Abstract
Background:
Deviation of host immune response by engagement of inhibitory receptors is one of the well-known mechanisms of tumor cells for immune evasion and survival. PD-1/PD-L1 and Tim-3/Gal-9 axes are two major pathways in this area which their contribution has been documented in a variety of malignancies. In this study, Gal-9 and PD-L1 expression was investigated in leukemic cells from patients with Chronic Lymphocytic Leukemia (CLL).
Methods:
Peripheral blood mononuclear cells (PBMCs) were obtained from 25 untreated CLL patients and 15 sex- and age-matched healthy controls. CLL patients were classified into different clinical stages based on the Rai staging system. Total RNA was extracted from all samples and applied for cDNA synthesis. Relative expression of Gal-9 and PD-L1 mRNA was determined by Real-Time PCR using β-actin as a housekeeping gene.
Results:
Gal-9 and PD-L1 mRNA was significantly more expressed in CLL patients compared to healthy controls (p<0.0001 and p=0.005, respectively). CLL patients in advanced clinical stages showed higher expression of Gal-9 and PD-L1 in comparison to patients in early clinical stages (p<0.0001 and p=0.004, respectively).
Conclusion:
Our promising results regarding over-expression of Gal-9 and PD-L1 in CLL patients call future complementary studies to more evaluate and confirm these pathways for immunotherapy approaches of this malignancy. Upregulation of both Gal-9 and PD-L1 in CLL patients with advanced clinical stages introduces them as useful prognostic biomarkers for disease progression.
Keywords: Exhausted T cell, galectin-9, PD-L1, chronic lymphocytic leukemia
Introduction
According to the cancer immune-surveillance theory, the host immune system can identify neoplastic cells and eliminate them when they arise (Sakuishi et al., 2010; Brusa et al., 2013). Anti-tumor immune response mechanisms play substantial roles to prevent tumor progression. But, tumor microenvironments inhibit the host anti-tumor immune responses through several immunosuppressive mechanisms (Wherry, 2011). One of the most important immunosuppressive mechanisms is the induction of T cell exhaustion (Wherry, 2011). Chronic Lymphocytic Leukemia (CLL) is the most common adult leukemia in western countries, and accounting for about 25-30% of all cases of adult leukemia (Ghia et al., 2007). Recent studies have indicated dysfunction of cell mediated immunity in CLL patients because of T cell exhaustion (Riches et al., 2013). T cell exhaustion describes a state of T lymphocyte dysfunction which commonly develops from persistence of antigens in chronic infections and tumors (Jin et al., 2011). Exhaustion is associated with the loss of T cell function in terms of proliferation, cytokine production, cytotoxicity as well as high level expression of multiple inhibitory receptors, including T cell immunoglobulin and mucin-domain containing-3 (Tim-3), programmed death-1 (PD-1, CD279), lymphocyte activation gene-3 (LAG-3), cytotoxic T lymphocyte associated protein-4 (CTLA-4), 2B4 and CD160 (Yi et al., 2010). Among these inhibitory receptors, PD-1 and Tim-3 are two important immune checkpoint molecules which have already been introduced as major T cell exhaustion markers in various chronic pathological conditions (Sakuishi et al., 2010; Zhou et al., 2011). PD-1, a member of CD28 receptor super-family, is a major immune checkpoint receptor in exhausted T cells (Butte et al., 2007; Mumprecht et al., 2009). PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273) are two identified ligands of PD-1 which are commonly expressed on the surface of T cells, B cells, dendritic cells, macrophages and tumor cells (Butte et al., 2007; Mumprecht et al., 2009). When PD-1 binds to PD-L1, an inhibitory signal is transmitted