Abstract
Background:
Meningiomas are common central nervous system (CNS) tumors that account for thirty percent of primary intracranial tumors.. The accuracy of predicting meningioma recurrence and progression is not enough. So, there is a real need for discovering recent factors for identification of the relapse risk, progression rates, which patients will need aggressive treatment and predicting and improving patients’ survival. Thioredoxin-interacting-protein [TXNIP] is an alpha-arrestin-protein family member that is mapped on chromosome 1-q21–22 and is found to participate in cellular redox reactions regulations and control. Transglutaminase 2 (TGM2) is a transglutaminase enzyme family member that is found in many human cells, it may act as an enzyme, a structural protein and also has multiple roles in many cellular activities.
Aim of our study:
It was to explore the expression of TXNIP, TGM2 and Ki-67 using immunohistochemistry in different pathological grades of meningiomas, and to investigate the relevance between their expressions, clinicopathological criteria, disease recurrence and prognosis of meningioma patients.
Methods:
we included 50 cases of meningioma of different pathological grades; all patients were managed according to their grade by surgery alone, with radiotherapy or combined modalities. Sections from paraffin blocks prepared from samples of all patients stained by TXNIP, TGM2 and Ki-67 using immunohistochemistry.
Results:
high expression of TXNIP in 28 out of 50 (56%) cases of meningioma of different pathological grades and was positively correlated with meningioma lower grade, low KI labeling index (p=0.000), adequacy of resection, negatively correlated with high incidence of recurrence after surgery and it was negatively correlated with meningioma higher pathological grades (p=0.000). We detected high expression of TGM2 in 21 out of 50 (42%) cases of meningioma and it was positively correlated with meningioma higher grade (p= 0.002), high KI labeling index (p=0.000), high incidence of recurrence after surgery, progression to higher pathological grades and was negatively correlated with adequacy of resection of meningioma (p=0.000).
Conclusion:
There is inverse relation between both [TXNIP and TGM2 expression in meningiomas and the combination of decreased expression of TXNIP and increased expression of TGM2 could predict risk of meningioma recurrence and progression in to higher pathological grades.
Keywords: TXNIP, TGM2, meningioma, immunohistochemistry, recurrence, progression, response to therapy
Introduction
Meningiomas are common central nervous system (CNS) tumors that account for thirty percent of primary intracranial tumors. According to the World Health Organization (WHO, 2016) classification system criteria; they are divided into benign (WHO grade I), atypical (WHO grade II) and anaplastic (WHO grade III) meningioma (Louis et al., 2016). Atypical and anaplastic meningiomas have a poorer prognosis and more liability for recurrence than the benign meningiomas. Despite advancements in therapies for meningiomas grade II or III, that include surgery with or without radiotherapy, patients prognosis is still disappointing (Soussain et al., 2009), with liability for recurrence even with aggressive surgical resection of the tumor which is a major cause of morbidity, mortality and progression to higher grades, but rates of recurrence markedly differ between WHO pathological grades (Riemenschneider et al., 2006; (Yang et al., 2008).
Factors that allow expecting recurrence and progression of meningiomas are WHO pathological grade, inadequate surgical resection and a high Ki67 labeling index (Simpson, 1957; Oya et al., 2012). But the accuracy of such factors in predicting meningioma recurrence especially in higher-grade is not enough. So, there is a real need for discovering recent biomarkers for identification of the relapse risk, progression rates and which patients will need aggressive management (Van de Vijevr et al., 2002; Wen et al., 2010). In our research we evaluated two recent markers expression in meningioma: Thioredoxin-interacting protein (TXNIP), transglutaminase 2 (TGM2) as their roles in meningioma progression and prognosis had not sufficiently clarified and no previous studies had studied them together in meningiomas, and we correlate their expression together with the Ki-67 labeling index. Thioredoxin-interacting-protein [TXNIP] is an alpha-arrestin-protein family member that is mapped on chromosome 1-q21–22 (Zhou et al., 2011). It is found to participate in cellular redox reactions regulations and control (Lee et al., 2010). Also, it has been found to play a tumor inhibitory role in many kinds of human cancers (Zhou et al., 2011). Transglutaminase 2 (TGM2) is a transglutaminase enzyme family member that is found in many human cells (Thomázy and Fésüs, 1989). TGM2 may act as an enzyme, a structural protein and also has multiple roles in many cellular activities (Mishra and Murphy 2006). Ki-67 is essential for proliferation of cells as it is found during all the cell cycle active phases, but is not present in the G0 phase. It had significant associations with cancer growths, proliferation, progression relapse and recurrence of a wide range of human tumors (Wiemels et al., 2010; Koshland, 1993).
Aim of our study; was to explore the expression of TXNIP, TGM2 and Ki-67 using immunohistochemistry in different pathological grades of meningiomas, and to investigate the relevance between their expressions, clinicopathological criteria, disease recurrence and prognosis of meningioma patients.
Materials and Methods
Patients and Methods
We carried out this extended retrospective cohort-study at pathology, clinical oncology and nuclear medicine and neurosurgery departments, Zagazig University, faculty of medicine, approved by the institutional review board (IRB) ethical Committee.
We included 50 patients that have been diagnosed as meningioma with different grades. We collected all the patients’ data as; patient gender, age, tumor size, site, degree of surgical resection, recurrence and recurrence-free survival rates, in five years from the archives of the shared departments. Patients’ management differed according to their grade as they were managed by surgery alone, with or without radiotherapy. All patients performed a written consent before tumor resection. World Health Organization (WHO) classification 2016 was used for meningiomas subtyping and pathological grading (Louis et al., 2016)
Simpson grading system was used for evaluation of the range of surgical resection (Simpson, 1957). In Clinical Oncology Department, radiotherapy was performed when indicated; in in benign tumors (grade I) if just biopsied or incompletely resected and in all cases of grade IIandIII tumors even if completely resected. All cases were treated by 3-D conformal technique by linear accelerator 6 MV energy. For benign meningioma the target volume was the gross tumor volume GTV that, defined by brain MRI and CT scan, plus 1.5 cm additional margin (clinical target volume CTV+ planning target volume PTV) the total dose was 54 Gy at 1.8 Gy per fraction daily for 5 days a week. While in case of atypical and anaplastic meningioma the additional margin (CTV+PTV) was 2 cm around GTV with total dose 60 Gy at 1.8 Gy per fraction daily for 5 days a week.
