Abstract
Background
Vaginal discharge and vulvitis are common presenting symptoms in general practice. Few studies have specifically looked at the validity of self-taken low vulvovaginal swabs (LVS) for the diagnosis of vulvovaginal candidiasis (VVC) and bacterial vaginosis (BV).
Aim
To assess if patient self-taken LVS are a valid alternative to clinician-taken high vaginal swabs (HVS) for the detection of VVC and BV.
Design and setting
Case-control study with the patient acting as their own control in an urban sexual health centre in Newcastle upon Tyne, UK.
Method
Females aged 16–65 years attending with symptomatic vaginal discharge, vulval irritation, genital pain, and an offensive genital smell were recruited into the study. Participants took a self-taken LVS before vaginal examination, during which a clinician took an HVS (reference standard). Main outcome measures were the diagnosis of BV or VVC infection.
Results
A total of 104 females were enrolled. Of those, 45 were diagnosed with VVC and 26 with BV. The sensitivities of self-taken LVS for VVC and BV were 95.5% and 88.5% respectively. Cohen’s κ coefficient showed ‘strong agreement’ for the detection of both VVC and BV. Vulval itching was the most common symptom associated with VVC (69%), whereas 50% of females diagnosed with BV presented with an offensive discharge. Both symptoms had poor positive predictive values (0.63 and 0.50, respectively).
Conclusion
Self-taken LVS appears to be a valid alternative to clinician-taken HVS for detecting VVC and BV infections. Symptoms were found to be a poor indicator of underlying infection.
Keywords: bacterial vaginosis, general practice, vaginal examinations, vulvovaginal candidiasis
INTRODUCTION
For a female presenting for the first time with a change in vaginal discharge, current guidelines for management in general practice do not generally advocate high vaginal swab (HVS) as a diagnostic tool.1 However, a number of clinical scenarios do require microbiological confirmation for the diagnosis of abnormal discharge.2 Bacterial vaginosis (BV) is the commonest cause of infective vaginal discharge in females of reproductive age.3 Vulvovaginal candidiasis (VVC) is the second most common and particularly affects females aged 20 to 30 years.3,4 Symptomatic vulvovaginal discharge and vulval irritation are frequent and often distressing presenting symptoms in females attending both general practice surgeries5 and sexual health services.4,6 Classical symptoms of WC are vulval itching associated with a thick, white, curdy discharge whereas BV typically presents as a non-irritant, thin, grey, offensive discharge.4,6 However, vaginal symptoms and signs are not a reliable indicator of underlying aetiology. BV may cause vulval irritation7 whereas VVC may present solely with a change in discharge.8 Even females with previously confirmed episodes of VVC are poor at self-diagnosis9 and as few as 16% of females with recurrent symptoms typical of candida have VVC confirmed on culture.10 Other infective causes of a discharge should always be considered and screening is offered for chlamydia, gonorrhoea, and trichomonas, particularly in females <25 years of age.2 Non-infective causes of vulval irritation/itching are common (up to half the females in one study presenting with symptoms suggestive of VVC were shown to have another condition).4 These include atopy, eczema, lichen sclerosis, and vulval carcinoma. In order to make a definitive diagnosis, clinicians should ideally perform a genital examination that includes the insertion of a speculum and the collection of bacteriological samples for microscopy, culture, and sensitivity.
In general practice, HVS has a place in the first-line management of a number of specific clinical scenarios. Box 1 shows instances where HVS is recommended for the detection of vaginal flora in females of reproductive age with a vaginal discharge.2 In the management of an uncomplicated first presentation of abnormal vaginal discharge it is however of debatable use, particularly in the diagnosis of BV.2,3 The flora typical of BV can be found in up to 40% of asymptomatic females in the UK6 whereas Candida albicans is an asymptomatic commensal in 10–20% of females.4
Box 1. First-line management indications for taking a high vaginal swab in primary care.
Previous treatment failure.
Recurrent (≥4 episodes/year).
Pre- or post-gynaecological surgery.
Pre- or post-termination of pregnancy.
Postnatal or post-termination.
Symptoms not characteristic of bacterial vaginosis or vulvovaginal candidiasis.
Vaginitis without discharge.