which reduces T cell response such as cytokine production and proliferation (Butte et al., 2007). Tumor cells exploit this immune checkpoint pathway to evade from host immune responses (Brusa et al., 2013). It has been documented in previous study that blockade of PD-1/PD-L1 pathway could partially inhibit tumor growth and thus alternative pathways are involved (Koyama et al., 2016). Tim-3 is another inhibitory receptor which is mainly expressed on activated T cells, B cells and natural killer cells (Monney et al., 2002). Among different ligands identified for Tim-3, Galectin-9 (Gal-9) is the most studied one which is expressed on various tumor cells (Zhu et al., 2005; Zitvogel and Kroemer, 2012). Several previous studies have shown that PD-1/ PD-L1 and Tim-3/Gal-9 interactions attenuate T cell expansion and effectors function in tumor microenvironment and chronic infection (Sakuishi et al., 2010; Wherry, 2011; Brusa et al., 2013). Moreover, recent studies have indicated that co-expression of Tim-3 and PD-1 on T cells is associated with more severe exhaustion features (Jin et al., 2010). In our previous studies, the increased expression of Tim-3 and PD-1 together with functional defects have been documented in T-CD8+ (Taghiloo et al., 2017) and T-CD4+ (manuscript under review) of CLL patients. In this study, we hypothesized that leukemic cells from CLL patients increase the expression of Gal-9 and PD-L1 to interact with the inhibitory receptors, Tim-3 and PD-1, for induction of T cell exhaustion. A more comprehensive understanding about the inhibitory receptors and their ligands involved in T cell exhaustion may reveal a potential therapeutic target to restore the T cell function and control tumor progression.
Materials and Methods
Patients and healthy donors
Peripheral blood was obtained from 25 CLL patients who had not received any chemotherapy regimen attending the Hematology and Oncology Clinic of Imam Khomeini Hospital, affiliated to Mazandaran University of Medical Sciences, and 15 age and sex-matched healthy controls. Written informed consents were taken from all participants and the study was approved by the Ethical Committee of Mazandaran University of Medical Sciences. CLL diagnosis was done based on clinical evaluation, peripheral blood cell count, cell morphology, and immunophenotyping analysis according to the criteria outlined by WHO. CLL patients were clinically classified into different stages according to the Rai staging system and National Cancer Institute Working Group (NCI-WG) criteria. The Major clinical and laboratory characteristics of CLL patients and healthy controls are summarized in Table 1. The immunophenotyping profile of all CLL patients was previously described (Allahmoradi et al., 2017).
Table 1.
Major Clinical and Laboratory Characteristics of CLL Patients and Healthy Controls
| Characteristics | CLL Patients | Healthy Controls | p-value |
|---|---|---|---|
| Number of subjects | 25 | 15 | |
| Sex | |||
| Male | 13 | 9 | 0.24 |
| Female | 12 | 4 | |
| Age (years) | |||
| Mean ± SEM | 62.24 ± 2.12 | 58.13 ± 2.53 | 0.34 |
| Range | 48 – 84 | 35 -77 | |
| WBC×103/mm3 | |||
| Mean ± SEM | 39.79 ± 4.79 | 7.50 ± 0.49 | < 0.0001 |
| Range | 13.48 – 112.0 | 4.20 – 9.79 | |
| Lym (%) | |||
| Mean ± SEM | 80.61 ± 2.18 | 35.83 ± 1.20 | < 0.0001 |
| Range | 56 – 95 | 27 – 40 | |
| PLT×103/mm3 | |||
| Mean ± SEM | 173.9 ± 15.39 | 214.6 ± 12.59 | 0.04 |
| Range | 38 – 365 | 131 – 270 | |
| Hb (g/dl) | |||
| Mean ± SEM | 12.19 ± 0.44 | 13.47 ± 0.46 | 0.04 |
| Range | 6 – 15 | 11 – 15 | |
CLL, chronic lymphocytic leukemia; WBC, white blood cell count; Lym, lymphocytes percent in peripheral blood; Hb, hemoglobin; PLT, platelet count; SEM, standard error of mean; P-values < 0.05 were considered significant.