Sections from paraffin blocks prepared from samples of all patients were stained by routine haematoxylin and eosin (Hand E) for adequate evaluation and grading of meningioma, and then sections put on positively charged slides for immunohistochemistry that was performed in pathology department faculty of medicine, Zagazig University.
Immunohistochemical technique of staining
Streptavidin-biotin method were used for immunohistochemistry (Hsu et al., 1981), incubated slides overnight with primary rabbit monoclonal anti- TXNIP antibody (ab185544) [EPR15225] dilution 1:500, primary mouse mono-clonal anti- TGM2 antibody [CUB 7402] ab2386 dilution 1:50 (Abcam, MA, USA-Cambridge) and anti-Ki-67 (NeoMarkers ready to use Inc.; clone SP6) human breast carcinoma tissue were used as positive control for both TXNIP and TGM2.
Evaluation of TXNIP and TGM2 expressions
We assessed the extent and intensity of positive tumor cells. The extent of positive tumor cells was classified into 5 classes: from 0 to 5 for, <5%, 5%-25%, 26%-50%, 51%-75% and >75% cells stained, respectively. Stain intensity was graded into one to three for faint, moderate, and strong stain, respectively. To acquire a final staining score from 0 to 12 for TXNP, we multiplied both the stain extent and intensity scores and then we considered final score from zero-three as low expression, and scores of four to twelve as high expression (Li et al., 2015).
To acquire the final staining score of 0 to 7 for TGM2, we summated both the stain extent and intensity scores. Then consider final score from zero-three as low expression, and scores of four to seven as high expression (Erdem et al., 2015).
Evaluation of Ki-67 (MIB-1) labeling index
We calculated Ki-67 (MIB-1) labeling index as positive tumor cell nuclei percentage by counting the stained nuclei in areas of maximum stain in 5 high power field (magnificationx400) (Perry et a;l., 2001). The Ki-67 labeling index was considered low when less than 10% nuclei of the tumor cells were stained and were considered high when more than 10% nuclei of the tumor cells were stained.
Statistical-analysis
We performed our statistics by using the program of SPSS 22.0 for windows (SPSS Inc., Chicago, IL, USA) and MedCalc windows (MedCalc Software bvba 13, Ostend, Belgium). Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). We compared the percent of categorical variables by using Pearson’s Chi-square test or Fisher’s exact test. We calculated the Recurrence Free Survival (RFS) rate as the time from surgery to date at which local recurrence was detected. We calculated the Overall Survival (OS) rate as the time from diagnosis to death. Stratification of both rates was done according to all clinicopathological features and immunohistochemical markers, and these rates were estimated using the method of Kaplan-Meier plot, and compared using two-sided exact log-rank test. A p-value <0.05 was considered significant.
Results
Patient features
We illustrated the clinic-pathological criteria of our patients in Table 1.
Table 1.
Demographic and Follow Up Data of Our Patients
| Characteristics | All patients (N=50) | Characteristics | All patients (N=50) | ||
|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||
| Age (years) | Surgery | ||||
| Mean ± SD | 42.34 | ±11.59 | Gross tumor resection | 27 | 54% |
| Median (Range) | 39.5 | (24-65) | Near total resection | 11 | 22% |
| < 40 years | 26 | 52% | Subtotal resection | 7 | 14% |
| > 40 years | 24 | 48% | Biopsy | 5 | 10% |
| Sex | Simpson grading system | ||||
| Male | 15 | 30% | Grade I | 11 | 22% |
| Female | 35 | 70% | Grade II | 16 | 32% |
| Site | Grade III | 11 | 22% | ||
| Parasagittal | 14 | 28% | Grade IV | 7 | 14% |
| Anterior Fossa | 12 | 24% | Grade V | 5 | 10% |
| Convexity | 8 | 16% | Radiotherapy | ||
| Middle fossa | 8 | 16% | No | 26 | 52% |
| Posterior fossa | 8 | 16% | Yes | 24 | 48% |
| Calcification | Response | (N=23) | |||
| Absent | 28 | 56% | OAR | 19 | 82.60% |
| Present | 22 | 44% | NR | 4 | 17.40% |
| Grade | CR | 17 | 73.90% | ||
| Grade I | 31 | 62% | PR | 2 | 8.70% |
| Grade II | 12 | 24% | SD | 2 | 8.70% |
| Grade III | 7 | 14% | PD | 2 | 8.70% |
| Ki67 | Follow-up (months) | ||||
| Low | 30 | 60% | Mean ± SD | 36.16 | ±15.85 |
| High | 20 | 40% | Median (Range) | 30 | (11-60) |
| Outcome | |||||
| Recurrence (out of 45patients) | 13 | 28.90% | |||
| Died (out of 50patients) | 17 | 34% | |||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD & median (range).
We included 50 patients having meningiomas in our study, which were divided into 35 (70%) females and 20 (30%) males with age ranged from (24-65) years (Mean: 42.3±11.6 years). Our cased divided into 31 (62%) cases of benign meningioma, 12 (24%) cases of atypical meningioma and 7 (14%) cases of anaplastic meningioma. Regarding Simpson grades; our cases were divided into 11 (22%) cases grade I and 16 (32%) cases grade II that undergoes gross total resection, 11 (22%) cases grade III that undergoes near total resection, 7(14%) cases grade IV that undergoes subtotal resection and 5(10%) cases grade V that undergoes only biopsy. We found significant positive correlations between meningioma recurrence and higher Simpson grade. 24 (48%) of our cases have received different doses of irradiation according to their Simpson grades. We found no significant correlations between meningioma recurrence, age, sex of patients, site, pathological grade of the tumor or presence of calcification.
Interpretation of immunohistochemical results TXNIP expression: Tables 2 and 3; Figures 1 and 3.
Table 2.
