Abstract
Background
Regional variations in the presentation of uncomplicated urinary tract infection (UTI) and pathogen sensitivity to antibiotics have been cited as reasons to justify differences in how the infections are managed, which includes the prescription of broad-spectrum antibiotics.
Aim
To describe presentation and management of UTI in primary care settings, and explore the association with patient recovery, taking microbiological findings and case mix into account.
Design and setting
Prospective observational study of females with symptoms of uncomplicated UTI presenting to primary care networks in England, Wales, the Netherlands, and Spain.
Method
Clinicians recorded history, symptom severity, management, and requested mid-stream urine culture. Participants recorded, in a diary, symptom severity each day for 14 days. Time to recovery was compared between patient characteristics and between countries using two-level Cox proportional hazards models, with patients nested within practices.
Results
In total, 797 females attending primary care networks in England (n = 246, 30.9% of cohort), Wales (n = 213, 26.7%), the Netherlands (n = 133, 16.7%), and Spain (n = 205, 25.7%) were included. In total, 259 (35.8%, 95% confidence interval 32.3 to 39.2) of 726 females for whom there was a result were urine culture positive for UTI. Pathogens and antibiotic sensitivities were similar. Empirical antibiotics were prescribed for 95.1% in England, 92.9% in Wales, 95.1% in Spain, and 59.4% in the Netherlands There were no meaningful differences at a country network level before and after controlling for severity, prior UTIs, and antibiotic prescribing.
Conclusion
Variation in presentation and management of uncomplicated UTI at a country primary care network level is clinically unwarranted and highlights a lack of consensus concerning optimal symptom control and antibiotic prescribing.
Keywords: antibiotic prescribing; antibiotic resistance; Gram-negative infections; practice patterns, physicians’; primary care; urinary tract infections
INTRODUCTION
Variation in the presentation and management of symptoms of uncomplicated urinary tract infection (UTI) has been identified,1–3 but it is not known whether such variation is warranted by differences in symptom presentation, prevalence of microbiologically confirmed UTI, or characteristics of infecting pathogens between settings, and whether such factors are associated with patient recovery. Variation in antibiotic prescribing that is not warranted on clinical grounds could waste resources, put patients at unnecessary risk of delayed recovery and adverse events, and unnecessarily drive forward antimicrobial resistance, particularly where broad-spectrum antibiotics are used. Antibiotic resistance is a growing international problem.4
The authors previously investigated variation in antibiotic prescribing for acute cough/lower respiratory tract infection (LRTI) in Europe, and found a four-fold variation between primary care networks in 14 countries that was not meaningfully associated with patient recovery, and huge variation in the choice of first-line antibiotics.5 This highlighted the need for standardising clinical care and promoting self-care.6 Although that analysis controlled for presentation and case mix, it was not able to take microbiological findings into account — this is important as clinicians may justify their antibiotic prescribing on the basis of assumed differences in patient characteristics as well as aetiology and presumed bacterial antibiotic susceptibility.7,8
Uncomplicated UTI is one of the most common bacterial infections managed in primary care. Nearly 40% of females report having had at least one UTI in their lifetime.9 More than 10% report at least one episode and some 3% report having had ≥3 episodes (recurrent UTI) in the past year.9 Most females in the UK consult a health professional when they have symptoms attributable to a UTI, and about three-quarters of these have some form of urine test and are prescribed an antibiotic for their symptoms.10 However, up to 70% of females with symptoms attributable to UTI are found not to have a UTI confirmed microbiologically when routine urine culture is performed — this is dependent on the thresholds and criteria used by laboratories, study design, and population.11–15
How this fits in
Regional differences in the presentation of uncomplicated urinary tract infection (UTI) and pathogen sensitivity to antibiotics in primary care have been used to justify variation in management. However, such differences have not been prospectively described, and the association with patient recovery, taking microbiological findings into account, is unknown. This study found little variation in patient presentation, or aetiology and sensitivity of urinary pathogens cultured in the urine of females with symptoms of uncomplicated UTI in four European primary care settings. However, the proportion of urine cultures meeting laboratory definitions of UTI, patients prescribed an antibiotic, antibiotic classes commonly prescribed, whether antibiotic choice was concordant with culture results, and subsequent consulting and prescribing did differ markedly. Despite these differences, patient-reported recovery measures did not vary at the country network level, before and after controlling for severity, prior urine infections, and antibiotic prescribing.
Antimicrobial stewardship interventions and clinical practice guidelines aimed at optimising standard routine care would, therefore, be enhanced by a better understanding of the variation in presentation and care (for example, patient characteristics, dipstick results and requesting urine culture, proportion and appropriateness of antibiotic prescribing, non-antibiotic prescribing, planned follow-up arrangement, subsequent antibiotic prescribing, and re-consultations), and the association with microbiological findings and recovery. This study, therefore, aimed to describe the variation in the presentation and management of the condition, as well as the association with outcomes for females presenting with symptoms of uncomplicated UTI to primary care research networks in four European settings.
METHOD
Setting and participants
This study was conducted in primary care general practices that were part of primary care networks in England, Wales, Spain, and the Netherlands between November 2012 and February 2014. These primary care research networks were selected on the basis of having well-established primary care research capability and reflected the countries in which the investigators were based.
Each primary care network aimed to recruit approximately 10 general practices based on their interest and capacity to deliver the study protocol. Each country network was set a target to recruit 200 eligible females. The primary care clinicians in the practices were asked to:
sequentially recruit adult females presenting with symptoms of uncomplicated UTI;
record patient demographics;
record their usual care diagnostic procedures and treatment; and
collect and send a urine sample for laboratory culture.