RNA isolation and cDNA synthesis
Peripheral blood mononuclear cells (PBMCs) were separated via Ficoll-Histopaque density gradient centrifugation according to the manufacturer’s instructions (Biosera, Nuaille, France). Total RNA was isolated from 8×106 PBMCs using RNeasy kit (CinnaGen, Tehran, Iran) based on the manufacturer’s protocol. The quality of isolated RNA was confirmed by nano-spectrophotometer (WPA, Cambridge, England) and electrophoresis. Complementary DNA (cDNA) was reverse-transcribed from 1 microgram of total RNA in a 20µl reaction mixture containing 1μl random hexamer primer, 4μl of 5x reaction buffer, 1μl RNase inhibitor, 2 μl dNTP 10mM, 200 unit RevertAid M-MuLV reverse transcriptase enzyme and appropriate RNase/DNase free water. The mixture was then incubated at 25°C for 5 min, 42°C for 1 hour and 70°C for 5 min using the Thermo Scientific RevertAid first strand cDNA synthesis kit (Thermo Scientific, Massachusetts, USA).
Semi-quantitative Real-Time PCR
Real-Time PCR was performed using Thermo Scientific Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, Massachusetts, USA) reagent in an iCycler iQ5 Real-Time PCR system (Bio-Rad, California, USA) with the following primers: Gal-9, forward: CAG TGC TCA GAG TTC CAC A, reverse: TGA GGC AGT GAG CTT CAC AC; PD-L1, forward: CTA TGG TGG TGC CGA CTA CAA, reverse: CTG CTT GTC CAG ATG ACT TCG; β-actin, forward: CCT TCC TGG GCA TGG AGT CCT, reverse: TGG GTG CCA GGG CAG TGA T. PCR reactions were amplified at 95°C for initial denaturation followed by 40 cycles at 94°C for 30 seconds, 59°C (Gal-9), 61°C (PD-L1) and 57°C (β-actin) for 30 seconds, and extension at 72°C for 30 seconds. The PCR amplicon sizes were 118 bp, 159 bp and 174 bp for Gal-9, PD-L1 and β-actin, respectively. Each run was completed with a melting curve analysis to confirm the specificity of the amplification and the absence of the primer dimers. Relative expression level of Gal-9 and PD-L1 mRNA was determined with 2-ΔCt value using β-actin as an internal housekeeping gene.
Statistical analysis
All statistical analyses were performed using SPSS 20 for Windows. Normality distribution of the obtained data was determined by Kolmogorov-Smirnov test. Mann-Whitney U test was considered to compare the mean differences between two groups. Spearman’s rank correlation analysis was used to calculate the correlation coefficients. Data are expressed as means ± standard error of mean (SEM) and p-values less than 0.05 were considered significant. All graphs were prepared using the Graphpad Prism 6 software.
Results
Upregulation of Gal-9 and PD-L1 mRNA in peripheral blood of CLL patients
To investigate the mRNA expression profile of Gal-9 and PD-L1, PBMCs were isolated from CLL patients and healthy controls. Relative expression of Gal-9 and PD-L1 mRNA was evaluated in all samples by a semi-quantitative Real-Time PCR method using β-actin as an internal control. The primer efficiencies were defined as 100.4% and 104% for Gal-9 and PD-L1, respectively. Amplification curve and melting curve analysis results obtained for Gal-9, PD-L1 and β-actin are illustrated in supplementary figure. Our results demonstrated that Gal-9 was significantly over-expressed in CLL patients compared to healthy controls (p < 0.0001, Figure 1A and B). Similar to Gal-9, our results showed the upregulation of PD-L1 immune checkpoint molecule in CLL patients in comparison with healthy controls (p = 0.005, Figure 1C and D).
Figure 1.
