Abstract
Burkholderia pseudomallei and its close relative B. mallei are human pathogens that are classified as Tier 1 bio-threat agents. Both organisms have previously been shown to constitutively produce a capsular polysaccharide (CPS) that is both a virulence determinant and protective antigen. Extraction and purification of CPS for use as a potential vaccine candidate requires containment level 3 laboratories which is expensive and time-consuming. B. thailandensis strain E555 is closely related to B. pseudomallei and B. mallei, but is non-pathogenic to humans and based on immunological cross-reactivity has previously been shown to express a B. pseudomallei-like CPS. In this study, capsular polysaccharide isolated from an O-antigen deficient strain of B. thailandensis E555 was identified by 1H and 13C NMR spectroscopy as -3-)-2-O-acetyl-6-deoxy-β-d-manno-heptopyranose-(-1, and identical to that produced by B. pseudomallei. This was further substantiated by anti-CPS monoclonal antibody binding. In connection with the production of CPS fragments for use in glycoconjugate vaccines, we set out to assess the importance or otherwise of the CPS 2-OAc groups in immune protection. To this end conjugates of the native and de-O-acetylated CPS with the Hc fragment of tetanus toxin (TetHc) were used as vaccines in a mouse model of melioidosis. The level of protection provided by deacetylated CPS was significantly lower than that from native, acetylated CPS. In addition, sera from mice vaccinated with the deacetylated CPS conjugate did not recognise native CPS. This suggests that CPS extracted from B. thailandensis can be used as antigen and that the acetyl group is essential for protection.
Keywords: Melioidosis, Burkholderia pseudomallei, Burkholderia thailandensis, Capsular polysaccharide, Glycoconjugate vaccine
Graphical abstract
Highlights
-
•
B. thailandensis E555 CPS is shown to be structurally indistinguishable from CPS expressed by B. pseudomallei.
-
•
Monoclonal antibody recognition of B. thailandensis E555 CPS was also indistinguishable from B. pseudomallei CPS.
-
•
The CPS 2-OAc group is crucial for recognition by four murine anti-CPS monoclonal antibodies.
-
•
The 2-OAc group is also crucial for protective immunogenicity of TetHc-CPS in a murine model of acute melioidosis.
-
•
B. thailandensis CPS is a credible and readily accessible candidate for melioidosis/glanders vaccine development.
Content includes material subject to © Crown copyright (2017), Dstl. This material is licensed under the terms of the Open Government Licence except where otherwise stated. To view this licence, visit http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3 or + write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: psi@nationalarchives.gsi.gov.uk.
1. Introduction
Burkholderia pseudomallei is a tropical pathogen and the causative agent of melioidosis, which results in an estimated 90,000 human deaths per year [1], [2]. Melioidosis can present itself in a number of forms, ranging from acute pneumonia or septicaemia to chronic infections characterised by abscess formation at multiple sites on the body. Sub-clinical infections have also been reported which may remain undiagnosed for a number of years [3], [4]. This organism is naturally resistant to a wide range of commonly used antibiotics, including penicillins, rapamycins, aminoglycosides and third-generation cephalosporins [5]. B. mallei, a close relative of B. pseudomallei, is the etiologic agent of glanders, a disease primarily of horses and related solipeds, where it is associated with a high mortality rate without antibiotic therapy [6]. Infection in humans can be very severe and is characterised by extreme pain, fever and abscesses primarily of the spleen and liver [7]. B. pseudomallei and B. mallei are classified as Tier 1 select agents by the Department of Health and Human Services [8]; no vaccines are available for either melioidosis or glanders.
Both B. pseudomallei and B. mallei organisms produce multiple cell surface glycans, one of which is common to both species; capsular polysaccharide (CPS) with the repeating homopolymer structure based on -3)-2-O-acetyl-6-deoxy-β-d-manno-heptopyranose-(1- [9]. CPS is one of the main surface-associated antigens of B. pseudomallei and B. mallei [10], with anti-CPS antibodies commonly present in convalescent patient antisera [11]. CPS is also a known virulence determinant of both of these species, as loss of capsule production results in strong attenuation in animal models of disease [3], [12]. CPS has also been demonstrated to be a protective antigen in animal models against both B. pseudomallei and B. mallei challenge; passive transfer of anti-CPS monoclonal antibody also provides partial protection [13], [14], [15]. However, despite recent advances in methods for CPS purification from pathogenic Burkholderia, [9] the yields are low and the requirement for high level containment facilities when cultivating these organisms at scale is problematic and costly. Several studies have, however, utilised the 6-deoxyheptan CPS extracted from non-pathogenic, select-agent excluded strains of B. pseudomallei [16], [17].
Burkholderia thailandensis is closely related to B. pseudomallei and B. mallei, but with a >105-fold reduction in virulence, with recent data suggesting that B. thailandensis lacks a critical pathogenic activity common to B. mallei and B. pseudomallei [18], [19]. As B. thailandensis can be handled at lower levels of containment and is very similar genetically to B. pseudomallei, it is often used as a substitute in melioidosis research [4], [20], [21], [22]. Although most strains of B. thailandensis do not produce CPS, the E555 strain produces a polysaccharide that cross-reacts with an anti-B. pseudomallei CPS monoclonal antibody [23]. In combination with the high sequence similarity of six CPS genes between these two species [23], this offers the potential for a source of CPS from an essentially avirulent organism, which would be less expensive, quicker and safer to produce. However, confirmation of its CPS structure is required. Compared to non-pathogenic, select-agent excluded strains of B. pseudomallei; B. thailandensis has the advantage of being a naturally avirulent strain with no potential for reversion, which may be more palatable for use in pharmaceutical scale-up.