Eligible participants were females:
aged ≥16 years;
able to provide written informed consent;
presenting to primary care with at least one of three key urinary tract symptoms (dysuria, urgency including nocturia, and frequency); and
in whom the clinician suspected uncomplicated UTI (no known urological abnormalities, non-pregnant females).16
Exclusions were: terminal illness, receiving treatment for life-threatening cancer, severe systemic symptoms, on long-term antibiotic treatment or had received antibiotics for UTI within the past 4 weeks, bladder surgery (including cystoscopy) within the past 4 weeks, significant immune compromise (for example, long-term corticosteroid or chemotherapy, insulin-dependent diabetes), functional or anatomical abnormalities of the genitourinary tract, history of pyelonephritis, and pregnancy. Fever was not an exclusion criterion.
The sample size was based on achieving a 95% confidence interval (CI) of 45% to 55% around a prevalence of antibiotic prescribing estimate of 50%; 50% was chosen as this gave the most conservative estimate — higher or lower percentages would have produced narrower CIs. This required 385 participants but was inflated to a total of 800 to account for an estimated practice-level intra-cluster correlation coefficient (ICC) of 0.057. This value is in line with previous work.17,18 No additions were made to this sample size for potential dropout, as data on prescribing antibiotics were collected at the initial baseline visit immediately after recruitment.
Clinical examination
On a case report form, clinicians were asked to record details of the participant’s presenting clinical symptoms including fever, pain in the side, blood in urine, smelly urine, burning or pain when passing urine, urgency, daytime frequency, night-time frequency, tummy pain, restricted activities, and feeling generally unwell. Each feature was to be rated using a scale ranging from 0 (‘normal/not affected’) to 6 (‘as bad as it could be’). Doctors were also asked to record temperature, their antibiotic management for the suspected UTI, and any planned follow-up. The scale used was similar to the one used in the patient diary, and represented a slight modification of previously used instruments. The severity of three symptoms (daytime frequency, night-time frequency, and urgency) were summed to create a GP-rated symptom severity score ranging from 0 to 18 (more information about this is available from the authors on request).
Antibiotic prescribing
Antibiotic prescribing was assessed on:
index consultation (yes/no); and
whether prescriptions were ‘concordant’ (a UTI on laboratory culture and prescribed antibiotics matching pathogen sensitivity, or no UTI on culture with no antibiotic prescribed) or ‘not concordant’ (a UTI on laboratory culture and prescribed an antibiotic to which the pathogen was resistant, or a UTI on culture and no antibiotic prescribed, or no UTI on culture and an antibiotic prescribed).
Urine dipstick and culture
Participants were asked to provide a mid-stream urine sample at baseline, in addition to any urine samples the responsible clinician wished to obtain to guide usual care. Clinicians were asked to record:
whether they undertook urine dipstick testing and the results of dipstick tests performed; and
whether the urine was cloudy or had an offensive smell.
Urine samples (stored in a boric acid sample container for microbiological investigation) were then referred by usual post to a microbiology laboratory — samples for England and Wales were sent to Public Health Wales Specialist Antimicrobial Chemotherapy Unit (PHW SACU); in Spain, samples were tested by microbiological departments of the hospitals Ramón y Cajal (Madrid), Joan XXIII (Tarragona), and Bon Pastor (Barcelona); and University Medical Center (UMC) Utrecht tested samples for females in the Netherlands.
Isolated bacteria considered to be causing a UTI were frozen and subsequently sent to PHW SACU, where sensitivities to urinary tract antimicrobials were determined using agar dilution and the European Committee on Antimicrobial Susceptibility Testing breakpoints. Urine samples were considered positive for UTI if there was a pure or predominant (103 difference between the first and the second most abundant isolate on any subsequent pathogens) culture at ≥105 colony-forming unit (CFU)/mL of any organisms.19 A sensitivity analysis was conducted using a European definition that required a lower quantification threshold: ≥103 CFU/mL of any organism cultured.20
Participant follow-up
Participants were asked to complete a paper diary each day for 14 days, recording their symptoms (fever, pain in the side, blood in urine, smelly urine, burning or pain when passing urine, urgency, daytime frequency, night-time frequency, tummy pain, restricted activities, and feeling generally unwell) and rating them on a scale of 0 (‘no problem’) to 6 (‘as bad as it could be’). Any follow-up consultations for their UTI and medication use (including medication purchased over the counter) were also recorded in the diary. Participants were contacted by telephone by the research team if diaries were not returned 2 weeks after the due date.
All data collection forms were translated for use in Spain and the Netherlands, then translated back into English so the meaning and validity of translations could be checked.
Patient-reported recovery
Recovery was assessed in terms of:
time to full recovery — the first day that all 11 symptoms were scored 0 (normal/not a problem);
time to resolution of moderately bad symptoms — the first day that all 11 symptoms were scored 2 (slight problem or less); and
time to resolution of daytime frequency, night-time frequency, and urgency — the first day that all three symptoms were scored 0.
The last recovery outcome was derived following a factor analysis of all 11 symptoms. (More information on this can be obtained from the authors on request.)
Data analysis
Descriptive statistics by country and overall were calculated using means and standard deviations (SDs) inflated for clustering, medians (interquartile ranges [IQRs]), and proportions, as appropriate. The odds of having the following were compared between various patient characteristics and between countries using two-level logistic regression models, with patients nested within practices. The patient characteristics were:
a dipstick test performed;
a microbiologically confirmed UTI;
being prescribed antibiotics;
receiving an antibiotic prescription concordant with urine culture results;
having a urine sample that would have normally been sent for culture by a GP;
having a planned follow-up arrangement;
being prescribed subsequent antibiotics; and
re-consulting in the 2 weeks following the index consultation.