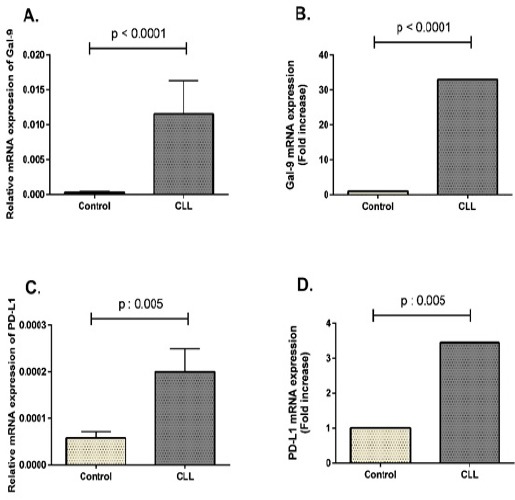
Gal-9 and PD-L1 mRNA Expression Profile in CLL Patients and Healthy Controls. Total RNA was extracted from peripheral blood and single-strand cDNA was synthesized. Real-Time PCR was performed with specific primers for Gal-9, PD-L1 and β-actin. (A) Relative mRNA transcript levels of Gal-9 in CLL patients and healthy controls. (B) Fold increase of Gal-9 mRNA expression in CLL patients compared to healthy groups. (C) Relative mRNA transcript levels of PD-L1 in CLL patients and healthy controls. (D) Fold increase of PD-L1 mRNA expression in CLL patients compared to healthy groups. Gene expression results are represented as mean ± SEM of 2-ΔCt after normalization with β-actin as an internal control.
Gal-9 and PD-L1 mRNA were more expressed in CLL patients at advanced clinical stages
In our previous study, we have indicated more expression of Tim-3 and PD-1 in T-CD8+ (Taghiloo et al., 2017) and T-CD4+ (manuscript under review) lymphocytes of CLL patients at advanced clinical stages compared to early stages. In the current study, the mRNA expression profile of their ligands, Gal-9 as Tim-3 ligand and PD-L1 as a ligand for PD-1, was explored in different clinical stages of CLL patients. As shown in Figure 2, the relative expression levels of both Gal-9 and PD-L1 were significantly higher than in CLL patients at advanced clinical stages when compared to patients at early stages of the disease (p < 0.0001 and p = 0.004, respectively).
Figure 2.
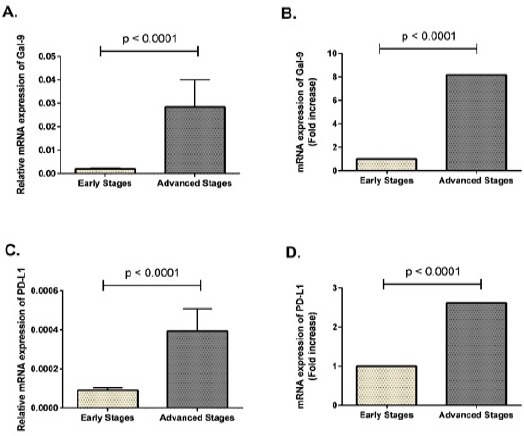
Gal-9 and PD-L1 Were More Expressed in Advanced Clinical Stages of CLL Patients. Total RNA was extracted from peripheral blood and single-strand cDNA was synthesized. Real-Time PCR was performed with specific primers for Gal-9, PD-L1 and β-actin. (A) Relative mRNA transcript levels of Gal-9 in early and advanced clinical stages of CLL patients. (B) Fold increase of Gal-9 mRNA expression in advanced clinical stages of CLL patients compared to early stages. (C) Relative mRNA transcript levels of PD-L1 in early and advanced clinical stages of CLL patients. (D) Fold increase of PD-L1 mRNA expression in advanced clinical stages of CLL patients compared to early stages. Gene expression results are represented as mean ± SEM of 2-ΔCt after normalization with β-actin as an internal control.