Whilst extraction of CPS from avirulent B. thailandensis E555 would be an improvement over current methods, the CPS itself is very much larger than carbohydrate antigens used in licensed vaccines which utilise oligosaccharides, and is not ideal or optimal for melioidosis glycoconjugate development considering the reported success with a synthetic hexamer of 1524 Da [24]. The use of short chain, oligosaccharide fragments of CPS would assist development of a defined glycoconjugate and provide more vaccine doses per mg of CPS extracted. While cutting-edge chemical synthesis, including of a hexasaccharide fragment of CPS has recently been reported [24], [25], such approaches remain challenging at this time. Plausible acid- or base-mediated conditions for partial depolymerisation of native CPS give rise to de-O-acetylation (unpublished observations). If the 2-OAc group could be demonstrated to have limited impact on vaccine efficacy, this would open up the use of CPS hydrolysis fragments for glycoconjugate vaccine production. A challenge to this prospect lies in recent work with synthetic CPS fragments, which show that the 2-OAc group is essential in the interaction with an anti-CPS monoclonal antibody; absence of the acetyl moiety resulted in complete loss of antibody affinity [26]. While this observation does not necessarily mean a loss of immune protection as a result of CPS deacetylation, this observation clearly merits attention. In other systems, the impact of polysaccharide antigen acetylation on immune responses is variable and has had some impact on vaccine design [27], [28], [29], [30], [31]. In the current study, we have investigated the structure of the CPS produced by B. thailandensis E555. Our studies have addressed the immunogenicity and the protective potential of this material conjugated to the Hc fragment of tetanus toxin (TetHc), a carrier protein used in previous work [24], in comparison to the corresponding deacetylated CPS conjugate in a murine model of acute melioidosis.
2. Results
2.1. 6-Deoxy-d-manno-heptose
In support of analytical and structural studies on the B. thailandensis E555 CPS, defined 6-deoxy-manno-heptose was chemically synthesised. The synthetic route employed was essentially that described by Aspinall et al. [32] In outline, this involved C-6 extension of a suitably protected 6-deoxy-6-iodo-α-d-mannopyranoside with cyanide, followed by nitrile reduction-hydrolysis-reduction, proceeding via imine and aldehyde intermediates. Final global deprotection afforded the desired monosaccharide.
2.2. Extraction and structural characterisation of CPS from B. thailandensis E555:: wbiI (pKnock-KmR)
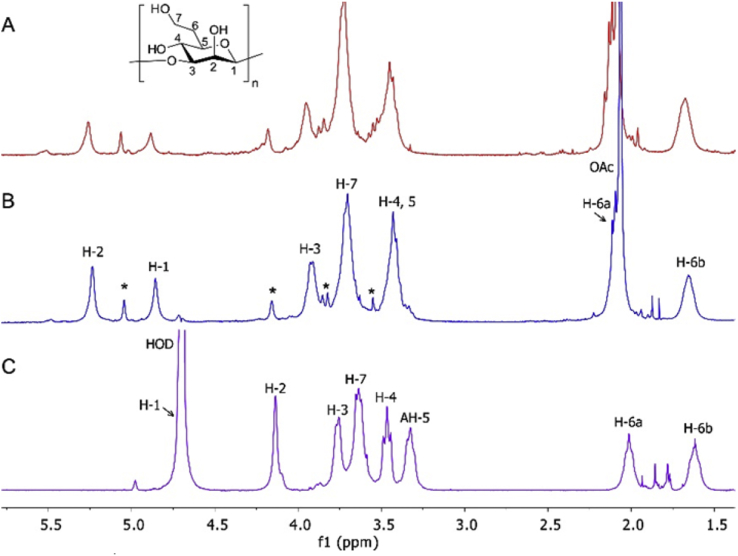
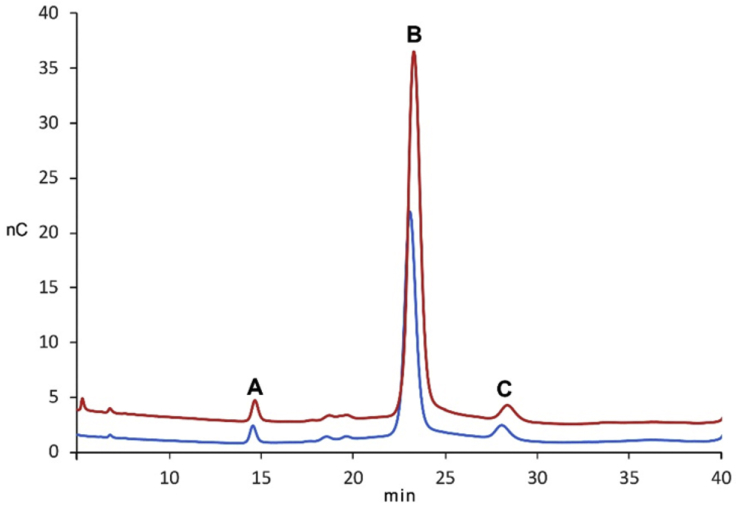
Analysis of 1H NMR spectra of CPS extracted from B. thailandensis E555:: wbiI (pKnock-KmR) demonstrated the presence of essentially the same (1 → 3)-linked 2-O-acetyl-6-deoxy-β-d-manno-heptopyranose repeat unit as found in CPS extracted from B. pseudomallei strain 1026b (Fig. 1) and from B. pseudomallei BP2683 [9]. Corresponding HSQC and 13C NMR data (Figure S1 and S2 in supplementary information) also supported that structure. An additional spin system (highlighted with asterisks in Fig. 1) present in CPS 1H NMR spectra can be identified as a set of signals belonging to α-1,3-mannan, a polymer which is known to be produced by B. pseudomallei and B. mallei strains and is also present in purified B. thailandensis CPS [9]. Monosaccharide analysis of fully hydrolysed CPS from B. thailandensis E555:: wbiI (pKnock-KmR), using high-performance anion-exchange chromatography with pulse amperometric detection (HPAEC-PAD) established the presence of 6-deoxy-manno-heptose, which was confirmed by spiking experiment of the hydrolysed sample with defined 6-deoxy-manno-heptose (Fig. 2, peak B). Also identified was d-mannopyranose (peak C) by co-injection of the sample with D-mannose (data not shown).
Fig. 1.
Carbohydrate regions of 1H NMR spectra (400 MHz, D2O, 25 °C) of CPS isolated from (A) B. pseudomallei 1026b, (B) B. thailandensis E555:: wbiI (pKnock-KmR), and (C) deacetylated CPS from B. thailandensis E555. Signals of α-1,3-mannan present in CPS spectra are is indicated by asterisks. Spectra of native CPS are recorded using water suppression experiment.
Fig. 2.