The practice-level ICC was estimated using the standard π2/3 estimator.21
Time to recovery was compared between various participant characteristics and between countries using two-level Cox proportional hazards models, with participants nested within practices.
Candidate variables related to case mix comprised:
age of participant at baseline;
temperature of participant at baseline;
clinician-rated symptom severity score;
number of days off work (0 or >1));
previous number of days with symptoms (0–7, 8–14, 15–21, ≥22);
level of leukocytes found in urine on dipstick testing (negative, +, ++, +++);
nitrites, protein, and blood were either negative or positive, and the PH level was between 5.0–7.0, and between 7.5–8.5 (results are given as whole numbers, for example, 7.0 going up at 0.5 increments);
cloudy urine;
offensive smelling urine;
diagnosed with a urine infection in the past;
number of treated urine infections in the past year (zero, one, two, three, or >3).
Candidate variables related to patient management comprised:
performed a dipstick test;
would have collected urine sample under normal circumstances;
prescribed an antibiotic; and
organised follow-up.
All candidate variables that were associated with the response variable at the 10% significance level (P-value <0.1) in a univariable model were entered into a multivariable model. Findings from the univariable analyses are available from the authors on request.
Each country was compared with the overall average in the regression models using a sum-to-zero contrast. However, each country was also compared with England (the country from where the highest number of participants were recruited) to ensure the findings were not strongly influenced by the choice of contrast, as indicated by Hardy.22
Data management was performed using IBM SPSS Statistics for Windows, version 20. All analyses were performed using R (version 3.0.1) and the lme4 package.
RESULTS
A total of 797 females were included, with the smallest proportion being recruited in the Netherlands: 246 females were recruited in England, 213 in Wales, 205 in Spain, and 133 in the Netherlands. Baseline data were returned for 793 participants (Table 1).
Table 1.
Participants’ characteristics at baseline
| Characteristic | Wales | England | Spain | The Netherlands | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| n | n | n | n | n | |||||||
| Median age in years (IQR) | 211 | 39 (27–54) | 245 | 50 (31– 63) | 205 | 45 (30–61) | 133 | 45 (34–62) | 794 | 45 (30–61) | |
|
| |||||||||||
| GP symptom severity score, mean (SD) | Urgency | 204 | 3.6 (1.9) | 239 | 3.7 (1.6) | 205 | 2.9 (1.6) | 133 | 3.2 (1.8) | 781 | 3.4 (1.7) |
| Daytime frequency | 203 | 3.8 (1.7) | 239 | 3.6 (1.4) | 205 | 3.1 (1.48) | 133 | 3.4 (1.58) | 780 | 3.5 (2.45) | |
| Night-time frequency | 202 | 3.0 (2.0) | 239 | 2.9 (1.8) | 205 | 2.1 (1.6) | 132 | 2.5 (1.9) | 778 | 2.7 (1.9) | |
| Mean scorea | 202 | 10.5 (4.6) | 239 | 10.1 (4.0) | 205 | 8.1 (3.7) | 132 | 9.1 (4.2) | 778 | 9.5 (4.2) | |
|
| |||||||||||
| Paid employment, % | Yes | 132 | 62.6 | 147 | 60.2 | 88 | 42.9 | 84 | 63.2 | 451 | 56.9 |
| No | 79 | 37.4 | 97 | 39.8 | 117 | 57.1 | 49 | 36.8 | 342 | 43.1 | |
|
| |||||||||||
| Of those who work, number of days taken off work due to this illness, % | 0 days | 114 | 90.5 | 107 | 78.7 | 72 | 84.7 | 77 | 96.3 | 370 | 86.7 |
| ≥1 days | 12 | 9.5 | 29 | 21.3 | 13 | 15.3 | 3 | 3.8 | 57 | 13.3 | |
|
| |||||||||||
| Median number of days with symptoms before consulting (IQR) | 210 | 3 (2–7) | 240 | 4 (2–6) | 204 | 2.5 (1–5) | 131 | 5 (3–10) | 785 | 3 (2–7) | |
|
| |||||||||||
| Mean temperature at baseline in °C (SD) | 204 | 36.6 (0.5) | 239 | 36.7 (0.5) | 205 | 36.2 (0.4) | 122 | 36.7 (0.5) | 770 | 36.5 (0.5) | |
|
| |||||||||||
| Managing their UTI with cranberry juice,b % | Yes | 61 | 46.6 | 81 | 45.8 | 2 | 1.3 | 40 | 38.1 | 184 | 32.5 |
| No | 70 | 53.4 | 96 | 54.2 | 153 | 98.7 | 65 | 61.9 | 384 | 67.5 | |
Summary score is based on results for the three listed items.
Based on participants who returned diaries. IQR = interquartile range. SD = standard deviation. UTI = urinary tract infection.
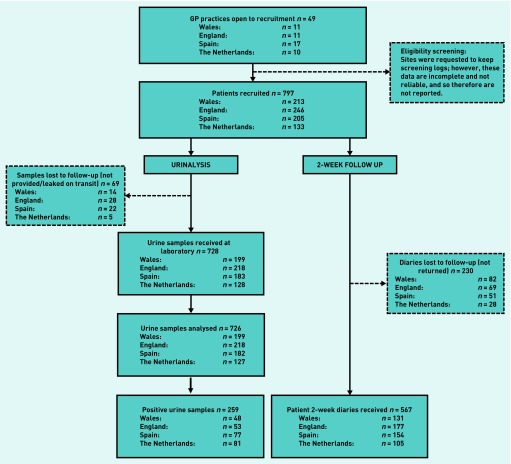
Urine samples that were analysed for the primary UTI identification were provided by 726 participants (91.1%). For the remainder, samples were either not provided (n = 39), leaked in transit to the laboratory (n = 24), or were unable to be processed by the laboratory (n = 6).