Gal-9 and PD-L1 expression was positively correlated with Tim-3 and PD-1 expression in CLL patients
We have shown in our previous study that Tim-3 and PD-1 are highly expressed on T lymphocytes of our CLL patients (Taghiloo et al., 2017). To find any correlations between Tim-3 and PD-1 expression with the expression of their ligands in CLL patients, Gal-9 and PD-L1, the current results were analyzed with Tim-3 and PD-1 expression data of CLL patients from our previous findings. As shown in Figure 3A, Gal-9 mRNA expression was significantly associated with the frequency of Tim-3+/CD8+ T cells (r = 0.426, p = 0.04). Moreover, PD-L1 mRNA expression was also significantly correlated with the frequency of PD-1+/CD4+ T cells in CLL patients (r = 0.608, p = 0.001) (Figure 3B). There were no significant correlations between Gal-9 and Tim-3 expression in CD4+ T-cells and also between PD-L1 and PD-1 expression in CD8+ T-cells of CLL patients.
Figure 3.
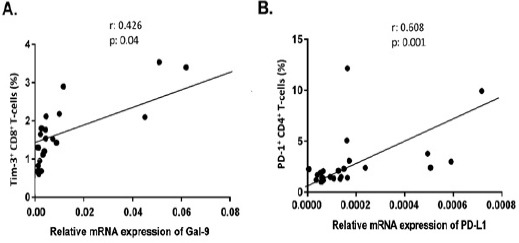
Correlation Analysis of Gal-9 and PD-L1 mRNA Expression with the Frequency of Tim-3+ or PD-1+ T lymphocytes in CLL patients. Gal-9 mRNA expression was significantly associated with the frequency of Tim-3+/CD8+ T cells (r = 0.426, p = 0.04) (4A). PD-L1 mRNA expression was also significantly correlated with the frequency of PD-1+/CD4+ T cells in CLL patients (r = 0.608, p = 0.001) (4B).
Discussion
T cell exhaustion is a state of acquired T cell dysfunction originally described in the context of chronic viral infections, solid tumors and hematologic malignancies (Sakuishi et al., 2010; Leite et al., 2015). In the tumor microenvironment, exhausted cells are characterized by upregulation of inhibitory receptors, decreasing in production of effector cytokines and low cytotoxic activity leading to the failure of cancer elimination (Jiang et al., 2015). Among the various inhibitory receptors, Tim-3/Gal-9 and PD-1/PD-L1 inhibitory axes are two important regulatory pathways which play key roles in the induction of exhaustion features during numerous chronic infections and tumors (Wherry, 2011; Kahan et al., 2015). In our previous studies, upregulation of both Tim-3 and PD-1 were detected on CD8+ T cells (Taghiloo et al., 2017) and CD4+ T cells (manuscript under review) of patients with CLL. To more confirm the involvement of these regulatory pathways in CLL, the current study has demonstrated the over-expression of Gal-9 and PD-L1 as the main ligands of Tim-3 and PD-1 in this chronic B cell leukemia. Furthermore, Gal-9 and PD-L1 were predominantly displayed at advanced clinical stages of CLL introducing them as possible prognosis biomarkers.
Accumulating evidence indicates that galectins family fall into the category of immune regulatory molecules in tumors, autoimmune diseases, chronic inflammatory problems and other immune pathologic conditions (Liang et al., 2008; Merani et al., 2015). Previous reports have demonstrated upregulation of Gal-9 in various tumors including renal cell carcinoma (Fu et al., 2015), cervical squamous cell carcinoma (Liang et al., 2008), as well as in murine AML (Zhou et al., 2011). Although the expression profile of some other galectin family members has been studied in CLL patients (Asgarian-Omran et al., 2010), there are not any reports evaluating Gal-9 expression pattern and involvement of this regulatory molecule in CLL patients. In our previous study, we have observed that Tim-3+/CD8+ T cells are increased in CLL patients and their frequency is correlated with more dysfunction of isolated CD8+ T cells (Taghiloo et al., 2017). So, possibly upregulation of Gal-9 by CLL leukemic cells and engagement of Tim-3 on immune cells help them to induce exhaustion processes in infiltrated CD8+ T cells and evade from immune effector anti-tumor mechanisms. Similar to Gal-9, unusual expression of PD-L1 has also been indicated in various human tumors, including breast cancer, ovarian carcinoma, melanoma, lung cancer, colon cancer (Dong et al., 2002; Leite et al., 2015) as well as in CLL patients (Brusa et al., 2013; Grzywnowicz