Monosaccharide analysis of B. thailandensis E555:: wbiI (pKnock-KmR) CPS by HPAE-PAD on Dionex Carbopac PA 20 column. The bottom trace shows the ion chromatogram of CPC hydrolysis products and the top one is the same sample spiked with 25 μM 6-deoxy-d-manno-heptose standard. Peaks correspond to (A) – unidentified sugar, (B)-6-deoxy-d-manno-heptose, and (C) – mannose. The analysis was performed on Dionex Carbopac PA 20 column (3 × 150 mm) at 0.25 mL/min in 8 mM NaOH at 25 °C.
To investigate any potential differences in CPS secondary structure, the comparative binding of an anti-CPS monoclonal antibody generated against heat-killed B. pseudomallei (DSTL189) to both B. pseudomallei and B. thailandensis purified CPS samples was assessed. There was no significant difference over a range of B. pseudomallei 1026b and B. thailandensis E555:: wbiI (pKnock-KmR) purified CPS concentrations (supplementary information, Figure S3).
2.3. Role of the CPS 2-OAc group in antibody recognition
After confirmation of structural similarity between B. thailandensis E555 and B. pseudomallei 1026b CPS by NMR and antibody recognition, attention turned to investigation of the influence of the acetyl moiety on CPS antibody recognition. Following recent work which demonstrated the importance of CPS acetylation to antibody recognition [26], a sample of deAc CPS was prepared by treatment of native CPS with aqueous ammonia. In these mild conditions the main structure of CPS remains unaffected as followed from monitoring the reaction by 1H NMR. Furthermore, the 1H NMR spectrum of a product of deacetylation (Fig. 1C) revealed a characteristic upfield shift of the resonance of H-2 and disappearance of OAc signal confirming a complete removal of acetyl group. Deacetylated CPS was probed with four anti-CPS monoclonal antibodies (DSTL187, 188, 189, 190)13 and essentially complete loss of antibody affinity to the deacetylated CPS was apparent by ELISA (supplementary information, Figure S4).
2.4. Role of the CPS 2-OAc group in antibody elicitation and immune protection
Whilst CPS is a promising vaccine candidate, polysaccharide immunogenicity can be improved by conjugation to an appropriate protein carrier, such as the Hc fragment of tetanus toxin [33]. Such conjugates provide T-cell dependent immunogenicity, including immunological memory, avidity maturation and isotype switching to generate complement-activating antibody isotypes such as IgG1 [34]. In order to determine the effect of CPS acetylation on immune protection against Burkholderia infection, glycoconjugate vaccines were produced with carrier protein TetHc chemically conjugated by reductive amination [16] to purified B. thailandensis E555:: wbiI (pKnock-KmR) CPS or its deacetylated counterpart (deAc CPS), which other than the absence of the O-acetyl group had exactly the same structure as native CPS (Fig. 1C). The glycoconjugate vaccines were analysed by SDS-PAGE and western blot (supplementary information, Figure S5-S6), and the ratio sugar: protein determined by phenol-sulfuric acid and BCA assays, respectively (supplementary information, Table S1). BALB/c mice were vaccinated with native and deAc CPS-TetHc vaccines containing 10 μg of CPS per dose on three occasions, 2 weeks apart, followed by a 5-week rest period and then challenged intraperitoneally with B. pseudomallei K96243 (1.17 × 105 CFU/mouse). There was a statistically significant difference in comparative efficacy between the TetHc conjugates containing CPS or deAc CPS (p = 0.0015, Fig. 3). In addition, the protective efficacy of deAc CPS-TetHc was not significantly different to adjuvant alone (p = 0.1694, Fig. 3).
Fig. 3.
Survival of BALB/c mice vaccinated with CPS-TetHc (acetylated and deacetylated CPS), TetHc and alum followed by challenge with 1.17 × 105 CFU via the IP route of B. pseudomallei K96243 (approximately 157 x median lethal doses (MLDs)). Mice were vaccinated three times in two-week intervals prior to challenge. Vaccination with acetylated CPS conjugate offered significantly greater protection than the deacetylated CPS conjugate [p = 0.0015 Log-Rank (Mantel Cox test [35])]. The deacetylated CPS-TetHc conjugate did not offer any significantly greater protection than adjuvant or TetHc alone [p = 0.1694 and p = 0.0872 Log-Rank, respectively (Mantel Cox test)].
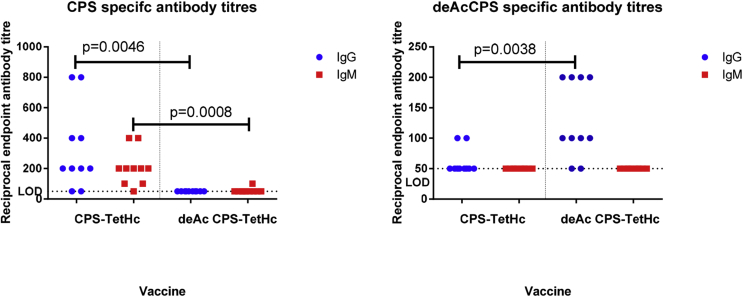
Analysis of the relevant CPS-specific IgG and IgM titres from mouse sera following the third vaccination showed an IgG and IgM response in mice vaccinated with native CPS-TetHc and only an IgG response in mice that received deAc CPS-TetHc. CPS-specific antibody responses in mice vaccinated with deAc CPS-TetHc were significantly lower than mice that received native CPS-TetHc. Analysis of the deAc CPS-specific antibody response showed a very low IgG titre in native CPS-TetHc vaccinated mice which was significantly lower than in mice that received deAc CPS-TetHc (Fig. 4).
Fig. 4.
ELISA analysis of acetylated and deacetylated CPS-specific IgG and IgM immune responses following vaccination with CPS conjugate vaccines. Mice were vaccinated with native CPS-TetHc or deacetylated CPS-TetHc three times at 2-week intervals. Serum was obtained from mice 14 days after the third vaccination, and titres of IgG and IgM specific for CPS or deacetylated CPS were determined by ELISA. Individual symbols represent a single immunised mouse. Significance was determined by unpaired t-test. LOD, limit of detection.