The 2-week follow-up diary was returned by 567 participants (71.1%) (Figure 1). Those who did not return their diaries were younger on average (median age 34 years, IQR 23–48 years, versus median age 50 years, IQR 35–64 years), but had similar GP-rated symptom severity scores at enrolment compared with those who did return their diaries. Diary return rates were lower in Wales and females recruited there tended to be younger than in the other networks.
Figure 1.
STROBE participant flowchart.
Presentation
Symptom severity at baseline, as rated by recruiting GPs, were lowest for participants in Spain (mean 8.1, SD 3.7), followed by the Netherlands (mean 9.1, SD 4.2), England (mean 10.1, SD 4.0), and Wales (mean 10.5, SD 4.6) (Table 1). Participants in the Netherlands were symptomatic for longer before consulting (median 5 days, IQR 3–10 days) versus responders from all four countries (median 3 days, IQR 2–7 days).
Median age ranged from 39 years (IQR 27–54 years) in Wales to 50 years (IQR 31–63 years) in England (Table 1). The proportion of participants in paid employment was similar in Wales, England, and the Netherlands, but slightly lower in Spain, while the proportion that had taken ≥1 days off work was highest in England and lowest in the Netherlands (Table 1).
Participants for whom outcome data were available were older but had similar symptom severity scores at inclusion compared with those lost to follow-up.
Before consulting, of the 567 participants who completed the follow-up diary, 184 (32.5% — ranging from 1.3% [2/155] in Spain to 46.6% [61/131] in Wales) — reported trying to manage their UTI with cranberry juice (Table 1).
Mean body temperature at baseline was normal in all networks.
Dipstick testing
A total of 669/791 (84.6%) participants had a dipstick test performed at baseline; the highest number of tests performed was in the Netherlands (127/133, 95.5%) and the lowest in Spain (141/205, 68.8%).
Microbiological confirmation of a UTI
Overall, 259/726 (35.7%, 95% CI = 32.3 to 39.2) participants were identified with a UTI according to the primary microbiological definition used in this study, with similar proportions in England (24.3%, 95% CI = 19.1 to 30.4) and Wales (24.1%, 95% CI = 18.7 to 30.5) but larger ones in Spain (42.3%, 95% CI = 35.4 to 49.6) and in the Netherlands (63.8%, 95% CI = 55.1 to 71.6) (Table 2). Enterobacteriaceae (most commonly Escherichia coli) were implicated in 88.8% (230/259) of UTIs and Coagulase-negative staphylococci in 5.8% (15/259) of UTIs (Table 2).
Table 2.
Prevalence of UTI and urinary pathogens
| Wales N= 199 | England N= 218 | Spain N= 182 | Netherlands N= 133 | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| n | % | n | % | n | % | n | % | n | % | ||
| No UTI confirmed | Mixed growth (≥2 organisms) | 103 | 51.8 | 118 | 54.1 | 9 | 4.9 | 37 | 29.1 | 267 | 36.8 |
| Single organism growth at <105 | 34 | 17.1 | 37 | 17.0 | 26 | 14.3 | 2 | 1.6 | 99 | 13.6 | |
| No growth | 14 | 7.0 | 10 | 4.6 | 34 | 18.7 | 7 | 5.5 | 65 | 9.0 | |
| Unclear organism names (mixed growth) | 0 | 0.0 | 0 | 0.0 | 36 | 19.8 | 0 | 0.0 | 36 | 5.0 | |
| Total | 151 | 75.9 | 165 | 75.7 | 105 | 57.7 | 46 | 36.2 | 467 | 64.3 | |
|
| |||||||||||
| UTI confirmed | Pure culture at ≥105 | 34 | 17.1 | 38 | 17.4 | 77 | 42.3 | 81 | 63.8 | 230 | 31.7 |
| Predominant culture at ≥105 | 14 | 7.0 | 15 | 6.9 | 0 | 0.0 | 0 | 0.0 | 29 | 4.0 | |
| Total | 48 | 24.1 | 53 | 24.3 | 77 | 42.3 | 81 | 63.8 | 259 | 35.7 | |
|
| |||||||||||
| Urinary pathogen identificationa | Enterobacteriaceae | 44 | 91.7 | 48 | 90.6 | 66 | 85.7 | 72 | 88.9 | 230 | 88.8 |
| Coagulase negative staphylococci (S. saprophyticus) | 2 | 4.2 | 1 | 1.9 | 9 | 11.7 | 3 | 3.7 | 15 | 5.8 | |
| Other pathogens | 2 | 4.2 | 4 | 7.5 | 2 | 2.6 | 6 | 7.4 | 14 | 5.4 | |
| Total | 48 | 100.0 | 53 | 100.0 | 77 | 100.0 | 81 | 100.0 | 259 | 100.0 | |
Based on those who have a microbiologically confirmed UTI (as per primary definition of UTI in this study). UTI = urinary tract infection.
Resistance to at least one of the tested antibiotics was recorded in 52.6% (110/209) of isolated strains (Table 3). Trimethoprim resistance was similar between countries (16.7% [8/48] in England to 22.7% [10/44] in Wales), but nitrofurantoin resistance was higher in England and the Netherlands; numbers, however, are small (Table 3).
Table 3.