Correlation between Clinicopathological Features, TGM2, TXNIP Expression, Ki Labeling Index of Our Patients
| Characteristics | All | TGM2 | p-value | TXNIP | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | |||||||||
| (N=50) | (N=29) | (N=21) | (N=22) | (N=28) | ||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Age (years) | ||||||||||||
| Mean ± SD | 42.34 ±11.59 | 40.34 ±10.70 | 45.09 ±12.46 | 0.155* | 45.45 ±11.89 | 39.89 ±10.95 | 0.093* | |||||
| Median (Range) | 39.5 | (24-65) | 39 | (24-65) | 43 | (24-65) | 44 | (24-65) | 39 | (24-65) | ||
| < 40 years | 26 | (52%) | 18 | (69.20%) | 8 | (30.80%) | 0.094‡ | 7 | (26.90%) | 19 | (73.10%) | 0.011‡ |
| > 40 years | 24 | (48%) | 11 | (45.80%) | 13 | (54.20%) | 15 | (62.50%) | 9 | (37.50%) | ||
| Sex | ||||||||||||
| Male | 15 | (30%) | 5 | (33.30%) | 10 | (66.70%) | 0.021‡ | 12 | (80%) | 3 | (20%) | 0.001‡ |
| Female | 35 | (70%) | 24 | (68.60%) | 11 | (31.40%) | 10 | (28.60%) | 25 | (71.40%) | ||
| Site | ||||||||||||
| Parasagittal | 14 | (28%) | 12 | (85.70%) | 2 | (14.30%) | 0.117‡ | 3 | (21.40%) | 11 | (78.60%) | 0.262‡ |
| Anterior Fossa | 12 | (24%) | 7 | (58.30%) | 5 | (41.70%) | 5 | (41.70%) | 7 | (58.30%) | ||
| Convexity | 8 | (16%) | 3 | (37.50%) | 5 | (62.50%) | 5 | (62.50%) | 3 | (37.50%) | ||
| Middle fossa | 8 | (16%) | 4 | (50%) | 4 | (50%) | 4 | (50%) | 4 | (50%) | ||
| Posterior fossa | 8 | (16%) | 3 | (37.50%) | 5 | (62.50%) | 5 | (62.50%) | 3 | (37.50%) | ||
| Calcification | ||||||||||||
| Absent | 28 | (56%) | 14 | (50%) | 14 | (50%) | 0.196‡ | 13 | (46.40%) | 15 | (53.60%) | 0.696‡ |
| Present | 22 | (44%) | 15 | (68.20%) | 7 | (31.80%) | 9 | (40.90%) | 13 | (59.10%) | ||
| Grade | ||||||||||||
| Grade I | 31 | (62%) | 24 | (77.40%) | 7 | (22.60%) | 0.002§ | 6 | (19.40%) | 25 | (80.60%) | <0.001§ |
| Grade II | 12 | (24%) | 3 | (25%) | 9 | (75%) | 9 | (75%) | 3 | (25%) | ||
| Grade III | 7 | (14%) | 2 | (28.60%) | 5 | (71.40%) | 7 | (100%) | 0 | 0%) | ||
| Ki67 | ||||||||||||
| Low | 30 | (60%) | 24 | (80%) | 6 | (20%) | <0.001‡ | 5 | (16.70%) | 25 | (83.30%) | <0.001‡ |
| High | 20 | (40%) | 5 | (25%) | 15 | (75%) | 17 | (85%) | 3 | (15%) | ||
| TGM2 | ||||||||||||
| Low | 29 | (58%) | 3 | (10.30%) | 26 | (89.70%) | <0.001‡ | |||||
| High | 21 | (42%) | 19 | (90.50%) | 2 | (9.50%) | ||||||
| TXNIP | ||||||||||||
| Low | 22 | (44%) | 3 | (13.60%) | 19 | (86.40%) | <0.001‡ | |||||
| High | 28 | (56%) | 26 | (92.90%) | 2 | (7.10%) | ||||||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD & median (range)
Independent samples Student’s t-test
Chi-square test
Chi-square test for trend; p< 0.05 is significant.
Table 3.
Correlation Between Clinicopathological Features, TGM2, TXNIP Expression, Ki Labeling Index, Disease Recurrence, Survival and Response to Therapy in Our Patients
| Outcome | All | TGM2 | p-value‡ | TXNIP | p-value‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | |||||||||
| (N=50) | (N=29) | (N=21) | (N=22) | (N=28) | ||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Surgery | ||||||||||||
| Gross tumor resection | 27 | (54%) | 25 | (86.20%) | 2 | (9.50%) | <0.001 | 3 | (13.60%) | 24 | (85.70%) | <0.001 |
| Near total resection | 11 | (22%) | 2 | (6.90%) | 9 | (42.90%) | 8 | (36.40%) | 3 | (10.70%) | ||
| Subtotal resection | 7 | (14%) | 2 | (6.90%) | 5 | (23.80%) | 6 | (27.30%) | 1 | (3.60%) | ||
| Biopsy | 5 | (10%) | 0 | 0%) | 5 | (23.80%) | 5 | (22.70%) | 0 | 0%) | ||
| Simpson grading system | ||||||||||||
| Grade I | 11 | (22%) | 11 | (37.90%) | 0 | 0%) | <0.001 | 0 | 0%) | 11 | (39.30%) | <0.001 |
| Grade II | 16 | (32%) | 14 | (48.30%) | 2 | (9.50%) | 3 | (13.60%) | 13 | (46.40%) | ||
