major problems to produce recombinant t-PA is low biological activity of the expressed protein due to its complex structure and the existence of 17 disulphide bands (Goojani et al., 2013). For example transformed E. coli produce large amount of recombinant t-PA protein but owing to create inclusion bodies, the expressed protein is not biologically active (Zhang et al., 2011). Whilst in this study, zymography test, a sensitive analytical assay (detect less than 10 pg of protease enzymes), confirmed proteolytic and biological activity of the KM71H produced recombinant t-PA. In the mentioned test, one sample of cell supernatant which had been inactive with 2ME and boiling, was used for the accuracy assessment of zymography test, which didn’t create white band correspond to gelatin hydrolysis. Negative proteolytic activity of the supernatant (lane 5, Figure 5) proved the accuracy of zymography test for differentiation between active and inactive protein.
In conclusion, the successful cloning and expression of biologically active recombinant t-PA protein using Pichia pastoris KM71H strain was accomplished in this study. It should be noted that enzyme activity quantification of recombinant protein, optimization of the Pichia pastoris expression system, and further process optimization at larger scales production must be done in order to obtain high yield and cost effective production of recombinant t-PA protein.
Conflict of interest
There is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors would like to acknowledge and appreciate the Faculty of Medicine, AJA University of Medical sciences and Tasnim Biotechnology Research Center (TBRC), for their support and contribution to this study.
References
- Ahmad M, Hirz M, Pichler H, et al. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol. 2014;98:5301–17. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MR, Salim Hossain M, Islam MA, et al. Aspect of thrombolytic therapy: a review. Scientific World J. 2014;2014:1–8. doi: 10.1155/2014/586510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RA, Cartwright SP, Dilworth MV, et al. In ‘Recombinant Protein Production in Yeast: Methods and Protocols’. Totowa, NJ: Eds Humana Press; 2012. Which yeast species shall I choose? Saccharomyces cerevisiae versus Pichia pastoris (Review) pp. 11–23. [DOI] [PubMed] [Google Scholar]
- Davami F, Sardari S, Majidzadeh A, et al. A novel variant of t-PA resistant to plasminogen activator inhibitor-1;expression in CHO cells based on in silico experiments. BMB Rep. 2011;44:34–9. doi: 10.5483/BMBRep.2011.44.1.34. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran S, Doherty TM, Kenneth J. Comparison of different standards for real-time PCR-based absolute quantification. J Immunol Methods. 2010;354:34–9. doi: 10.1016/j.jim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Gasser B, Prielhofer R, Marx H, et al. Pichia pastoris: protein production host and model organism for biomedical research. Future Microbiol. 2013;8:191–208. doi: 10.2217/fmb.12.133. [DOI] [PubMed] [Google Scholar]
- Gebbink M. Tissue-type plasminogen activator-mediated plasminogen activation and contact activation, implications in and beyond haemostasis. J Thromb Haemost. 2011;9:174–81. doi: 10.1111/j.1538-7836.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- Goojani HG, Javaran MJ, Nasiri J, et al. Expression and large-scale production of human tissue plasminogen activator (t-PA) in transgenic tobacco plants using different signal peptides. Appl Biochem Biotechnol. 2013;169:1940–51. doi: 10.1007/s12010-013-0115-4. [DOI] [PubMed] [Google Scholar]
- Gupta V, Sengupta M, Prakash J, et al. in ‘basic and applied aspects of biotechnology’. Singapore: Springer Singapore; 2017. Production of recombinant pharmaceutical proteins; pp. 77–101. [Google Scholar]
- he Zhu W, nan Sun M, sheng Wang Y, et al. Expression and functional characterization of a recombinant targeted toxin with an uPA cleavable linker in Pichia pastoris. Protein Expr Purif. 2011;76:184–9. doi: 10.1016/j.pep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Hou J, Tyo KE, Liu Z, et al. Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res. 2012;12:491–510. doi: 10.1111/j.1567-1364.2012.00810.x. [DOI] [PubMed] [Google Scholar]