Resistance profiles of identified urinary pathogensa
| Wales (N= 44) | England (N= 48) | Spain (N= 44) | The Netherlands (N= 73) | Total (N= 209) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Amoxicillin | 15 | 34.1 | 25 | 52.1 | 27 | 61.4 | 18 | 24.7 | 85 | 40.7 |
| Trimethoprim | 10 | 22.7 | 8 | 16.7 | 8 | 18.2 | 13 | 17.8 | 39 | 18.7 |
| Co-amoxiclav | 0 | 0.0 | 4 | 8.3 | 12 | 27.3 | 0 | 0.0 | 16 | 7.7 |
| Nitrofurantoin | 0 | 0.0 | 4 | 8.3 | 1 | 2.3 | 6 | 8.2 | 11 | 5.3 |
| Fosfomycin | 3 | 6.8 | 2 | 4.2 | 3 | 6.8 | 3 | 4.1 | 11 | 5.3 |
| Ciprofloxacin | 2 | 4.5 | 1 | 2.1 | 2 | 4.5 | 2 | 2.7 | 7 | 3.3 |
| Gentamicin | 1 | 2.3 | 2 | 4.2 | 1 | 2.3 | 1 | 1.4 | 5 | 2.4 |
| Cefalexin | 0 | 0.0 | 2 | 4.2 | 2 | 4.5 | 1 | 1.4 | 5 | 2.4 |
| Meticillin | 0 | 0.0 | 2 | 4.2 | 3 | 6.8 | 0 | 0.0 | 5 | 2.4 |
| Cefotaxime | 0 | 0.0 | 2 | 4.2 | 0 | 0.0 | 2 | 2.7 | 4 | 1.9 |
| Ceftazidime | 0 | 0.0 | 1 | 2.1 | 1 | 2.3 | 2 | 2.7 | 4 | 1.9 |
| Ertapenem | 0 | 0.0 | 1 | 2.1 | 2 | 4.5 | 0 | 0.0 | 3 | 1.4 |
| Temocillin | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sensitive to all tested antibiotics | 24 | 54.5 | 16 | 33.3 | 13 | 29.5 | 46 | 63.0 | 99 | 47.4 |
| Resistant to single antibiotic | 12 | 27.3 | 18 | 37.5 | 13 | 29.5 | 14 | 19.2 | 57 | 27.3 |
| Resistant to >1 antibiotic | 8 | 18.2 | 14 | 29.2 | 18 | 40.9 | 13 | 17.8 | 53 | 25.4 |
Based on those who have a microbiologically confirmed UTI. UTI = urinary tract infection.
Slightly more participants had a microbiologically confirmed UTI according to the European definition for a UTI, which requires a lower quantification threshold of 103 CFU/mL (285 participants, 39.3%) compared with the primary definition used in the study (urine samples were considered positive for UTI if pure or predominant (103 difference between the first and the second most abundant isolate on any subsequent pathogens) culture at ≥105 CFU/mL of any organisms). The prevalence of UTI in the Netherlands (65.4%, 83/127) remained highest when compared with other countries (England: 22.5%, 49/218; Wales: 26.6%, 53/199; Spain: 54.9%, 100/182) using this definition.
Antibiotic prescribing
A total of 232/244 participants in England (95.1%), 196/211 in Wales (92.9%), 195/205 in Spain (95.1%), and 79/133 in the Netherlands (59.4%) were prescribed empirical antibiotics (Table 4).
Table 4.
Antibiotic prescriptions at initial consultation
| Wales N= 211 | England N= 244 | Spain N= 205 | The Netherlands N= 133 | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| n | % | n | % | n | % | n | % | n | % | |
| No prescribed antibiotics | 15 | 7.1 | 12 | 4.9 | 10 | 4.9 | 54 | 40.6 | 91 | 11.5 |
|
| ||||||||||
| Prescribed antibiotics | 196 | 92.9 | 232 | 95.1 | 195 | 95.1 | 79 | 59.4 | 702 | 88.5 |
|
| ||||||||||
| Fosfomycin | 0 | 0.0 | 0 | 0.0 | 148 | 75.9 | 5 | 6.3 | 153 | 21.8 |
|
| ||||||||||
| Trimethoprim | 150 | 76.5 | 107 | 46.1 | 0 | 0.0 | 9 | 11.4 | 266 | 37.9 |
|
| ||||||||||
| Nitrofurantoin | 34 | 17.3 | 113 | 48.7 | 6 | 3.1 | 63 | 79.7 | 216 | 30.8 |
|
| ||||||||||
| Co-amoxiclav | 2 | 1.0 | 1 | 0.4 | 19 | 9.7 | 1 | 1.3 | 23 | 3.3 |
|
| ||||||||||
| Cephalosporins | 3 | 1.5 | 5 | 2.2 | 2 | 1.0 | 0 | 0.0 | 10 | 1.4 |
|
| ||||||||||
| Ciprofloxacin | 2 | 1.0 | 0 | 0.0 | 18 | 9.2 | 1 | 1.3 | 21 | 3.0 |
|
| ||||||||||
| Other antibiotica | 5 | 2.6 | 6 | 2.6 | 2 | 1.0 | 0 | 0.0 | 13 | 1.9 |
|
| ||||||||||
| Receiving antibiotic prescription | ||||||||||
| OR (95% CI) P-valueb,c | 0.70 (0.34 to 1.46) 0.346 | 2.50 (1.11 to 5.62) 0.027 | 3.22 (1.32 to 7.86) 0.010 | 0.18 (0.08 to 0.39) <0.001 | 1.00 | |||||
|
| ||||||||||
| Concordant antibiotic prescriptions | ||||||||||
| UTI and antibiotic and sensitive | 33 | 17.1 | 40 | 19.0 | 38 | 25.7 | 51 | 41.5 | 162 | 24.0 |
| No UTI and no antibiotic | 13 | 6.7 | 12 | 5.7 | 7 | 4.7 | 31 | 25.2 | 63 | 9.3 |
|
| ||||||||||
| Total | 46 | 23.8 | 52 | 24.6 | 45 | 30.4 | 82 | 66.7 | 225 | 33.3 |
|
| ||||||||||
| Non-concordant antibiotic prescriptions | ||||||||||
| UTI and antibiotic and resistance | 10 | 5.2 | 8 | 3.8 | 4 | 2.7 | 6 | 4.9 | 28 | 4.1 |
| UTI and no antibiotic | 1 | 0.5 | 0 | 0.0 | 1 | 0.7 | 20 | 16.3 | 22 | 3.3 |
| No UTI and antibiotic | 136 | 70.5 | 151 | 71.6 | 98 | 66.2 | 15 | 12.2 | 400 | 59.3 |
|
| ||||||||||