| Grade III | 11 | (22%) | 2 | (6.90%) | 9 | (42.90%) | 8 | (36.40%) | 3 | (10.70%) | ||
| Grade IV | 7 | (14%) | 2 | (6.90%) | 5 | (23.80%) | 6 | (27.30%) | 1 | (3.60%) | ||
| Grade V | 5 | (10%) | 0 | 0%) | 5 | (23.80%) | 5 | (22.70%) | 0 | 0%) | ||
| Residual | ||||||||||||
| Absent | 27 | (54%) | 25 | (86.20%) | 2 | (9.50%) | <0.001 | 3 | (13.60%) | 24 | (85.70%) | <0.001 |
| Present | 23 | (46%) | 4 | (13.80%) | 19 | (90.50%) | 19 | (86.40%) | 4 | (14.30%) | ||
| Radiotherapy | ||||||||||||
| No | 26 | (52%) | 24 | (82.80%) | 2 | (9.50%) | <0.001 | 2 | (9.10%) | 24 | (85.70%) | <0.001 |
| Yes | 24 | (48%) | 5 | (17.20%) | 19 | (90.50%) | 20 | (90.90%) | 4 | (14.30%) | ||
| Response | (N=23) | (N=4) | (N=19) | (N=19) | (N=4) | |||||||
| OAR | 19 | (82.60%) | 2 | (50%) | 17 | (89.50%) | 0.125 | 15 | (78.90%) | 4 | (100%) | 1 |
| NR | 4 | (17.40%) | 2 | (50%) | 2 | (10.50%) | 4 | (21.10%) | 0 | 0%) | ||
| CR | 17 | (73.90%) | 2 | (50%) | 15 | (78.90%) | 0.289 | 13 | (68.40%) | 4 | (100%) | 0.635 |
| PR | 2 | (8.70%) | 0 | 0%) | 2 | (10.50%) | 2 | (10.50%) | 0 | 0%) | ||
| SD | 2 | (8.70%) | 1 | (25%) | 1 | (5.30%) | 2 | (10.50%) | 0 | 0%) | ||
| PD | 2 | (8.70%) | 1 | (25%) | 1 | (5.30%) | 2 | (10.50%) | 0 | 0%) | ||
| Recurrence | (N=45) | (N=28) | (N=17) | (N=17) | (N=28) | |||||||
| Absent | 32 | (71.10%) | 28 | (100%) | 4 | (23.50%) | <0.001 | 5 | (29.40%) | 27 | (96.40%) | <0.001 |
| Present | 13 | (28.90%) | 0 | 0%) | 13 | (76.50%) | 12 | (70.60%) | 1 | (3.60%) | ||
| Survival | (N=50) | (N=29) | (N=21) | (N=22) | (N=28) | |||||||
| Absent | 33 | (66%) | 29 | (100%) | 4 | (19%) | <0.001 | 6 | (27.30%) | 27 | (96.40%) | <0.001 |
| Present | 17 | (34%) | 0 | 0%) | 17 | (81%) | 16 | (72.70%) | 1 | (3.60%) | ||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD & median (range)
Chi-square test; p< 0.05 is significant.
Figure 1.
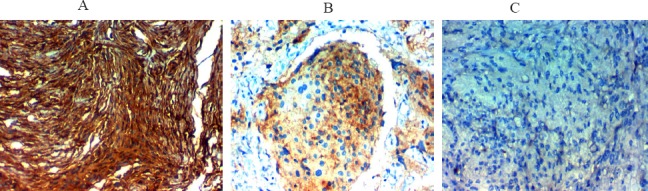
Immunohistochemical Expression of TXNIP in Meningioma; A. High TXNIP expression in benign meningiomax400, B. Low TXNIP expression in atypical meningiomax400, C. Low TXNIP expression in malignant meningioma. Magnification A, B & C original magnificationx 400
Figure 2.
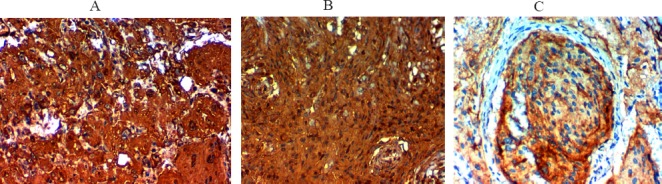
Immunohistochemical Expression of TGM2 in Meningioma; A and B high TGM2 expression in malignant meningiomax400, C, High TGM2 expression in atypical meningiomax400, D, low TGM2 expression in benign meningioma
Figure 3.
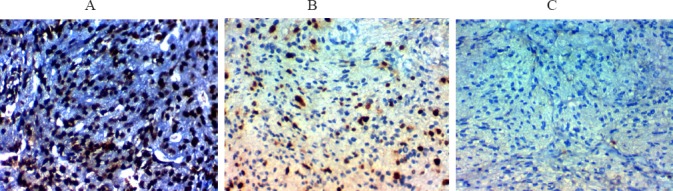
Ki67 Labeling Index in Meningioma. A, High Ki67 labeling index in malignant meningiomax400, B, High Ki67 labeling index in malignant meningiomax400, C, Low ki67 labeling index in benign meningiomax400
We detected high expression of TXNIP in 28 out of 50 (56%) cases of meningioma of different pathological grades.
TXNIP expression was higher in female patients (p<0.001) and in patients with younger age (p= 011).
TXNIP over expression was associated with lower grade and low KI labeling index (p=0.000).
We found no significant correlations between TXNIP expression, site or presence of calcification in meningioma.
Interpretation of immunohistochemical results TGM2 expression: Tables 2 and 3; Figures 2 and 3.
We detected high expression of TGM2 in 21 out of 50 (42%) cases of meningioma.
TGM2 expression was significantly higher in male patients (p=0.02).
TGM2 over expression was significantly positively correlated with higher meningioma grade (p= 0.002) and high KI labeling index (p=0.000).
We found no significant correlations between TGM2expression, age of the patient, site or presence of calcification in meningioma.
Progression and survival analysis in relation to TXNIP expression: Tables 4, 5 and 6, Figures 4 and 5.
Table 4.