et al., 2015; Li et al., 2017). Over-expression of PD-L1 is required for tumor cells to interact with regulatory receptor PD-1 on tumor infiltrated lymphocytes and escape from immune surveillance mechanisms (Brusa et al., 2013; Zeng et al., 2016). In a therapeutic perspective, blockade of the PD-1/PD-L1 and Tim-3/Gal-9 pathways by monoclonal antibodies have been described as a successful strategy to improve the anti-tumor immune responses in murine model of human malignancies (Zhou et al., 2011; Grzywnowicz et al., 2012). Now, it has been well accepted that blocking of PD-1/PD-L1 pathway alone does not completely restore T cell function indicating the involvement of supplementary negative regulatory pathways such as Tim-3/Gal-9 in T cell exhaustion (Koyama et al., 2016). Blocking of Tim-3/Gal-9 pathway has shown synergistic effects with PD-1/PD-L1 pathway inhibition to restore the function of exhausted CD8+ T cells (Sakuishi et al., 2010; Zhou et al., 2011). So, our findings from this study regarding Gal-9 and PD-L1 over-expression together with our previous results about Tim-3 and PD-1 upregulation (Taghiloo et al., 2017) in CLL patients may introduce these pathways as potential targets for combined immunotherapy. Consistent with this statement, Sakuishi et al., (2010) have reported that combined targeting of Tim-3/Gal-9 and PD-1/PD-L1 pathways is highly effective to control the proliferation of solid tumor growth such as colon adenocarcinoma, mammary adenocarcinoma and melanoma.
We further determined the levels of Gal-9 and PD-L1 expression in different clinical stages of CLL patients and their possible correlation with disease prognosis. We observed that both Gal-9 and PD-L1 were significantly more expressed at advanced clinical stages of CLL patients compared to early stages. Similar studies on renal cell carcinoma, melanoma and chronic lymphocytic leukemia have also demonstrated that PD-L1 expression on tumor cells is significantly associated with adverse prognosis (Thompson et al., 2006; Oba et al., 2014; Li et al., 2017). However, a contradictory study was reported that there is no correlation between PD-L1 expression and clinical stage of CLL patients. They found that PD-L1 expression is not associated with prognostic factors of CLL patients including the expression of ZAP-70, CD38 and the mutational status of IGHV (Grzywnowicz et al., 2015). Previous reports have also indicated that Gal-9 expression is a biomarker of disease outcome and correlated with poor prognosis (Klibi et al., 2009; Cedeno-Laurent and Dimitroff, 2012). So, Gal-9 and PD-L1 as the main ligands of immune inhibitory receptors, Tim-3 and PD-1, are highly expressed on tumor cells and more engagement of these pathways help tumor cells to escape from immune effector mechanisms. Our findings suggest the application of Gal-9 and PD-L1 as biomarkers of disease prognosis which their upregulation is correlated with worse prognosis of the CLL patients.
In summary, our results have demonstrated for the first time the upregulation of Gal-9 in CLL patients and also the over-expression of PD-L1 in this chronic leukemia. More expression of Gal-9 and PD-L1 in CLL patients at advanced clinical stages may introduce these molecules as suitable biomarkers for disease prognosis. Our data may warrant future strategies based on the manipulation of negative immune regulatory pathways for T cell reactivation in human cancer. Furthermore, the combination therapy of targeting Tim-3/Gal-9 and PD-1/PD-L1 pathways with conventional antitumor reagents or newly proposed vaccination methods should be considered and may be more effective in clinical cancer treatment. Hence, further studies are needed to investigate the detailed role of Gal-9 and PD-L1 in hematologic malignancies and their potential therapeutic effects to restore the function of exhausted T cells.
Conflict of Interest
The authors declare that the current research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Figure.

Amplification Curve, Melting Curve and Peak Analysis of Gal-9, PD-L1 and β-actin.