In primary care, various constraints such as time pressure and lack of a chaperone, combined with a patient’s reluctance to be examined, can conspire to make a genital inspection with speculum examination difficult if not impossible. Clinicians may therefore opt to treat vaginal discharge and vulval irritation syndromically without microbiological evidence of infection.5 In cases where vulval itching is not in fact due to candidiasis but is triggered by other pathology such as atopy, atrophic vaginitis, or lichen sclerosis, females may experience symptomatic relief from the moisturising action of antifungal creams, particularly if combined with the anti-inflammatory action of hydrocortisone, further muddying the waters with regard to diagnosis.
How this fits in
In general practice a number of constraints such as time pressure and lack of a chaperone may limit the suitability of examination of a female presenting with vaginal discharge. This study aimed to determine the validity of a self-taken vaginal swab. The findings confirm that a self-taken low vulvovaginal swab (LVS) is a valid alternative to a clinician-taken high vaginal swab (HVS) in assisting with the diagnosis of bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC).
If an HVS is required there is a general consensus in current guidelines that a blind swab is acceptable.1,2 Two large, well-conducted studies in Leeds in 2012 showed that a self-taken low vaginal swab (LVS) is in fact superior to a clinician-taken endocervical swab for the detection of chlamydia and gonorrhoea,11,12 and current guidelines have changed to reflect this with regard to sexually transmitted infection (STI) screening.13 There is a reasonable body of research to support the use of a self-taken LVS for detection of abnormal vaginal bacteria but very little on the validity of this method for the detection of candida.14,15 The current study was therefore designed to determine if a patient self-taken LVS is as reliable as clinician-taken HVS in the diagnosis of both VVC and BV.
METHOD
Study population
From May to August 2015, females between 16 and 65 years of age who presented to the New Croft Centre for Sexual Health in Newcastle upon Tyne, UK, with symptoms of vaginal discharge, genital irritation, or offensive genital smell were recruited into the study, after providing informed consent. Females already diagnosed with VVC or BV, and those with established immunodeficiency, were excluded from the trial. No patient was entered more than once. The study was approved by the NHS Research Ethics Committee (REC).
Data collection
Those enrolled in the trial were seen by either a doctor or a nurse trained in genitourinary medicine (GUM) and were given both verbal and written instructions on how to perform an LVS. They were advised to insert the cotton end of the swab stick 6 cm into the vagina, rotate it for 10 seconds, and then place the swab into Amies transport medium. The females then underwent a speculum examination and an HVS was collected from the posterior fornix by the examining clinician. This was also placed into different Amies transport medium. Symptom data were collated by summarising the presenting complaints into four categories:
vulval irritation/itching;
offensive discharge;
genital pain with abnormal discharge; and
any other changes to the female’s normal discharge.
Laboratory assessment
Both self-taken and physician-collected swabs were sent to the microbiology laboratory for microscopy and culture for candida species and organisms causing BV. For the diagnosis of candida, the HVS specimen was cultured on Sabouraud culture medium incubated in air at 35–38ºC for 48 hours and any growing colonies analysed for candida.16 The diagnosis of BV was made by Gram staining the swab specimens and then using the Hay–Ison scoring methodology.17 In addition to swabs being sent for laboratory diagnosis, all patients had in-house wet-mount phase microscopy for trichomoniasis and gram staining of specimens looking for evidence of candida plus Hay–Ison scoring for BV. Patients with candidiasis were treated with a single dose of oral fluconazole 150 mg; those with BV were given oral metronidazole 400 mg twice daily for 7 days.
Data analysis
Data were analysed using VassarStats online statistical computation version 2017. Descriptive analyses were conducted for all relevant variables and outcomes, using appropriate measures of location (mean or median) and dispersion (standard deviation or range) for continuous variables. Categorical variables were summarised using absolute frequencies and proportions. The patient self-taken swab diagnostic test performance was assessed using the sensitivity, specificity, and positive and negative predictive values. The Cohen’s kappa (κ) statistic18 was used to investigate the level of agreement between the two test methods.
The interpretation of Cohen’s κ suggested by Cohen was followed: values ≤0 as indicating no agreement, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement. When applicable, 95% confidence intervals (CIs) were reported.