Correlation Between TGM2, TXNIP Expression, Ki Labeling Index and Response to Therapy in Our Patients
| Characteristics | All | Response | p-value | Response | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OAR | NR | CR | PR | SD | PD | |||||||||||
| (N=23) | (N=19) | (N=4) | (N=17) | (N=2) | (N=2) | (N=2) | ||||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Ki67 | ||||||||||||||||
| Low | 4 | (17.40%) | 4 | (100%) | 0 | 0%) | 1.000‡ | 4 | (100%) | 0 | 0%) | 0 | 0%) | 0 | 0%) | 0.635‡ |
| High | 19 | (82.60%) | 15 | (78.90%) | 4 | (21.10%) | 13 | (68.40%) | 2 | (10.50%) | 2 | (10.50%) | 2 | (10.50%) | ||
| TGM2 | ||||||||||||||||
| Low | 4 | (17.40%) | 2 | (50%) | 2 | (50%) | 0.125‡ | 2 | (50%) | 0 | 0%) | 1 | (25%) | 1 | (25%) | 0.289‡ |
| High | 19 | (82.60%) | 17 | (89.50%) | 2 | (10.50%) | 15 | (78.90%) | 2 | (10.50%) | 1 | (5.30%) | 1 | (5.30%) | ||
| TXNIP | ||||||||||||||||
| Low | 19 | (82.60%) | 15 | (78.90%) | 4 | (21.10%) | 1.000‡ | 13 | (68.40%) | 2 | (10.50%) | 2 | (10.50%) | 2 | (10.50%) | 0.635‡ |
| High | 4 | (17.40%) | 4 | (100%) | 0 | (0%) | 4 | (100%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | ||
| TGM2/TXNIP | ||||||||||||||||
| Low/Low | 2 | (8.70%) | 0 | 0%) | 2 | (100%) | 0.011§ | 0 | 0%) | 0 | 0%) | 1 | (50%) | 1 | (50%) | 0.018§ |
| Low/High | 2 | (8.70%) | 2 | (100%) | 0 | 0%) | 2 | (100%) | 0 | 0%) | 0 | 0%) | 0 | 0%) | ||
| High/Low | 17 | (73.90%) | 15 | (88.20%) | 2 | (11.80%) | 13 | (76.50%) | 2 | (11.80%) | 1 | (5.90%) | 1 | (5.90%) | ||
| High/High | 2 | (8.70%) | 2 | (100%) | 0 | 0%) | 2 | (100%) | 0 | 0%) | 0 | 0%) | 0 | 0%) | ||
| TGM2/TXNIP | ||||||||||||||||
| High/Low or High | 19 | (82.60%) | 17 | (89.50%) | 2 | (10.50%) | 0.125‡ | 15 | (78.90%) | 2 | (10.50%) | 1 | (5.30%) | 1 | (5.30%) | 0.289‡ |
| Low/Low or High | 4 | (17.40%) | 2 | (50%) | 2 | (50%) | 2 | (50%) | 0 | 0%) | 1 | (25%) | 1 | (25%) | ||
Categorical variables were expressed as number (percentage); continuous variables were expressed as mean ± SD & median (range); * Independent samples Student’s t-test; • Kruskal Wallis H test
Chi-square test
Chi-square test for trend; p< 0.05 is significant.
Table 5.
Correlation Between TGM2, TXNIP Expression, Ki Labeling Index and Disease Recurrence in Our Patients
| Characteristics | All | Recurrence | p-value | Recurrence Free Survival (RFS) | p-value† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Present | Mean RFS | 3 y RFS | 5 y RFS | |||||||
| (N=45) | (N=32) | (N=13) | (months) | (%) | (%) | ||||||
| No. | (%) | No. | (%) | No. | (%) | ||||||
| All patients | 50 | (100%) | 32 | (71.10%) | 13 | (28.90%) | 48.09 | (75.20%) | (68.90%) | ||
| Ki67 | |||||||||||
| Low | 30 | (66.70%) | 24 | (80%) | 6 | (20%) | 0.086‡ | 52.9 | (86.40%) | (78.20%) | 0.016 |
| High | 15 | (33.30%) | 8 | (53.30%) | 7 | (46.70%) | 38.84 | (52.50%) | (52.50%) | ||
| TGM2 | |||||||||||
| Low | 28 | (62.20%) | 28 | (100%) | 0 | 0%) | <0.001‡ | 60 | (100%) | (100%) | <0.001 |
| High | 17 | (37.80%) | 4 | (23.50%) | 13 | (76.50%) | 27.21 | (34.30%) | (11.40%) | ||
| TXNIP | |||||||||||
| Low | 17 | (37.80%) | 5 | (29.40%) | 12 | (70.60%) | <0.001‡ | 31 | (41.20%) | (24.70%) | <0.001 |
| High | 28 | (62.20%) | 27 | (96.40%) | 1 | (3.60%) | 58.68 | (96.40%) | (96.40%) | ||
| TGM2/TXNIP | |||||||||||
| Low/Low | 2 | (4.40%) | 2 | (100%) | 0 | 0%) | <0.001§ | 60 | (100%) | (100%) | <0.001 |
| Low/High | 26 | (57.80%) | 26 | (100%) | 0 | 0%) | 60 | (100%) | (100%) | ||
| High/Low | 15 | (33.30%) | 3 | (20%) | 12 | (80%) | 26.42 | (33.30%) | (11.10%) | ||
| High/High | 2 | (4.40%) | 1 | (50%) | 0 | 0%) | 24 | (50%) | (50%) | ||
| TGM2/TXNIP | |||||||||||
| High/Low or High | 17 | (37.80%) | 4 | (23.50%) | 13 | (76.50%) | <0.001‡ | 27.2 | (34.30%) | (11.40%) | <0.001 |
| Low/Low or High | 28 | (62.20%) | 28 | (100%) | 0 | 0%) | 60 | (100%) | (100%) | ||
| Response | (N=18) | (N=7) | (N=11) | ||||||||
| CR | 17 | (94.40%) | 6 | (35.30%) | 11 | (64.70%) | 0.389‡ | 31.08 | (32.30%) | (32.30%) | 0.299 |
| SD | 1 | (5.60%) | 1 | (100%) | 0 | 0%) | 60 | (100%) | 100%) | ||
Categorical variables were expressed as number (percentage); continuous variables were expressed as mean ± SD & median (range); * Independent samples Student’s t-test
Chi-square test
Chi-square test for trend
Log rank test; p< 0.05 is significant.
Table 6.