- Keyvani H, Saroukalaei ST, Mohseni AH. Assessment of the Human cytomegalovirus UL97 gene for identification of resistance to ganciclovir in Iranian immunosuppressed patients. Jundishapur J Microbiol. 2016;9:31733–9. doi: 10.5812/jjm.31733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmuth BE, McDonald KA. Production and characterization of Acidothermus cellulolyticus endoglucanase in Pichia pastoris. Protein Expr Purif. 2011;77:153–8. doi: 10.1016/j.pep.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhao B, Zhang Y, et al. High-level expression, purification, and enzymatic characterization of truncated human plasminogen (Lys531-Asn791) in the methylotrophic yeast Pichia pastoris. BMC Biotechnol. 2015;15:50–8. doi: 10.1186/s12896-015-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A, Bursomanno S, Lopardo T, et al. Pichia pastoris as a host for secretion of toxic saporin chimeras. FASEB J. 2010;24:253–65. doi: 10.1096/fj.08-118042. [DOI] [PubMed] [Google Scholar]
- Majidzadeh-a K, Khalaj V, Fatemeh D, et al. Cloning and expression of functional full-length human tissue plasminogen activator in Pichia pastoris. Appl Biochem Biotechnol. 2010;162:2037–48. doi: 10.1007/s12010-010-8979-z. [DOI] [PubMed] [Google Scholar]
- Majidzadeh K, Mohseni A, Soleimani M. Construction and evaluation of a novel internal positive control (IPC) for detection of Coxiella burnetii by PCR. Jundishapur J Microbiol. 2014;7:8849–55. doi: 10.5812/jjm.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Salzig D, Czermak P. Considerations for the process development of insect-derived antimicrobial peptide production. Biotechnol Prog. 2015;31:1–11. doi: 10.1002/btpr.2002. [DOI] [PubMed] [Google Scholar]
- Mutch NJ, Engel R, de Willige SU, et al. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115:3980–8. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- Pedro A, Oppolzer D, Bonifacio M, et al. Evaluation of MutS and Mut+Pichia pastoris strains for membrane-bound catechol-O-methyltransferase biosynthesis. Appl Biochem Biotechnol. 2015;175:3840–55. doi: 10.1007/s12010-015-1551-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro M, Gomes K, Dusse L. Fibrinolytic system in preeclampsia. Clin Chim Acta. 2013;416:67–71. doi: 10.1016/j.cca.2012.10.060. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor laboratory press; 2001. pp. 48–52. [Google Scholar]
- Soleimani M, Mahboudi F, Davoudi N, et al. Expression of human tissue plasminogen activator in the trypanosomatid protozoan Leishmania tarentolae. Biotechnol Appl Biochem. 2007;48:55–61. doi: 10.1042/BA20060217. [DOI] [PubMed] [Google Scholar]
- Steinle A, Witthoff S, Krause JP, et al. Establishment of cyanophycin biosynthesis in Pichia pastoris and optimization by use of engineered cyanophycin synthetases. Appl Environ Microbiol. 2010;76:1062–70. doi: 10.1128/AEM.01659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vhanmarathi AM, Matlani R, Lali AM. cloning and extracellular expression of recombinant tissue plasminogen activator (rt-pa) using a methylotrophic yeast pichia pastoris. Biosci Eng Int J (BIOEJ) 2015;2:1–11. [Google Scholar]
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–72. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Zhu Q, Chen K, et al. Improving the secretory production of the heterologous protein in Pichia pastoris by focusing on protein folding. Appl Biochem Biotechnol. 2015;175:535–48. doi: 10.1007/s12010-014-1292-5. [DOI] [PubMed] [Google Scholar]
- Yuzbashev TV, Yuzbasheva EY, Sobolevskaya TI, et al. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol Bioeng. 2010;107:673–82. doi: 10.1002/bit.22859. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chou CP, Moo-Young M. Disulfide bond formation and its impact on the biological activity and stability of recombinant therapeutic proteins produced by Escherichia coli expression system. Biotechnol Adv. 2011;29:923–9. doi: 10.1016/j.biotechadv.2011.07.013. [DOI] [PubMed] [Google Scholar]