3. Discussion and conclusions
B. pseudomallei and its close relative B. mallei are Tier 1 select agents due to the severity of disease, infection by the aerosol route, antibiotic resistance and lack of available vaccines [8]. For these reasons the development of medical countermeasures against these organisms is a priority. The manno-heptose-based CPS expressed by B. pseudomallei and B. mallei has shown to be partially protective in animal models of melioidosis, as have anti-CPS monoclonal antibodies in passive transfer studies [13], [14]. As B. pseudomallei and B. mallei are classified by the Advisory Committee on Dangerous Pathogens (ACDP) as hazard group 3 pathogens, extraction of CPS in containment level 3 laboratories is required: this is expensive and time consuming, although several studies have described the extraction of CPS from non-pathogenic, select-agent excluded strains of B. pseudomallei [16], [17]. Identification of B. thailandensis E555 by Sim et al. [23], which expresses a B. pseudomallei-like CPS provides an opportunity for a less expensive, quicker route of CPS extraction and purification given the lower levels of containment required for B. thailandensis E555. In this study, we have shown through the use of NMR and monosaccharide analysis of hydrolysed polysaccharide that at a chemical level, the capsular polysaccharide produced from B. thailandensis E555 is essentially identical to that from the pathogenic Burkholderia species B. pseudomallei. Furthermore, B. thailandensis E555 also expresses the α-1,3 mannan found in its pathogenic relatives. Recognition of CPS extracted from B. thailandensis E555 with a monoclonal antibody raised against B. pseudomallei confirms previous work by Sim et al. [23] and infers that both polysaccharides have an epitope of similar structure. The implications of this finding mean that capsular polysaccharide can now be extracted from B. thailandensis E555, a naturally avirulent bacterium, which should reduce the cost of obtaining this polysaccharide and may also make licensure easier than the use of hazard group 3 bacteria, or select-agent excluded strains of B. pseudomallei where reversion to virulence is possible. The purity of CPS extracted from B. thailandensis E555 was >90% and similar to the reported purity of CPS extracted from Bp82 [16]. CPS yields from B. thailandensis E555 varied between 0.5 and 18 mg per litre of bacterial culture, which is a wider range than the 10–15 mg per litre of culture reported from Bp82 [16]. This may be due to slight procedural differences in the extraction method rather than a difference in CPS expression between these two species. This laboratory has not extracted CPS from B. pseudomallei and so a true comparison has not been made.
NMR analysis of B. thailandensis E555 extracted CPS shows that the sample is approximately 90% 6-deoxy-manno-heptose and 10% α,1-3 mannan. This composition is more in agreement with CPS produced by B. pseudomallei than B. mallei, where for the latter the ratio is approximately 43:57,9 indicating that B. thailandensis could be more suitable as a proxy for B. pseudomallei than B. mallei. This is also supported by Sim et al. [23], where PCR analysis with oligonucleotide primers against six B. pseudomallei CPS genes (BPSL2791 to BPSL2797) were amplified in B. thailandensis E555. DNA sequencing of the PCR products confirmed nucleotide similarities of at least 92% to the CPS genes of B. pseudomallei K96243.
A solution to utilising native CPS as a melioidosis/glanders vaccine antigen may lie in producing the polysaccharide, or fragments thereof, by chemical synthesis or alternatively, naturally occurring CPS extracted from non-pathogenic B. thailandensis could be fragmented into minimal antigenic units (vide infra). This would facilitate progress of the antigen through the regulatory process, given that the vaccine candidate would be highly defined and would not originate from the pathogen. In addition, set chain lengths would lower the cost of production by increasing the number of doses per mg of CPS based on the results of a cost-benefit analysis commissioned by Dstl (unpublished). However, yields of CPS following sizing and the final vaccine carbohydrate content would have to be considered as optimal chain length and CPS content per dose is currently unknown.
In spite of the huge progresses achieved in the chemical synthesis of CPS fragments [24], [25], the CPS molecule still represents a challenge for carbohydrate chemistry, given the ambiguity surrounding whether the α-1,3-mannan and 6-deoxy-d-manno-heptose-based glycans are part of the same chain or are closely associated chains that are difficult to separate; in addition, the β-manno configuration also presents a challenge, particularly given the presence of the 2-O-acetyl group. As recently reported by Marchetti et al., [26] the acetyl group is required for 3C5 monoclonal antibody recognition of the molecule, although this study did not determine whether the acetyl group was important for achieving immune protection. In the current study, we found that a panel of four anti-CPS monoclonal antibodies (DSTL187, 188, 189, 190) have no binding affinity for deacetylated CPS, which supports the work by Marchetti et al. [26] and also demonstrates that these antibodies do not bind the α-1,3-mannan which is present in these samples.
Whilst the acetyl group is demonstrably important for antibody recognition, extrapolating this finding to protection against bacterial challenge is difficult on this basis alone and does not address the importance of potential antibodies raised against the CPS backbone. In the present study, we have shown that the acetyl moiety is essential for protection in an animal model of melioidosis: CPS conjugated to the carrier protein TetHc provided significantly greater protection in vaccinated mice than a deacetylated CPS conjugate. Furthermore, vaccination with the deacetylated CPS conjugate offered no greater protection to bacterial challenge than vaccination with adjuvant alone. Antibody analysis of sera from mice vaccinated with the acetylated CPS conjugate showed minimal cross-reactivity to purified de-acetylated CPS, which suggests that the acetyl group is an integral part of an immunodominant epitope. The lack of an IgM antibody response to deacetylated CPS above the assay limit of detection, which was seen for CPS in this work, also suggests that the deacetylated CPS is less immunogenic. The essentiality of the CPS 2-OAc group for immune protection presents challenges for fragmenting this large (>200 kDa, as judged by PAGE) [36] polysaccharide into efficacious fragments. Both acid- or base-catalysed polysaccharide fragmentation is associated with concomitant de-O-acetylation (unpublished observations).
In summary, the use of capsular polysaccharide from Burkholderia species as a vaccine against melioidosis and glanders remains a tangible prospect. This study presents clear evidence that B. thailandensis E555 extracted CPS can be used as a substitute vaccine candidate to CPS from pathogenic species. We have shown that the acetyl group of CPS forms an immunodominant, protective, epitope which is an important finding for chemical efforts to produce synthetic CPS and also places limitations on the methods that can be employed to fragment native CPS polysaccharide.