RESULTS
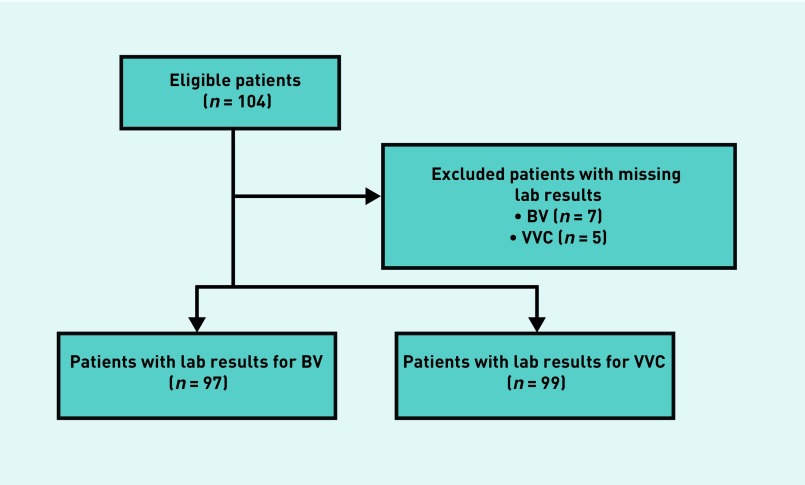
Figure 1 summarises the enrolment figures of the patients included in the study, while the resulting outcomes of both diagnostic tests for VVC and BV are summarised in Table 1.
Figure 1.
CONSORT chart outlining the study plan and enrolment figures.
BV = bacterial vaginosis. VVC = vulvovaginal candidiasis.
Table 1.
Summary of the outcome of self-taken LVS and clinician-taken HVS
| Clinician-taken HVS not detected, n | Clinician-taken HVS detected, n | Total, n | ||
|---|---|---|---|---|
| VVC | Self-taken LVS not detected, n | 50 | 2 | 52 |
| Self-taken LVS detected, n | 4 | 43 | 47 | |
| Total | 54 | 45 | 99 | |
|
| ||||
| BV | Self-taken LVS not detected, n | 68 | 3 | 71 |
| Self-taken LVS detected, n | 3 | 23 | 26 | |
| Total | 71 | 26 | 97 | |
BV = bacterial vaginosis. HVS = high vaginal swab. LVS = low vulvovaginal swab. VVC = vulvovaginal candidiasis.
The median age of the participants was 26 years old (range 17–49). Out of the 104 females that were enrolled during the study period, 97 had complete laboratory data for BV and 99 for VVC. (Data were incomplete for seven patients due to loss of one or both swabs in transit between the authors’ community-based site and the main hospital laboratory.)
Using the clinician HVS as the reference standard, the prevalence of VVC was 45.5% (n = 45) whereas the prevalence of BV was 26.8% (n = 26). Five females had both VVC and BV and 31 females had neither BV nor candida. In addition, eight patients (8.7%) were diagnosed with chlamydia, two (2.2%) with chlamydia and gonorrhoea, and two (2.2%) with herpes.
Performance of patient self-taken LVS
For VVC, four patients had a false positive result and two were false negatives, whereas, for BV, three resulted in false positives and three in false negatives (Table 1). Using the clinician-taken HVS as the reference standard, the sensitivities of self-taken vulvovaginal swabs for BV and VVC were 88.5% (95% CI = 68.7 to 97.0) and 95.5% (95% CI = 83.6 to 99.2) respectively, as reported in Table 2. Specificity of self-taken swab for BV and VVC was 95.8% (CI = 87.3 to 99.0) and 92.6% (CI = 81.3 to 97.6) respectively, giving a PPV of 88.5% for BV and 91.5% for VVC.
Table 2.
Performance measures for the patient self-taken LVS (clinician-taken HVS as the reference standard)
| Infection | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % | NPV % | Cohen’s kappa κ (95% CI) |
|---|---|---|---|---|---|
| BV | 88.5 (68.7 to 97.0) | 95.8 (87.3 to 99.0) | 88.5 | 95.8 | 0.84 (0.72 to 0.96) |
| VVC | 95.5 (83.6 to 99.2) | 92.6 (81.3 to 97.6) | 91.5 | 96.2 | 0.88 (0.78 to 0.97) |
BV = bacterial vaginosis. CI = confidence interval. HVS = high vaginal swab. LVS = low vulvovaginal swab. NPV = negative predictive value. PPV = positive predictive value. VVC = vulvovaginal candidiasis.