| Total | 147 | 76.2 | 159 | 75.4 | 103 | 69.6 | 41 | 33.3 | 450 | 66.7 |
|
| ||||||||||
| Overall | 193 | 100.0 | 211 | 100.0 | 148 | 100.0 | 123 | 100.0 | 675 | 100.0 |
|
| ||||||||||
| Receiving concordant antibiotic prescription | ||||||||||
| OR (95% CI) P-valueb,d | 0.57 (0.43 to 0.77) <0.001 | 0.60 (0.45 to 0.79) <0.001 | 0.80 (0.59 to 1.08) 0.144 | 3.66 (2.67 to 5.02) <0.001 | 1.00 | |||||
Other antibiotic includes: amoxicillin, metronidazole, pipemidic acid, and doxycycline.
Two-level model, with centre as the second level and participants as the first level.
Compared with the overall average. Adjustment made for participant characteristics including age, clinician-rated symptom severity score, previous number of days with symptoms, positive protein test, and positive blood test. Model based on 455 participants (57.1%) nested within 47 practices, practice-level ICC = 0.140.
Adjusted for country. ICC = intra-cluster correlation coefficient. OR = odds ratio. UTI = urinary tract infection.
After adjusting for participant characteristics, the odds of being prescribed an antibiotic were 150% higher for participants in England (odds ratio [OR] 2.50, 95% CI = 1.11 to 5.62, P = 0.027) and 222% higher for participants in Spain (OR 3.22, 95% CI = 1.32 to 7.86, P = 0.010) compared with the overall average. The odds of being prescribed an antibiotic in the Netherlands were 82% lower (OR 0.18, 95% CI = 0.08 to 0.39, P<0.001) compared with the overall average. Changing the comparator from the overall average to England, it was found that participants in Wales and the Netherlands had lower odds of receiving an antibiotic prescription (multivariable OR for Wales 0.28, 95% CI = 0.08 to 0.97; the Netherlands 0.07, 95% CI = 0.02 to 0.27; and Spain 1.29, 95% CI = 0.32 to 5.19) (data not shown). England was chosen as the reference category as it is the country where most participants were recruited.
The odds of being prescribed an antibiotic were also higher for those participants with a positive dipstick test for blood in urine (OR 2.95, 95% CI = 1.42 to 6.14, P = 0.004) or a higher clinician-rated symptom severity score (for one-unit increase OR 1.20, 95% CI = 1.10 to 1.31, P<0.001) (data not shown).
Trimethoprim was the most commonly prescribed antibiotic in Wales (76.5%, 150/196), fosfomycin in Spain (75.9%, 148/195), nitrofurantoin in the Netherlands (79.7%, 63/79), and trimethoprim and nitrofurantoin in England (46.1%, 107/232 and 48.7%, 113/232 respectively) (Table 4). Spain had the highest proportion of co-amoxiclav prescribing (9.7%, 19/195) and ciprofloxacin prescribing (9.2%, 18/195) (Table 4). Ten participants (1.4%) received a prescription for cephalosporins (Table 4). Overall, 13/702 (1.9%) participants were given a delayed antibiotic prescription (data not shown).
A total of 225/675 (33.3%) participants were prescribed an antibiotic that was concordant with the culture result (antibiotic class matched to a microbiological definition for UTI on culture and to pathogen sensitivity, as well as those who did not have a microbiological UTI and were not prescribed an antibiotic). The Netherlands had the highest proportion of concordant prescribing and Wales had the lowest (66.7%, 82/123 compared with 23.8%, 46/193) (Table 4).
In total 450/675 (66.7%) participants were prescribed antibiotics non-concordantly (Table 4). Overall, most non-concordant antibiotic prescribing related to females with a culture negative for UTI being prescribed an antibiotic (400 females, 59.3%), and few prescriptions were non-concordant because of resistance to the prescribed antibiotic (28/675, 4.8%) (data not shown). The proportion of participants prescribed a concordant antibiotic was almost identical (32.5%, 203/625) when the European laboratory criteria for UTI were used.
Non-antibiotic prescribed medication
Spain had the highest proportion of females who were prescribed paracetamol (20.5%, 42/205) or ibuprofen (5.9%, 12/205), while England had the highest proportion of females whose clinicians advised them to take paracetamol (28.5%, 70/246) or ibuprofen (10.6%, 26/246). Prescriptions for paracetamol or ibuprofen, or advice to self-medicate with these, was negligible in the other research networks.