4. Materials and methods
4.1. Bacterial growth and CPS isolation
The O-polysaccharide-deficient mutant of B. thailandensis E555 harbouring a kanamycin-resistance marked, in-frame deletion of its wbiI gene (B. thailandensis E555:: wbiI (pKnock-KmR)) [37] was grown in 2 L of LB broth overnight at 37 °C with shaking. Loss of O-polysaccharide expression had previously been confirmed by western immunoblot analysis, with bacterial stocks maintained in 35% (v/v) glycerol suspensions at −80 °C.
The CPS was extracted via a modified hot phenol method and purified as described previously [9], [38]. Briefly, the overnight culture was added in equal parts (v/v) to 90% (w/v) phenol and heated to 80 °C. The phenol was dialysed against distilled water using Spectra/Por 6–8K MWCO dialysis membrane and the resulting cell debris removed by centrifugation (10 min at 11,900×g). The samples were then enzymatically digested with 50 μg/mL DNAse and RNAse (Sigma) at 37 °C for 2 h and then 50 μg/mL proteinase K (Sigma) at 60 °C for 3 h. The sample was then centrifuged at 11,900×g for 40 min and the CPS isolated from the supernatant as precipitated gel following ultracentrifugation at 100,000×g for 6 h. The gel pellet was re-suspended in ultrapure water and lyophilised to concentrate. Rough LPS contaminants were then removed by acid hydrolysis with 2% (v/v) acetic acid at 100 °C for 2 h. The sample was centrifuged at 11,900×g for 40 min and the supernatants lyophilised to concentrate. The samples were then reconstituted at 20 mg/mL in ultrapure water and loaded onto a Sephadex G-50 column (40 cm × 2.6 cm). The purified CPS was eluted isocratically in ultrapure water at a rate of 4 mL/min. The fractions were analysed for carbohydrate using the phenol-sulphuric acid method [39] with the appropriate fractions combined and lyophilised.
CPS from B. pseudomallei 1026b for use as a reference in NMR analysis was kindly provided by Associate Professor P. Brett from the University of Nevada, Reno School of Medicine [9].
4.2. 1H and 13C NMR spectroscopy
The lyophilised CPS samples were reconstituted by dissolving in 700 μL D2O and NMR spectra were recorded with a Bruker Avance III HD 400 MHz spectrometer operating at 400 MHz (1H) or 100.6 MHz (13C). Assignments were made with the aid of homonuclear (COSY, NOESY) and heteronuclear (HSQC) two dimensional correlation spectroscopy.
4.3. Polysaccharide hydrolysis with strong acid
A solution of polysaccharide (20 μL of a 3 mg/mL solution in MQ-H2O) was added to a screw cap reaction vial containing trifluoroacetic acid (30 μL in 150 μL of MQ-H2O). The vial was sealed and heated to 100 °C for 4 h. The reaction was stopped and the solution was concentrated in vacuo using a SpeedVac® evaporation centrifuge (Thermo scientific). The polysaccharide was reconstituted in MQ-H2O (300 μL), vortexed for 30 s and centrifuged for 5 min (14,000×g).
4.4. Monosaccharide analysis by HPAEC-PAD chromatography
Polysaccharide hydroxylates (10 μL) were injected onto a Dionex® ICS-5000® HPAEC-PAD system and the monosaccharide analysis was done using an 8 mM NaOH (Fisher® HPLC grade) isocratic gradient (0.25 mL/min) for 40 min followed by a 6 min wash step (100 mM NaOH) and a 15 min re-equilibration step. Detection was achieved using pulsed amperometric detection (PAD). Spiking experiments for identification of 6-deoxy-manno-heptopyranose and d-mannopyranose were performed on a monosaccharide by monosaccharide basis. This was due to the impact of the addition of a monosaccharide on the retention times of the monosaccharide mixture. The polysaccharide hydroxylate samples were supplemented with monosaccharide standards (10/25 μM final concentration) and injected onto the column (10 μL).
4.5. Deacetylated CPS production
A sample of CPS (3 mg) in a glass sample vial was dissolved in H2O (0.4 mL), conc. aqueous NH3 (0.2 mL) was added and the vial was sealed and then heated at 50 °C for 2 h. The sample was subsequently freeze-dried and analysed by NMR. The disappearance of CPS OAc peak was observed within 80 min.
4.6. Generation of biotinylated monoclonal antibody DSTL189
DSTL189 antibody was diluted to 1 mg/mL in PBS. Using a syringe and needle, 1 mL of dimethylformamide (DMF) was introduced into a vial of biotin-N-Hydroxysulfosuccinimide (NHS) ester (Molecular Devices Corp.) and mixed until the label had completely dissolved. The dissolved label was removed using the syringe and introduced into a 1.5 mL Eppendorf tube and then placed in a foil wrapper to prevent UV degradation of the label. 10.8 μL of biotin-NHS label was then added to 1 mL of antibody solution and mixed thoroughly before incubating at room temperature for 2 h in the dark to achieve a molar coupling ration of 10. During this incubation, a PD-10 column (GE Healthcare) was equilibrated with 25 mL of PBS. A further 1–2 mL of PBS was added to the column and the bottom of the column capped (to prevent the upper disc from drying out over the remainder of the incubation period). Once incubation was complete, the cap was removed from the PD-10 column for the PBS to drain through. The 1 mL reaction volume was added to the columns, allowed to drain through and 1.25 mL of PBS added to each column to stop the reaction. Using graduated Eppendorfs to collect fractions, 3 mL of PBS was added to the column. The first 0.75 mL was collected in the first Eppendorf, 1.5 mL in the second Eppendorf and the remaining 0.75 mL in the third Eppendorf. This was repeated for the remaining reactions. The absorbance of the fractions was measured on a UV-VIS spectrophotometer at 280 nm and 362 nm. The labelled antibodies were stored at −70 °C until use.