Correlation Between TGM2, TXNIP Expression, Ki Labeling Index and Survival of Our Patients
| Characteristics | All | Mortality | p- value | Overall Survival (OS) | p- value† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Present | Mean OS | 3 y OS | 5 y OS | |||||||
| (N=50) | (N=33) | (N=17) | (months) | (%) | (%) | ||||||
| No. | (%) | No. | (%) | No. | (%) | ||||||
| All patients | 50 | (100%) | 33 | (66%) | 17 | (34%) | 46.35 | 68.60% | 62.90% | ||
| Ki67 | |||||||||||
| Low | 30 | (60%) | 24 | (80%) | 6 | (20%) | 0.010‡ | 53.69 | 86.20% | 77.90% | <0.001 |
| High | 20 | (40%) | 9 | (45%) | 11 | (55%) | 35.01 | 40.70% | 40.70% | ||
| TGM2 | |||||||||||
| Low | 29 | (58%) | 29 | (100%) | 0 | (0%) | <0.001‡ | 60 | 100% | 100% | <0.001 |
| High | 21 | ((42%)) | 4 | (19%) | 17 | (81%) | 27.2 | 25.70% | 8.60% | ||
| TXNIP | |||||||||||
| Low | 22 | (44%) | 6 | (27.30%) | 16 | (72.70%) | <0.001‡ | 30.11 | 32.70% | 19.60% | <0.001 |
| High | 28 | (56%) | 27 | (96.40%) | 1 | (3.60%) | 58.67 | 95.80% | 95.80% | ||
| TGM2/TXNIP | |||||||||||
| Low/Low | 3 | (6%) | 3 | (100%) | 0 | (0%) | <0.001§ | 60 | 100% | 100% | <0.001 |
| Low/High | 26 | (52%) | 26 | (100%) | 0 | (0%) | 60 | 100% | 100% | ||
| High/Low | 19 | (38%) | 3 | (15.80%) | 16 | (84.20%) | 26.43 | 25.30% | 8.40% | ||
| High/High | 2 | (4%) | 1 | (50%) | 1 | (50%) | 28 | 0% | 0% | ||
| TGM2/TXNIP | |||||||||||
| High/Low or High | 21 | (42%) | 4 | (19%) | 17 | (81%) | <0.001‡ | 27.2 | 25.70% | 8.60% | <0.001 |
| Low/Low or High | 29 | (58%) | 29 | (100%) | 0 | (0%) | 60 | 100% | 100% | ||
Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD & median (range); * Independent samples Student’s t-test
Chi-square test
Chi-square test for trend
Log rank test; p< 0.05 is significant.
Figure 4.
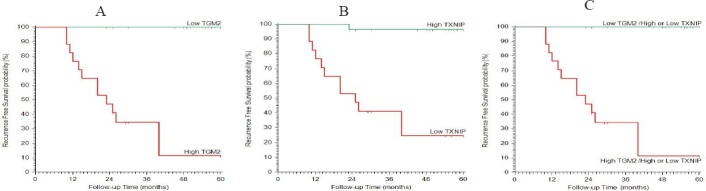
Kaplan-Meier Plot of Recurrence Free Survival. (A) stratified according to TGM2, (B) stratified according to TXNIP, (C) stratified according to TGM2 & TXNIP.
Figure 5.
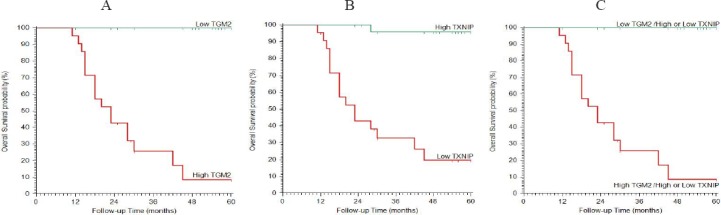
Kaplan-Meier Plot of Overall Survival. (A) stratified according to TGM2, (B) stratified according to TXNIP, (C) stratified according to TGM2 & TXNIP.
High TXNIP expression was negatively correlated with the presence of residuals after surgery, high incidence of recurrence after surgery, postoperative radiotherapy usage, meningioma progression to higher grades and with recurrence free survival (p=0.000).
We found no significant correlations between TXNIP expressions, dose of radiotherapy or therapy response.
Progression and survival analysis in relation to TGM2expression: Tables 4, 5 and 6, Figures 4 and 5.
High TGM2 expression was positively correlated with the presence of residuals after surgery, high incidence of recurrence after surgery, the postoperative radiotherapy usage, meningioma progression to higher grades and with recurrence free survival (p=0.000).
We found no significant correlations between TXNIP expressions, dose of radiotherapy or therapy response.
Discussion
Prognosis and quality of life of patients having meningioma have improved, recently, due to advancement in the treatment options. But recurrence rates of high grade meningiomas patients are still high (Soussain et al., 2009). So, important point in the recent research is to detect novel biomarkers that could help to identify patient with more liability for recurrence, discover new therapeutic targets to improve their outcomes. We have chosen to evaluate TXNIP expression in meningioma as it was found to have many biological roles in normal and neoplastic tissues; like the stimulation of cell proliferation, apoptosis, transcription process regulation and mediating the NK cells development (Kim et al., 2007). But its predictive role in meningioma needs clarifications. In our study, using immunohistochemistry, we showed that TXNIP expression was negatively correlated with meningioma pathological grade, KI labeling index meningioma progression to higher pathological grades. Furthermore, in this study, we also found that TXNIP expression was significantly negatively correlated with recurrence and recurrence free survival rates of patients with meningiomas. our results are similar to results of Cai Z et al., (2017), who find similar results in meningioma and results of Morrison et al., (2014), that identified TXNIP down regulation or even loss was positively correlated with thyroid tumors progression more aggressive and undifferentiated subtypes, which calcified its tumor suppressor role. TXNIP tumor suppressor role was explained by many studies which was related to disturbances in control of the cell cycle. TXNIP may stop the cell cycle in the G0/G1 phase by controlling a variety of regulatory proteins e.g. cyclin A, p27kip and p16 that could suppress tumor growth (Yam et al., 2002; Jeon et al., 2005), also, TXNIP control NK cells maturation and function. NK cells are parts of the immune system and had an important role against tumor progression and spread. So, TXNIP down regulation will lead to immune system suppression that stimulate and enhance tumor progression (Barber et al., 2007). TXNIP down regulation had been proved to be associated with progression, aggressive disease, advanced stage, shorter metastasis-free intervals and poor prognosis in many cancers (Nishizawa et al., 2011; Woolston et al., 2012; Lim et al., 2012), also, TXNIP mRNA down regulation was inversely correlated to progression of melanoma Goldberg et al., (2003). Down regulation of TXNIP could happen at several levels e.g. epigenetic, transcription or translation (Zhou et al., 2011). These results clarified the tumor suppressor role of TXNIP in cancers which explained our results that TXNIP over expression in meningioma could prevent its progression to higher grades and recurrence after adequate surgery. Another explanation of the tumor suppressor role of TXNIP that it could reduce invasion and tumor angiogenesis by down regulation of thioredoxin which decrease tumor cell survival by stimulating a pro-apoptotic factors (Goldberg et al., 2003; Dunn et al., 2010). So, TXNIP down regulation in a meningioma could stimulate tumor cell survival, invasion, and metastasis. Different from our study in Li Y et al., (2015) study, which find that high TXNIP expression was correlated to a shorter PFS rate in non-small lung cancer (NSCLC) patients; also they demonstrated that TXNIP expression was up-regulated in hypoxic condition and the mechanism through which TXNIP expression was affected by hypoxia has not been explained. These variable results proved the complex conflicting roles of TXNIP in cancer cell survival, apoptosis and progression that depend on cell types. Previous