With regard to assessing the level of agreement of the two diagnostic tests for BV, the number of observed agreements between clinician-taken HVS and patient-taken LVS were 91 (93.81% of the observations) and the number of agreements expected by chance were 58.9 (60.76% of the observations). Therefore κ = 0.84 for BV that indicates ‘almost perfect agreement’.
For VVC, the number of observed agreements were 93 (93.9%) whereas the number of agreements expected by chance were 49.7 (50.2%), which resulted in κ = 0.88, which again indicates ‘almost perfect agreement’.
Symptom data
Data relating to the symptoms presented by the patients are summarised in Table 3. The commonest presenting symptom was offensive discharge (n = 38; 39%) followed by vulvovaginal itching (n = 35; 36%). A change in normal discharge was present in 21% (n = 21) of patients and 3% (n = 3) presented with genital pain.
Table 3.
Symptoms presented by the patients when enrolled in the study
| BV | VVC | BV and VVC | Negative | Total | |
|---|---|---|---|---|---|
| Vulval irritation/itching, n (%) | 3 (9%) | 22 (63%) | 2 (6%) | 8 (20%) | 35 (36%) |
| Offensive discharge, n (%) | 17 (45%) | 6 (16%) | 2 (5%) | 13 (34%) | 38 (39%) |
| Genital pain (with abnormal discharge), n (%) | 0 (0%) | 2 (67%) | 0 (0%) | 1 (33%) | 3 (3%) |
| Any other changes to normal discharge, n (%) | 1 (4%) | 10 (48%) | 1 (4%) | 9 (44%) | 21 (21%) |
| Total, n | 21 | 40 | 5 | 31 | 97 |
BV = bacterial vaginosis. VVC = vulvovaginal candidiasis.
Looking at the relation between the symptoms and laboratory diagnosis, using the 97 patients for whom the authors had complete laboratory data for both VVC and BV, of the 35 females who presented with vulval itching/irritation, 69% were diagnosed with VVC. Of the 38 females who presented with an offensive discharge, 50% were diagnosed with BV.
With regard to the accuracy of symptoms in the syndromic management of symptomatic vaginal discharge, the sensitivity of vulval itching as an indicator of VVC was 0.533 (CI = 0.38 to 0.68), and specificity was 0.745 (CI = 0.61 to 0.85), giving a positive predictive value (PPV) of 0.631. The sensitivity of an offensive discharge as an indicator of BV was 0.73 (CI = 0.52 to 0.88); specificity was 0.733 (CI = 0.61 to 0.83), giving a PPV of 0.5.
DISCUSSION
Summary
An excellent level of agreement was found between self-taken and physician-collected vaginal swabs for the diagnosis of VVC and BV in the study population of females attending the clinic. The positive predictive value was 0.915 for VVC and 0.885 for BV, showing promising evidence supporting the use of a self-taken specimen for the diagnosis of VVC and BV.
Self-taken LVS are by no means a reliable substitute for a thorough genital examination but in a time-constrained service, combined with patient reluctance to be examined, they appear to have similar detection rates to HVS. This swab could be taken in conjunction with self-taken nucleic acid amplification tests (NAATs) for chlamydia, gonorrhoea, and trichomonas, thus allowing a number of infections to be investigated without the need for a genital examination. This is a particularly attractive screening method for adolescent females, up to 80% of whom prefer self-testing to a pelvic examination.19 The authors therefore conclude from this study that self-taken LVS appears to be a valid alternative to clinician-taken HVS for detecting VVC and BV infections.
Apart from the very strong agreement between the two swab techniques, an incidental finding of interest was the apparent invalidity of typical symptoms with regard to directing the diagnosis. Vulval irritation as an indicator of VVC showed a poor PPV of 0.63. Equally, offensive discharge appeared unreliable for the empirical diagnosis of BV, having a very poor PPV of 0.50. This supports other research which has shown that patient perception of their discharge is not a reliable indicator of likely pathology.9
Strengths and limitations
Although the sample size of the current study is relatively small, the 95% CI for Cohen’s κ indicates that the authors are confident that the level of agreement between the two testing methods is at least substantial (minimum κ = 0.72 for BV and minimum κ = 0.78 for VVC). The authors therefore surmise that a further extension of the study would show similar results.
A limitation of this study is that, although trichomoniasis was tested for using in-house wet-mount microscopy (the laboratory also used a wet-mount screening test), the authors did not use a NAAT, which is more reliable (wet-mount sensitivity 45–60% as opposed to NAAT sensitivity 98–99%).20 There is a low incidence of trichomoniasis in this service of <1% but it is possible that undiagnosed trichomoniasis may have impacted on the figures for symptom correlation with microbiological findings.