Planned follow-up with a GP or nurse
Overall, 225/779 (28.9%) participants had follow-up contact arranged with a GP or nurse (14 out of 793 were missing in planned follow-up with a GP or nurse questions). This varied widely between countries, from 12.4% (30/242) of participants in England to 55.0% (112/204) of those in Spain. After adjusting for participant characteristics, having a follow-up contact arranged was associated with:
age of the participant (OR for 10-year increase: 1.16, 95% CI = 1.01 to 1.32, P = 0.029);
presence of leukocytes (+++ result compared with a negative result: OR 0.43, 95% CI = 0.21 to 0.88, P = 0.021);
positive dipstick test for nitrites (OR: 0.55, 95% CI = 0.32 to 0.96, P = 0.035);
cloudy urine (OR 1.69, 95% CI = 1.00 to 2.86, P = 0.049); and
temperature of participant (OR for 1°C increase: 1.83, 95% CI = 1.10 to 3.04, P = 0.019).
Participant recovery
The median time to full recovery was 10 days (IQR 6–14 days). For those who had a microbiologically confirmed UTI, median recovery time was 9 days (IQR: 6–14 days); for those who did not, it was 10 days (IQR 6–14 days). Antibiotic prescription at the index consultation was associated with time to full recovery (adjusted hazard ratio 1.69, 95% CI = 1.05 to 2.72, P = 0.006). Those who were prescribed an antibiotic recovered faster than those who were not (median 9 days [IQR 5–14 days] versus 13 days [IQR 7–14 days]).
Although the median time to recovery in those who had a microbiologically confirmed UTI and were prescribed antibiotics was the shortest, there was no evidence of any differential association between antibiotic prescribing and a microbiologically confirmed UTI. (More information about this is available from the authors on request.)
There was also no evidence of any differences in recovery at a country level. Similarly, there was no evidence of any differences by country with regard to the time taken to resolve moderately bad symptoms (median 4 days, IQR 2–6 days) or daytime frequency/night-time frequency/urgency (median 8 days, IQR: 4–14 days). Findings were similar in unadjusted and adjusted models. (Further data are available from the authors on request.)
Subsequent antibiotic prescribing
In the 2 weeks following inclusion, 55/531 (10.4%) participants were prescribed at least one subsequent antibiotic for their UTI symptoms, with 19 of the 113 participants in Wales (16.8%), 24/165 in England (14.5%), 11/104 in Netherlands (10.6%), and 1/149 in Spain (0.7%) (data not shown).
Re-consultation
During the follow-up period, 130/547 (23.8%) participants reported that they had consulted with their GP or out-of-hours provider for their UTI symptoms: 41/121 participants in Wales (33.9%), 28/102 in the Netherlands (27.5%), 47/172 in England (27.3%), and 14/152 in Spain (9.2%).
DISCUSSION
Summary
This observational study of the presentation, management, and outcomes of uncomplicated UTI in primary care in four European countries involving almost 800 participants found remarkably little difference in GP-rated symptom severity at presentation, pathogens, and their sensitivity to antibiotics. However, considerable differences were found in UTI positivity on culture, antibiotic prescribing, subsequent antibiotic prescriptions, and re-consultations at the country primary care network level.
Antibiotic prescribing was favourably associated with recovery. However, there was no notable difference in participant recovery at the country level, after controlling for case mix and initial antibiotic prescribing. Delayed antibiotic prescribing was rare, as were non-antibiotic prescriptions. These findings indicate that there is considerable unwarranted clinical variation in care — particularly in the use of broad-spectrum antibiotics — and, as such, highlight the opportunity for determining the most cost-effective pathway of care for uncomplicated UTI. This could also help to minimise unnecessary exposure to antibiotics.
Strengths and limitations
To the authors’ knowledge, this is the first prospective study to describe the presentation and management of uncomplicated UTI in primary care settings in Europe, and to explore the association with patient recovery, taking microbiological findings and case mix into account. No attempt was made to standardise investigations and management across the centres because the goal was to describe variation and to explore whether any variation identified was associated with recovery and microbiological findings, thereby being clinically warranted.
Participants were recruited using the same eligibility criteria, outcome measures, and data collection tools in four European settings; sample numbers were adequately powered to determine variation at a primary care network level. In addition, susceptibility testing was standardised in a central microbiology research laboratory.
Clinicians may have altered their behaviour because of research conditions, despite clear communication that the purpose was to describe routine care. Their assessment of the patients’ symptoms at study inclusion may have been influenced by personal, interpersonal, and cultural factors. In addition, although this study largely met the authors’ pre-specified power requirements, relatively few patients from each network were included, and fewer participants were recruited in the Netherlands.
Primary care research networks in Eastern or Northern European countries were not included. Participating networks were local organising groups that recruited general practices into the study. Networks were selected partly because of their research experience and because of their ability to implement the study protocol to a high standard. However, it is important to be aware that each of the four networks does not necessarily reflect consulting behaviour and care of the whole country.
Study participants may have been selectively rather than sequentially invited to participate, and there are no reliable logs of those patients who were eligible to participate but not invited to do so. Studies in both hospitals and primary care that rely on opportunistic recruitment of acutely unwell patients during times of busy service delivery may be prone to selection bias that is difficult to fully assess. In addition, not all clinicians in the practices were participating in the study, and not all of those who did participate worked full time or recruited each time they were at work.
Although local laboratories followed their standard operating procedures for urinalysis and storage of microorganisms, sample transport times and arrangements may have differed, leading to variation in the proportion of samples that were considered positive for UTI.