4.7. Antibody analysis of purified CPS extracted from B. thailandensis E555:: wbiI (pKnock-KmR) and B. pseudomallei 1026b
A 96-well microtiter plate was coated overnight at 4 °C with 100 μL/well of purified monoclonal antibody (DSTL189) diluted to 5 μg/mL in PBS (Dulbecco's PBS 1 x without CaCl2, MgCl2). During all incubations, the microtiter plates were covered with plate sealers. Each well was washed three times with PBS-Tween 20 (0.05%) and blocked with 200 μL of PBS containing 2% (w/v) skimmed milk powder (BLOTTO) for 1 h at 37 °C. Purified CPS from B. thailandensis E555:: wbiI (pKnock-KmR)) and B. pseudomallei 1026b was diluted to an initial concentration of 10 μg/mL and serially diluted 1:3 in 100 μL aliquots on the plate. Following incubation for 1 h at 37 °C, each well was washed three times with PBS-Tween 20 (0.05%) and 100 μL/well of biotinylated DSTL189 antibody diluted in BLOTTO to 5 μg/mL was added. The plate was incubated for 1 h at 37 °C and each well then washed three times with PBS-Tween 20 (0.05%). A 1:1000 dilution of streptavidin peroxidase (Sigma) in 2% BLOTTO was then added and incubated at 37 °C for 1 h. Each well was washed six times with PBS-Tween 20 (0.05%) and bound conjugate detected with 100 μL/well of enzyme substrate (55 mg 2,2′-azino-bis(ethylbenzthiazoline-6-sulphonic acid) in 56 mL of 0.1 M citric acid, 44 mL of Na2HPO4 and 100 μL 30% (w/w) hydrogen peroxide, with incubation at room temperature for 15 min prior to optical density measurement at 414 nm.
4.8. Antibody analysis of purified CPS (acetylated and deacetylated) extracted from B. thailandensis E555::wbiI (pKnock-KmR)
A 96-well microtiter plate was coated overnight at 4 °C with 100 μL/well of B. thailandensis E555::wbiI (pKnock-KmR) purified CPS or B. thailandensis E555::wbiI (pKnock-KmR) purified deacetylated CPS at 10 μg/mL in PBS (Dulbecco's PBS 1 x, without CaCl2, MgCl2). During all incubations, the microtiter plates were covered with plate sealers. Each well was washed three times with PBS-Tween 20 (0.05%) and blocked with 200 μL of PBS containing 2% (w/v) skimmed milk powder (BLOTTO) for 1 h at 37 °C. Primary antibodies (DSTL187, 188, 189, 190)13 were diluted to 5 μg/mL in 2% BLOTTO and 100 μL was added to the plate in duplicate. The plate was then incubated for 1 h at 37 °C and each well washed three times with PBS-Tween 20 (0.05%). Goat anti-mouse IgG-HRP conjugate (Biorad) was diluted 1:2000 in 2% BLOTTO, 100 μL added to each well and the plate incubated at 37 °C for 1 h. Each well was washed six times with PBS-Tween 20 (0.05%) and bound conjugate detected with 100 μL/well of enzyme substrate (55 mg 2,2′-azino-bis(ethylbenzthiazoline-6-sulphonic acid)) in 56 mL of 0.1 M citric acid, 44 mL of Na2HPO4 and 100 μL 30% (w/w) hydrogen peroxide, with incubation at room temperature for 20 min prior to optical density measurement at 414 nm.
4.9. TetHc production
TetHc production was performed as previously reported [40]. Briefly, recombinant tetanus toxin Hc fragment was recovered from E. coli BL21 (pKS1-TetHc), kindly supplied by Dr N. Fairweather [41]. The recombinant His-tagged protein was eluted with up to 500 mM imidazole using HisTrap Columns (GE Healthcare) on an ATKA FPLC (GE Healthcare). Protein purity was assessed by Coomassie stained SDS PAGE gels (Expedeon) and protein concentration by BCA assay (Pierce).
4.10. CPS conjugation to TetHc
CPS and TetHc conjugation was performed as detailed previously [16], [17]. Briefly, purified CPS was solubilised at 5 mg/mL in PBS (Sigma) and sodium meta-periodate (Alpha Aesar) was added to a final 30 mM concentration. The mixture was gently stirred at room temperature for 40 min and afterwards, excess oxidising agent was removed by dialysis (GeBAflex-tube maxi). To the dialysed polysaccharide solution, TetHc protein was added to a final concentration of 5 mg/mL in PBS (Sigma). Sodium cyanoborohydride (Sigma: 1 M NaBH3CN in 10 mM NaOH) was then added at 10 μL per mL of conjugation mixture and then stirred at room temperature for 4 days. Following this, a further 10 μL of sodium borohydride (Sigma: 1 M NaBH4 in 10 mM NaOH) was added to each mL of conjugation mixture and stirring was continued at room temperature for 40 min. The conjugate reactions were then dialysed against distilled water (GeBAflex-tube maxi) and analysed by SDS-PAGE (12%, Expedeon) and western blot using anti-CPS monoclonal antibody DSTL189. The resulting products were then re-dissolved in ultrapure water and stored at −20 °C.
4.11. Conjugate analysis
CPS concentration was determined by the phenol-sulfuric acid assay [39] using 6-deoxy-manno-heptose to generate a standard curve and analysed against a known concentration of CPS. TetHc concentration was determined by the BCA protein assay (Pierce).
4.12. Animal challenge
Groups of 10 BALB/c female mice between 6 and 8 weeks old (Charles River UK) were acclimatised for two weeks prior to experimental start and vaccinated via the intra-muscular (IM) route on Day 0 with either B. thailandensis E555 CPS conjugated to TetHc or deacetylated B. thailandensis E555 CPS conjugated to TetHc, at a concentration of 10 μg CPS per mouse re-constituted in PBS and 18% v/v Alum (Invitrogen). Mice vaccinated with the acetylated CPS received 6 μg of TetHc and the mice that received the deacetylated CPS: 3.3 μg TetHc. Groups of 5 control mice were given adjuvant or TetHc only (6 μg per mouse per dose). Vaccine boosts were given on days 14 and 28 and the mice were challenged via the intra-peritoneal (IP) route with 0.1 mL of B. pseudomallei K96243 at 1.17 × 105 cfu per mouse on day 63 [157 x median lethal dose (MLD)]. We previously determined the MLD in the BALB/c mouse model to be 744 CFU by the IP route [17]. The mice were observed twice daily for a period of 35 days after challenge when surviving mice were culled. All mice were tail-bled 2 weeks post-vaccination. Survival data was analysed with GraphPad Prism and by the Log-Rank (Mantel-Cox) test [35]. All animal work was carried out according to the Animal (Scientific Procedures) Act 1986 and following challenge, the mice were handled within a containment level 3 half-suit isolator.