studies explained TXNIP pro-apoptotic roles by activating many death pathways (Chen et al., 2008). TGM2 has many biological functions, and disturbance in its expression has been related to occurrence of many diseases e.g. neurodegenerative diseases, celiac disease and cancer (Wang and Griffin, 2012). Consistent with our finding of that TGM2 over expression was significantly positively correlated with higher meningioma grade, high KI labeling index, the presence of residuals after surgery, with high incidence of recurrence after surgery, the postoperative radiotherapy usage, with meningioma progression to higher grades and positively correlated with recurrence free survival (p=0.000). Similar to our results Huang YC et al., (2014) and Wang et al., (2012) also revealed TGM2 over expression was present in aggressive high grade meningiomas more than low-grade meningioma. Also Park et al., (2012), that had explored TGM2 expression in clear cell renal cell carcinoma (ccRCC) and they proved that its over expression in CCRCC was positively correlated with Fuhrman nu¬clear grade and worse survival rates (Wang et al., 2012). Many studies revealed similar results to us, that TGM2 over expression was positively correlated with progression and dismal outcome of many cancers e.g. hepatocellular carcinoma, non-small cell lung cancer, or laryngeal cancer (Park et al., 2015; Sun et al., 2008; Choi et al., 2011)
Also, TGM2 over expression significantly related to chemotherapy-resistance in cancer breast (Jin et al., 2012) and cancer lung (Park et al., 2010). TGM2 over expression associated with poor survival rate in glioma patients, also, TGM2 inhibitors e.g. glucosamine, cystamine, or KCC009 increased malignant cell death in glioma (Yuan et al., 2007), cancer breast (Kim et al., 2009), and in pancreatic cancer (Fujisawa et al., 2012). These data in addition to our results roved that TGM2 over expression is correlated to aggressive course of many tumors, so it could be beneficial as a therapeutic target for cancers.
TGM2 is expressed in plethora of human tissues exerting many opposing roles on cellular growth and apoptosis through many enzymes and intermediate components, e.g. GTPase, transaminase, protein disulfide isomerase and kinase activities. But its exact role in cancer development still needs clarifications. Siegel and Khosla, (2007), found that TGM2 activates NF-kB, tyrosine kinase and focal adhesion kinase (FAK); that subsequently could activate anti-apoptotic pathways which make cancer cells immortalized, and that inhibition of TGM2 decreased meningioma cell growth, which was similar to results of Huang YC et al., (2014). Another mechanism of how TGM2 participated in cancer progression that it could control malignant cell migration into the ECM, invasion, angiogenesis and metastases (Lentini et al., 2013). Previous researchers have explained possible mechanisms. First, regarding TGM2 role in cancer cell adhesion, Park et al., (2015) found that its overexpression in breast cancer incresed cell invasion and spread by interaction with β integrins and fibronectin that are essential ECM components. Erdem et al., (2014) found that TGM2 over expression in RCC that subsequently lead to increase in syndecan-4 and β1 integrin levels increased RCC metastasis. A second role; TGM2 regulates apoptosis and its inhibition stabilizes p53 levels, increasing apoptosis in cancer cells (Ku et al., 2013). All these results proved the role of TGM2 in increasing cancer cells aggressiveness, metastasis and survival, which was similar to our results in that TGM2 over expression was related to tumor recurrence, high nuclear grade and poor prognosis of meningioma patients. So, future studies must focus on this TGM2 which might lead to discovering more efficient treatment options for patients with aggressive recurrent meningioma.
Summery In our study we investigated the expression levels of TXNIPand TGM2 corelating their expression with each other and with the proliferation marker Ki67; we found inverse significant correlation between TXNIP and TGM2 expression in meningioma patients and we explained their opposing role regarding apoptosis and progression of meningioma, and our study is the first to explain their roles together with the possibility of future use of them as therapeutic targets for adequate management of meningioma patients preventing its progression and recurrence. Further extended studies on large number of patients are needed to clarify roles of TXNIP and TGM2 expression in cancer cell proliferation, apoptosis and control of cell cycle.
References
- Ai L, Kim WJ, Demircan B, et al. The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis. 2008;29:510–8. doi: 10.1093/carcin/bgm280. [DOI] [PubMed] [Google Scholar]
- Barber MA, Zhang T, Gagne BA, et al. NK cells negatively regulate antigen presentation and tumor-specific CTLs in a syngeneic lymphoma model. J Immunol. 2007;178:6140–7. doi: 10.4049/jimmunol.178.10.6140. [DOI] [PubMed] [Google Scholar]
- Cai Z, Zhang C, Zou Y, et al. Tissue thioredoxin-interacting protein expression predicted recurrence in patients with meningiomas. Int J Clin Oncol. 2017 doi: 10.1007/s10147-017-1103-4. DOI 10.1007/s10147-017-1103-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Hui ST, Couto FM, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic β-cell mass and protects against diabetes. FASEB J. 2008;22:3581–94. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CM, Jang SJ, Park SY, et al. Transglutaminase 2 as an independent prognostic marker for survival of patients with nonadenocarcinoma subtype of non-small cell lung cancer. Mol Cancer. 2011;10:119. doi: 10.1186/1476-4598-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LL, Buckle AM, Cooke JP, et al. The emerging role of the thioredoxin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:2089–98. doi: 10.1161/ATVBAHA.110.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem M, Erdem S, Sanli O, et al. Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma. Urol Oncol. 2014;32:25e13–20. doi: 10.1016/j.urolonc.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Erdem S, Yegen G, Telci D, et al. The increased transglutaminase 2 expression levels during initial tumorigenesis predict increased risk of metastasis and decreased disease-free and cancer-specific survivals in renal cell carcinoma. World J Urol. 2015;33:1553–60. doi: 10.1007/s00345-014-1462-7. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Rubin B, Suzuki A, et al. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS One. 2012;7:e34437. doi: 10.1371/journal.pone.0034437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SF, Miele ME, Hatta N, et al. Melanoma metastasis suppression by chromosome 6: evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63:432–40. [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J HistochemCytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huang YC, Wei QC, Chang CN, et al. Transglutaminase 2 expression is increased as a function of malignancy grade and negatively regulates cell growth in meningioma. PLoS One. 2014;9:e108228. doi: 10.1371/journal.pone.0108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JH, Lee KN, Hwang CY, et al. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005;65:4485–9. doi: 10.1158/0008-5472.CAN-04-2271. [DOI] [PubMed] [Google Scholar]