Another limitation of this study was that the laboratory staff who analysed the swabs were not blinded as to whether the swab was self-collected or physician-collected. However, the authors do not think this would have impacted significantly on the data. All swabs were cultured using the same media and analysed in a way that was unlikely to have been biased.
Comparison with existing literature
Self-taken LVS have been shown in numerous studies to be accurate for the detection of chlamydia, gonorrhoea, and trichomoniasis. Two notable studies conducted in Leeds General Infirmary showed that self-taken LVS are superior to clinician-taken endocervical swabs for NAAT detection of chlamydia and gonorrhoea.11,12 A number of studies have also shown that self-taken LVS are highly acceptable to patients21, 22 and are extremely cost-effective.23 There are however only a few studies comparing the accuracy of self-taken LVS to clinician-taken HVS for the detection of BV14,15 and a particular paucity of evidence supporting self-taken LVS for the diagnosis of VVC.
Implications for practice
Recommended guidelines for the initial management of abnormal vaginal discharge in primary care rely on a combination of detailed clinical history with an examination that includes the use of pH paper and not necessarily the collection of an HVS.21 There are a number of clinical scenarios when an HVS is recommended.2 High vaginal swabs should be part of the management plan in recurrent candidiasis, screening for group B streptococcal infections, post-partum and post-instrumentation infections, vaginitis without discharge, symptoms not characteristic of BV or VVC, previous treatment failure, and recurrent vaginal discharge (≥4 episodes per year). In these instances if vaginal examination for whatever reason is deferred, the current study suggests that self-taken LVS may be as useful in assisting the diagnosis as clinician-taken HVS.
In the light of the finding of this study, the authors would also suggest that, in first presentation of cases suggestive of VVC, a LVS, particularly if it were to be negative, would be helpful in directing the diagnosis.
With regards to trichomonas (TV), infection with this sexually transmitted protozoan disturbs normal vaginal flora and commonly causes symptoms suggestive of BV, thereby creating the potential for misdiagnosis.24 Though the treatment for TV is the same as for BV (400 mg metronidazole twice a day for 7 days), TV being an STI requires partner notification. Although this study’s regional rate for TV is low, it is significantly higher in other areas such as London and the West Midlands.25 Interpretation of the current study findings should therefore be made with consideration of local rates for TV. In females presenting with recurrent symptoms suggestive of BV, trichomonas should be excluded.
Acknowledgments
The authors acknowledge with gratitude the patients who consented to take part in this study.
Funding
None given.
Ethical approval
Ethical approval was obtained from the NHS Research Ethics Committee North East — Newcastle and North Tyneside 2 (15/NE/0022).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
This study received 100 free Amies transport medium swabs from MWE Medical Wire.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Lazaro N, RCGP Sex, Drugs, HIV and Viral Hepatitis Group. British Association for Sexual Health and HIV . Sexually transmitted infections in primary care. 2nd edn. London: RCGP; 2013. https://www.bashh.org/documents/Sexually%20Transmitted%20Infections%20in%20Primary%20Care%202013.pdf (accessed 16 Oct 2017) [Google Scholar]
- 2.Public Health England, British Infection Association . Management and laboratory diagnosis of abnormal vaginal discharge. Quick reference guide for primary care. PHE, BIA; 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/345793/Vaginal_Discharge_treatment_guidance.pdf (accessed 3 Oct 2017) [Google Scholar]
- 3.Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit The management of women of reproductive age attending non-genitourinary medicine settings complaining of vaginal discharge. J Fam Plann Reprod Health Care. 2006;32(1):33–41. doi: 10.1783/147118906775275172. [DOI] [PubMed] [Google Scholar]
- 4.Clinical Effectiveness Group (Association for Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases) National guideline on the management of vulvovaginal candidiasis. 2007 http://www.bashh.org/documents/50/50.pdf (accessed 4 Oct 2017) [PubMed] [Google Scholar]