Amplification curve, melting curve and peak analysis of Gal-9 mRNA expression (A), PD-L1 mRNA expression (B) and β-actin mRNA expression (C) obtained from Real-Time PCR assay.
Acknowledgements
This study was supported by a grant from Mazandaran University of Medical Sciences (No: 1615).
References
- Allahmoradi E, Taghiloo S, Janbabaei G, et al. Immunophenotypic characterization of patients with chronic lymphocytic leukemia by flow cytometry;association with disease prognosis. J Mazandaran Univ Med Sci. 2017;26:9–19. [Google Scholar]
- Asgarian-Omran H, Forghani P, Hojjat-Farsangi M, et al. Expression profile of galectin-1 and galectin-3 molecules in different subtypes of chronic lymphocytic leukemia. Cancer Invest. 2010;28:717–25. doi: 10.3109/07357907.2010.494319. [DOI] [PubMed] [Google Scholar]
- Brusa D, Serra S, Coscia M, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–63. doi: 10.3324/haematol.2012.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj J. 2012;29:619–25. doi: 10.1007/s10719-012-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Fu H, Liu Y, Xu L, et al. Galectin-9 predicts postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Tumour Biol. 2015;36:5791–9. doi: 10.1007/s13277-015-3248-y. [DOI] [PubMed] [Google Scholar]
- Ghia P, Ferreri AJ, Caligaris-Cappio F. Chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 2007;64:234–46. doi: 10.1016/j.critrevonc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Grzywnowicz M, Karczmarczyk A, Skorka K, et al. Expression of programmed death 1 ligand in different compartments of chronic lymphocytic leukemia. Acta Haematol. 2015;134:255–62. doi: 10.1159/000430980. [DOI] [PubMed] [Google Scholar]
- Grzywnowicz M, Zaleska J, Mertens D, et al. Programmed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemia. PloS One. 2012;7:e35178. doi: 10.1371/journal.pone.0035178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H-T, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H-T, Jeong YH, Park HJ, et al. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011;44:217–31. doi: 10.5483/BMBRep.2011.44.4.217. [DOI] [PubMed] [Google Scholar]
- Kahan SM, Wherry EJ, Zajac AJ. T cell exhaustion during persistent viral infections. Virol J. 2015;479:180–93. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibi J, Niki T, Riedel A, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–66. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10510. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite KR, Reis ST, Junior JP, et al. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Ann Diagn Pathol. 2015;10:1–6. doi: 10.1186/s13000-015-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Pang NN, Zhang ZH, et al. PD-1/PD-L1 expression and its implications in patients with chronic lymphocytic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2017;38:198–203. doi: 10.3760/cma.j.issn.0253-2727.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Ueno M, Oomizu S, et al. Galectin-9 expression links to malignant potential of cervical squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134:899–907. doi: 10.1007/s00432-008-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merani S, Chen W, Elahi S. The bitter side of sweet: the role of Galectin-9 in immunopathogenesis of viral infections. Rev Med Virol. 2015;25:175–86. doi: 10.1002/rmv.1832. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Mumprecht S, Schürch C, Schwaller J, et al. Programmed death 1 signaling on chronic myeloid leukemia–specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- Oba J, Nakahara T, Abe T, et al. Expression of programmed death receptor ligand 1 in melanoma may indicate tumor progression and poor patient survival. J Am Acad Dermatol. 2014;70:954–6. doi: 10.1016/j.jaad.2014.01.880. [DOI] [PubMed] [Google Scholar]
- Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;21:1612–21. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghiloo S, Allahmoradi E, Tehrani M, et al. Frequency and functional characterization of exhausted CD8+T cells in chronic lymphocytic leukemia. Eur J Haematol. 2017;98:622–31. doi: 10.1111/ejh.12880. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nature Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–81. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zhang X, Chen H, et al. Expression of programmed cell death-ligand 1 and its correlation with clinical outcomes in gliomas. Oncotarget. 2016;7:8944–55. doi: 10.18632/oncotarget.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223–5. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]