- Jin T, Lin HX, Lin H, et al. Expression TGM2 and BNIP3 have prognostic significance in laryngeal cancer patients receiving surgery and postoperative radiotherapy: a retrospective study. J Transl Med. 2012;10:64. doi: 10.1186/1479-5876-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Park KS, Jeong KC, et al. Glucosamine is an effective chemo-sensitizer via transglutaminase 2 inhibition. Cancer Lett. 2009;273:243–9. doi: 10.1016/j.canlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Kim SY, Suh HW, Chung JW, et al. Diverse functions of VDUP1 in cell proliferation, differentiation, and diseases. Cell Mol Immunol. 2007;4:345–51. [PubMed] [Google Scholar]
- Koshland DE., Jr Molecule of the year. Science. 1993;262:1953. doi: 10.1126/science.8266084. [DOI] [PubMed] [Google Scholar]
- Ku BM, Kim DS, Kim KH, et al. Transglutaminase 2 inhibition found to induce p53 mediated apoptosis in renal cell carcinoma. FASEB J. 2013;27:3487–95. doi: 10.1096/fj.12-224220. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jeong EG, Choi MC, et al. Inhibition of histone deacetylase 10 induces thioredoxin-interacting protein and causes accumulation of reactive oxygen species in SNU-620 human gastric cancer cells. Mol Cells. 2010;30:107–12. doi: 10.1007/s10059-010-0094-z. [DOI] [PubMed] [Google Scholar]
- Lentini A, Abbruzzese A, Provenzano B, et al. Transglutaminases: key regulators of cancer metastasis. Amino Acids. 2013;44:25–32. doi: 10.1007/s00726-012-1229-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Miao LY, Xiao YL. Hypoxia induced high Expression of thioredoxin interacting protein (TXNIP) in non-small cell lung cancer and its prognostic effect. Asian Pac J Cancer Prev. 2015;16:2953–8. doi: 10.7314/apjcp.2015.16.7.2953. [DOI] [PubMed] [Google Scholar]
- Lim JY, Yoon SO, Hong SW, et al. Thioredoxin and thioredoxin-interacting protein as prognostic markers for gastric cancer recurrence. World J Gastroenterol. 2012;18:5581–8. doi: 10.3748/wjg.v18.i39.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Mishra S, Murphy LJ. The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem Biophys Res Commun. 2006;339:726–30. doi: 10.1016/j.bbrc.2005.11.071. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Pike LA, Sams SB, et al. Thioredoxin interacting protein (TXNIP) is a novel tumor suppressor in thyroid cancer. Mol Cancer. 2014;13:62. doi: 10.1186/1476-4598-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa K, Nishiyama H, Matsui Y, et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis. 2011;32:1459–66. doi: 10.1093/carcin/bgr137. [DOI] [PubMed] [Google Scholar]
- Oh JH, Chung AS, Steinbrenner H, et al. Thioredoxin secreted upon ultraviolet a irradiation modulates activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Arch Biochem Biophys. 2004;423:218–26. doi: 10.1016/j.abb.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Oya S, Kawai K, Nakatomi H, et al. Significance of simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012;117:121–8. doi: 10.3171/2012.3.JNS111945. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim HK, Lee JH, et al. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:493–502. doi: 10.1007/s00432-009-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Baek HW, Rhee YY, et al. Transglutaminase 2 expression and its prognostic significance in clear cell renal cell carcinoma. J Pathol Transl Med. 2015;49:37–43. doi: 10.4132/jptm.2014.10.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Chicoine MR, Filiput E, et al. Clinicopathologic assessment and grading of embolized meningiomas: a correlative study of 64 patients. Cancer. 2001;92:701–11. doi: 10.1002/1097-0142(20010801)92:3<701::aid-cncr1373>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–54. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther. 2007;115:232–45. doi: 10.1016/j.pharmthera.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatr. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639–51. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- Sun Y, Mi W, Cai J, et al. Quantitative proteomic signature of liver cancer cells: tissue transglutaminase 2 could be a novel protein candidate of human hepatocellular carcinoma. J Proteome Res. 2008;7:3847–59. doi: 10.1021/pr800153s. [DOI] [PubMed] [Google Scholar]
- Thomázy V, Fésüs L. Differential expression of tissue transglutaminase in human cells. An Immunohistochemical study. Cell Tissue Res. 1989;255:215–24. doi: 10.1007/BF00229084. [DOI] [PubMed] [Google Scholar]
- Van de Vijevr MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Wang X, Gong Y, Wang D, et al. Analysis of gene expression profiling in meningioma: deregulated signaling pathways associated with meningioma and EGFL6 overexpression in benign meningioma tissue and serum. PLoS One. 2012;7:e52707. doi: 10.1371/journal.pone.0052707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012;42:939–49. doi: 10.1007/s00726-011-1008-x. [DOI] [PubMed] [Google Scholar]
- Wen PY, Quant E, Drappatz J, et al. Medical therapies for meningiomas. J Neurooncol. 2010;99:365–78. doi: 10.1007/s11060-010-0349-8. [DOI] [PubMed] [Google Scholar]
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolston CM, Zhang L, Storr SJ, et al. The prognostic and predictive power of redox protein expression for anthracycline-based chemotherapy response in locally advanced breast cancer. Mod Pathol. 2012;25:1106–16. doi: 10.1038/modpathol.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–26. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Park C-K, Park S-H, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatr. 2008;79:574–80. doi: 10.1136/jnnp.2007.121582. [DOI] [PubMed] [Google Scholar]
- Yuan L, Siegel M, Choi K, et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–73. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yu Q, Chng WJ. TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int J Biochem Cell Biol. 2011;43:1668–73. doi: 10.1016/j.biocel.2011.09.005. [DOI] [PubMed] [Google Scholar]