- 5.Noble H, Estcourt C, Ison C, et al. How is the high vaginal swab used to investigate vaginal discharge in primary care and how do GPs’ expectations of the test match the tests performed by their microbiology services? Sex Transm Infect. 2004;80(3):204–206. doi: 10.1136/sti.2003.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Association for Sexual Health and HIV (BASHH) UK national guideline for the management of bacterial vaginosis 2012. https://www.bashh.org/documents/4413.pdf (accessed 16 Oct 2017)
- 7.Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for the diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304–1308. doi: 10.1128/JCM.43.3.1304-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291(11):1368–1379. doi: 10.1001/jama.291.11.1368. [DOI] [PubMed] [Google Scholar]
- 9.Ferris DG, Nyirjesy P, Sobel JD, et al. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Am Coll Obstet Gynecol. 2002;99(3):419–425. doi: 10.1016/s0029-7844(01)01759-8. [DOI] [PubMed] [Google Scholar]
- 10.Marrazzo J. Vulvovaginal candidiasis. BMJ. 2003;326(7397):993–994. doi: 10.1136/bmj.326.7397.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CMW, Schoeman SA, Booth RA, et al. Assessment of best single sample for finding chlamydia in women with and without symptoms: a diagnostic test study. BMJ. 2012;345:e8013. doi: 10.1136/bmj.e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoeman SA, Stewart CMW, Booth RA, et al. Assessment of self taken swabs versus clinician taken swab cultures for diagnosing gonorrhoea in women: single centre, diagnostic accuracy study. BMJ. 2012;345:e8107. doi: 10.1136/bmj.e8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BASHH Clinical Effectiveness Group . 2015 BASHH CEG guidance on tests for sexually transmitted infections. BASHH; 2015. https://www.bashhguidelines.org/media/1084/sti-testing-tables-2015-dec-update-4.pdf (accessed 16 Oct 2017) [Google Scholar]
- 14.Nelson DB, Bellamy S, Gray TS, Nachamkin I. Self-collected versus provider-collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. J Clin Epidemiol. 2003;56(9):862–866. doi: 10.1016/s0895-4356(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 15.Forney LJ, Gajer P, Williams CJ, et al. Comparison of self-taken and physician-collected vaginal swabs for microbiome analysis. J Clin Microbiol. 2010;48(5):1741–1748. doi: 10.1128/JCM.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandven P, Lassen J. Importance of selective media for recovery of yeasts from clinical specimens. J Clin Microbiol. 1999;37(11):3731–3732. doi: 10.1128/jcm.37.11.3731-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ison CA, Hay PE. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect. 2002;78(6):413–415. doi: 10.1136/sti.78.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 19.Holland-Hall CM, Wiesenfeld HC, Murray PJ. Self-collected vaginal swabs for the detection of multiple sexually transmitted infections in adolescent girls. J Paediatr Adolesc Gynecol. 2002;15(5):307–313. doi: 10.1016/s1083-3188(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 20.Sherrard J, Ison C, Moody J, et al. United Kingdom national guideline on the management of trichomonas vaginalis 2014. Int J STD AIDS. 2014;25(8):541–549. doi: 10.1177/0956462414525947. [DOI] [PubMed] [Google Scholar]
- 21.Brown L, Patel S, Ives NJ, et al. Is non-invasive testing for sexually transmitted infections an efficient and acceptable alternative for patients? A randomised controlled trial. Sex Transm Infect. 2010;86(7):525–531. doi: 10.1136/sti.2009.039479. [DOI] [PubMed] [Google Scholar]
- 22.Chernesky MA, Hook EW, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32(12):729–733. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 23.Blake DR, Maldeis N, Barnes MR, et al. Cost-effectiveness of screening strategies for Chlamydia trachomatis using cervical swabs, urine, and self-obtained vaginal swabs in a sexually transmitted disease clinic setting. Sex Transm Dis. 2008;35(7):649–655. doi: 10.1097/OLQ.0b013e31816ddb9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sujit DR, Krupp K, Klausner J, et al. Bacterial vaginosis and risk of trichomonas vaginalis infection: a longitudinal analysis. Sex Transm Dis. 2011;38(9):882–886. doi: 10.1097/OLQ.0b013e31821f91a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell HD, Lewis DA, Marsh K, Hughes G. Distribution and risk factors of Trichomonas vaginalis infection in England: an epidemiological study using electronic health records from sexually transmitted infection clinics, 2009–2011. Epidemiol Infect. 2014;142(8):1678–1687. doi: 10.1017/S0950268813002902. [DOI] [PMC free article] [PubMed] [Google Scholar]