4.13. Antibody analysis of animal sera
ELISAs were performed on sera collected 2 weeks after the third vaccination. 96 well plates were coated with either purified CPS or deacetylated CPS at 10 μg/mL in PBS (Dulbecco's PBS 1 x, without CaCl2, MgCl2) and incubated overnight at 4 °C. Each well was washed three times with PBS supplemented with 0.05% (v/v) Tween-20 (Sigma). The wells were then blocked with 2% (w/v) skimmed milk powder (Sigma) in PBS and incubated at 37 °C for 1 h. Following three further washes with PBS-Tween, two-fold dilutions of the mouse serum samples in PBS supplemented with 2% (w/v) skimmed milk powder were made across the plate. To act as a standard curve, one row of wells was coated with 5 μg/mL anti-fab antibody (Sigma), incubated and washed as described above. Appropriate isotype standards (Sigma) were diluted twofold across the plate. Also included into separate wells was serum from PBS and TetHc vaccinated control mice as negative controls. The plate was incubated for a further 1 h at 37 °C and washed three times in PBS-Tween. A 1:2000 dilution of isotype specific goat anti-mouse horseradish peroxidase conjugate (Biorad) in PBS supplemented with 2% (w/v) milk powder was added to each well and the plate incubated at 37 °C for 1 h. Following six washes in PBS-Tween, 100 μL of tetramethylbenzidine (KPL) substrate was added to each well according to the manufacturer's instructions, and incubated at room temperature for 20 min prior to measuring the absorbance at 620 nm.
Acknowledgements
This research was funded by the US Defence Threat Reduction Agency (DTRA), grant CBBAA12-VAXBT2-1-0032 and the United Kingdom Ministry of Defence. Studies at the JIC were supported by the UK BBSRC Institute Strategic Programme on Understanding and Exploiting Metabolism (MET) [BB/J004561/1] and the John Innes Foundation. MID was supported by a BBSRC Industrial CASE award with Mologic Ltd.
We would like to thank Associate Professor Paul Brett at the University of Nevada, Reno School of Medicine for providing purified CPS extracted from B. pseudomallei 1026b for use as a reference in NMR and for providing the method for CPS extraction.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.carres.2017.09.011.
Contributor Information
Marc Bayliss, Email: mbayliss@dstl.gov.uk.
Robert A. Field, Email: rob.field@jic.ac.uk.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Currie B.J., Kaestli M. Epidemiology: a global picture of melioidosis. Nature. 2016;529(7586):290–291. doi: 10.1038/529290a. [DOI] [PubMed] [Google Scholar]
- 2.Limmathurotsakul D., Golding N., Dance D.A., Messina J.P., Pigott D.M., Moyes C.L., Rolim D.B., Bertherat E., Day N.P., Peacock S.J., Hay S.I. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 3.Reckseidler S.L., DeShazer D., Sokol P.A., Woods D.E. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 2001;69(1):34–44. doi: 10.1128/IAI.69.1.34-44.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choh L.C., Ong G.H., Vellasamy K.M., Kalaiselvam K., Kang W.T., Al-Maleki A.R., Mariappan V., Vadivelu J. Burkholderia vaccines: are we moving forward? Front. Cell. Infect. Microbiol. 2013;3:5. doi: 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng A.C., Currie B.J. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005;18(2):383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Zandt K.E., Greer M.T., Gelhaus H.C. Glanders: an overview of infection in humans. Orphanet J. rare Dis. 2013;8:131. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waag D.M. Efficacy of postexposure therapy against glanders in mice. Antimicrob. agents Chemother. 2015;59(4):2236–2241. doi: 10.1128/AAC.04801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Services DoHaH Possession, use, and transfer of select agents and toxins; biennial review. Final rule. Fed. Regist. 2012;77(194):61083–61115. [PubMed] [Google Scholar]
- 9.Heiss C., Burtnick M.N., Wang Z., Azadi P., Brett P.J. Structural analysis of capsular polysaccharides expressed by Burkholderia mallei and Burkholderia pseudomallei. Carbohydr. Res. 2012;349:90–94. doi: 10.1016/j.carres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Tuanyok A., Stone J.K., Mayo M., Kaestli M., Gruendike J., Georgia S., Warrington S., Mullins T., Allender C.J., Wagner D.M. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS neglected Trop. Dis. 2012;6(1):e1453. doi: 10.1371/journal.pntd.0001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parthasarathy N., DeShazer D., England M., Waag D.M. Polysaccharide microarray technology for the detection of Burkholderia pseudomallei and Burkholderia mallei antibodies. Diagnostic Microbiol. Infect. Dis. 2006;56(3):329–332. doi: 10.1016/j.diagmicrobio.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeShazer D., Waag D.M., Fritz D.L., Woods D.E. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb. Pathog. 2001;30(5):253–269. doi: 10.1006/mpat.2000.0430. [DOI] [PubMed] [Google Scholar]
- 13.Jones S.M., Ellis J.F., Russell P., Griffin K.F., Oyston P.C. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 2002;51(12):1055–1062. doi: 10.1099/0022-1317-51-12-1055. [DOI] [PubMed] [Google Scholar]
- 14.Nelson M., Prior J.L., Lever M.S., Jones H.E., Atkins T.P., Titball R.W. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J. Med. Microbiol. 2004;53(Pt 12):1177–1182. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S., Feng S.H., Li B., Kim H.Y., Rodriguez J., Tsai S., Lo S.C. In Vitro and in Vivo studies of monoclonal antibodies with prominent bactericidal activity against Burkholderia pseudomallei and Burkholderia mallei. Clin. vaccine Immunol. CVI. 2011;18(5):825–834. doi: 10.1128/CVI.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtnick M.N., Heiss C., Roberts R.A., Schweizer H.P., Azadi P., Brett P.J. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front. Cell. Infect. Microbiol. 2012;2:108. doi: 10.3389/fcimb.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott A.E., Burtnick M.N., Stokes M.G., Whelan A.O., Williamson E.D., Atkins T.P., Prior J.L., Brett P.J. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect. Immun. 2014;82(8):3206–3213. doi: 10.1128/IAI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brett P.J., DeShazer D., Woods D.E. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 1998;48(Pt 1):317–320. doi: 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 19.Benanti E.L., Nguyen C.M., Welch M.D. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell. 2015;161(2):348–360. doi: 10.1016/j.cell.2015.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haraga A., West T.E., Brittnacher M.J., Skerrett S.J., Miller S.I. Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect. Immun. 2008;76(11):5402–5411. doi: 10.1128/IAI.00626-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brett P.J., Burtnick M.N., Heiss C., Azadi P., DeShazer D., Woods D.E., Gherardini F.C. Burkholderia thailandensis oacA mutants facilitate the expression of Burkholderia mallei-like O polysaccharides. Infect. Immun. 2011;79(2):961–969. doi: 10.1128/IAI.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French C.T., Toesca I.J., Wu T.H., Teslaa T., Beaty S.M., Wong W., Liu M., Schroder I., Chiou P.Y., Teitell M.A. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc. Natl. Acad. Sci. U. S. A. 2011;108(29):12095–12100. doi: 10.1073/pnas.1107183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim B.M., Chantratita N., Ooi W.F., Nandi T., Tewhey R., Wuthiekanun V., Thaipadungpanit J., Tumapa S., Ariyaratne P., Sung W.K. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 2010;11(8):R89. doi: 10.1186/gb-2010-11-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott A.E., Christ W.J., George A.J., Stokes M.G., Lohman G.J., Guo Y., Jones M., Titball R.W., Atkins T.P., Campbell A.S. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjugate Chem. 2016;27(6):1435–1446. doi: 10.1021/acs.bioconjchem.5b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamigney Kenfack M., Bleriot Y., Gauthier C. Intramolecular aglycon delivery enables the synthesis of 6-deoxy-beta-D-manno-heptosides as fragments of Burkholderia pseudomallei and Burkholderia mallei capsular polysaccharide. J. Org. Chem. 2014;79(10):4615–4634. doi: 10.1021/jo500640n. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti R., Dillon M.J., Burtnick M.N., Hubbard M.A., Kenfack M.T., Bleriot Y., Gauthier C., Brett P.J., AuCoin D.P., Lanzetta R. Burkholderia pseudomallei capsular polysaccharide recognition by a monoclonal antibody reveals key details toward a biodefense vaccine and diagnostics against melioidosis. ACS Chem. Biol. 2015;10(10):2295–2302. doi: 10.1021/acschembio.5b00502. [DOI] [PubMed] [Google Scholar]
- 27.Szu S., Li X., Stone A., Robbins J. Relation between structure and immunologic properties of the vi capsular polysaccharide. Infect. Immun. 1991;59(12):4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry D.S., Lynn F., Lee C.H., Frasch C.E., Bash M.C. Effect of O Acetylation of Neisseria meningitidis serogroup a capsular polysaccharide on development of functional immune responses. Infect. Immun. 2002;70(7):3707–3713. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusco P.C., Farley E.K., Huang C.H., Moore S., Michon F. Protective meningococcal capsular polysaccharide epitopes and the Role of O Acetylation. Clin. Vaccine Immunol. 2007;14(5):577–584. doi: 10.1128/CVI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauthier C., Chassagne P., Theillet F.X., Guerreiro C., Thouron F., Nato F., Delepierre M., Sansonetti P.J., Phalipon A., Mulard L.A. Non-stoichiometric O-acetylation of Shigella flexneri 2a O-specific polysaccharide: synthesis and antigenicity. Org. Biomol. Chem. 2014;12(24):4218–4232. doi: 10.1039/c3ob42586j. [DOI] [PubMed] [Google Scholar]
- 31.Boutet J., Blasco P., Guerreiro C., Thouron F., Dartevelle S., Nato F., Canada F.J., Arda A., Phalipon A., Jimenez-Barbero J. Detailed investigation of the immunodominant role of O-Antigen stoichiometric O-Acetylation as revealed by chemical synthesis, immunochemistry, solution conformation and STD-NMR spectroscopy for Shigella flexneri 3a. Chem. (Weinheim der Bergstrasse, Ger.) 2016;22(31):10892–10911. doi: 10.1002/chem.201600567. [DOI] [PubMed] [Google Scholar]
- 32.Aspinall O.G., McDonald A.G., Sood K.R. Syntheses of methyl glycosides of 6-deoxyheptoses. Can. J. Chem. 1994;72:5. [Google Scholar]
- 33.Hecht M.L., Stallforth P., Silva D.V., Adibekian A., Seeberger P.H. Recent advances in carbohydrate-based vaccines. Curr. Opin. Chem. Biol. 2009;13(3):354–359. doi: 10.1016/j.cbpa.2009.05.127. [DOI] [PubMed] [Google Scholar]
- 34.Lai Z., Schreiber J.R. Antigen processing of glycoconjugate vaccines; the polysaccharide portion of the pneumococcal CRM(197) conjugate vaccine co-localizes with MHC II on the antigen processing cell surface. Vaccine. 2009;27(24):3137–3144. doi: 10.1016/j.vaccine.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 36.Scott A.E., Laws T.R., D'Elia R.V., Stokes M.G.M., Nandi T., Williamson E.D., Tan P., Prior J.L., Atkins T.P. Protection against experimental melioidosis following immunisation with live Burkholderia thailandensis expressing manno-heptose capsule. Clin. Vaccine Immunol. 2013;20(7):1041–1047. doi: 10.1128/CVI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Champion O.L., Gourlay L.J., Scott A.E., Lassaux P., Conejero L., Perletti L., Hemsley C., Prior J., Bancroft G., Bolognesi M. Immunisation with proteins expressed during chronic murine melioidosis provides enhanced protection against disease. Vaccine. 2016;34(14):1665–1671. doi: 10.1016/j.vaccine.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Perry M.B., MacLean L.L., Schollaardt T., Bryan L.E., Ho M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 1995;63(9):3348–3352. doi: 10.1128/iai.63.9.3348-3352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S., Lee Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Scott A.E., Ngugi S.A., Laws T.R., Corser D., Lonsdale C.L., D'Elia R.V., Titball R.W., Williamson E.D., Atkins T.P., Prior J.L. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J. Immunol. Res. 2014;2014:392170. doi: 10.1155/2014/392170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha K., Box M., Lalli G., Schiavo G., Schneider H., Groves M., Siligardi G., Fairweather N. Analysis of mutants of tetanus toxin Hc fragment: ganglioside binding, cell binding and retrograde axonal transport properties. Mol. Microbiol. 2000;37(5):1041–1051. doi: 10.1046/j.1365-2958.2000.02091.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.