Differences in the proportion of urine samples considered positive for a UTI and proportion of patients prescribed an antibiotic between the Netherlands compared with the other countries may partly be explained by distinct characteristics of usual care in the Netherlands, where, for example, it is common for females who are symptomatic to leave a urine sample at the practice and, if it is positive for nitrite on dipstick, it is then tested with a dipslide culture. This is done before any antibiotic prescribing decision is made or the urine is sent for laboratory culture.
Comparison with existing literature
The authors’ systematic search in January 201411,12,14,15,23–30 and update in November 20162,31,32 found that this is the first prospective study to compare routine management of UTI in primary care between country settings, taking case mix and microbiological findings into account.
Daytime frequency and urgency were both the most prevalent and severely graded (rated ‘bad’) symptoms across all networks. Frequency and dysuria were the most prevalent symptoms reported in previous European studies, although urgency was reported by fewer studies and had a lower prevalence.12,24,25,30,32
Urinalysis dipsticks were the most commonly used tests across all four networks; the findings presented here are similar to those of studies undertaken in Spain, Sweden, and Germany, in which use of dipstick urinalysis ranged from 84% to 93%.23,27,30 UTI on culture was identified in just over a third of cases overall; similar proportions were identified in England (24.3%) and Wales (24.1%), whereas those in Spain (42.3%) and the Netherlands (63.8%) were much higher. Vellinga et al found that 70% of urine samples from patients with suspected UTI had no evidence of UTI on culture in a study in Ireland.15 Hummers-Pradier et al found 65.6% of patients in Germany had a positive result (using a definition of 103 CFU/mL and no more than two pathogens),27 and Etienne et al 31 found that 78% had a positive urine culture in a French study; however, both of these studies used a lower threshold for positivity compared with the primary definition used here in this current study. Three UK studies reported positivity of samples between 25% and 38%.11,14,25 Little et al ’s observational study, also conducted in the UK, found that 50% of females with symptoms attributed to a UTI met microbiological criteria for a UTI that was similar to that used in this current study;18 and, as with the current study, these authors also found that females treated with antibiotics recovered faster.12
As with the study presented here, Etienne et al found that Escherichia coli predominated, with generally high rates of sensitivity to commonly used antibiotics; 13% of isolates were resistant to trimethoprim-sulfamethoxazole compared with this study’s overall finding for trimethoprim resistance of 18.7%.31
In this curent study overall, antibiotics were prescribed for 88.5% (59.4% in the Netherlands and >92.0% in the other settings). Antibiotic prescribing ranged from 56.0% to 98.6% in previous European studies.15,24,27 Two English studies12,25 found prescribing rates similar to those reported here for the network in England, and a Spanish study found a similar proportion to the prescribing rate in the Spanish network in this current study.30 A Welsh study found a much lower prescribing rate than the one presented here, but that study relied on patient recall of antibiotic prescription rather than GPs’ recording it at the time of consultation.11
In the study presented here, trimethoprim (in Wales), nitrofurantoin (in Wales, England, and the Netherlands), and fosfomycin (in Spain) were most commonly prescribed, but quinolone antibiotics were fairly commonly prescribed in Spain, which confirms previous findings.28,31 Studies from across Europe also demonstrate the wide variation between countries in terms of the choice of antibiotics prescribed for uncomplicated UTI.2,23,24,26,28,29,31,32
Guideline-concordant antibiotic prescribing ranged from 23.8% in Wales to 66.7% in the Netherlands. Philips et al compared adherence to guidelines, regarding the type of antibiotics prescribed for the primary care out-of-hours management of UTI in four European countries, and found that it ranged from 25% to 100%.1 Other studies have similarly confirmed poor adherence to guidelines for managing uncomplicated UTI in primary care.2,23,24,28,30,33
Implications for research and practice
This study has demonstrated the little variation in presentation, pathogens, and sensitivity of pathogens to antibiotics causing UTI in four European settings. However, in contrast, the proportion of cases meeting laboratory definitions of UTI, the proportion of patients prescribed an antibiotic, the antibiotics commonly prescribed, subsequent antibiotics prescribed, and consulting behaviour did differ markedly. In spite of this, a variety of participant-reported recovery measures showed no variation at country level and antibiotics were associated with improved outcomes overall. Further research is needed to better define the relationship between microbiological findings (using optimal diagnostic testing), patient symptoms at presentation, prognosis, and response to antimicrobials.
Given the low rates of microbiologically confirmed UTI on culture (especially in the UK) and the response of some females with uncomplicated UTI to non-antibiotic treatment, such as ibuprofen,34 it is likely that symptoms of uncomplicated UTI represent a syndrome caused by a range of aetiology. This range could include infection that may or may not be routinely cultured,35 and inflammation at various sites in the urinary tract due to non-infectious causes.
Although more of the UTI treatment in the Netherlands was concordant, according to the definition used in this study, it was also at a cost of undertreating a greater proportion of cases of microbiologically confirmed UTI than other countries. The most cost-effective care pathway for managing symptoms of uncomplicated UTI should now be determined and care standardised to maximise symptom resolution, resource use, and better targeted antibiotic prescribing; current variation in care is not warranted on clinical grounds.
Acknowledgments
The POETIC (Point Of carE Testing for urinary tract Infection in primary Care) team comprises the authors, along with Charles Cowtan, Elinor Coulman, Rhys Thomas, Ana Moragas, José M Molero, and Josep M Cots. The authors would like to thank: the network microbiologists (Jennifer Richards, Rafael Cantón, Patricia Ruiz, Belén Viñado, Carolina Sarvisé, and Judith Vlooswijk); Freda McKenzie, the patient/public representative on the trial management group; study steering committee members (Alastair Hay, Andrew Lovering, and Toby Provost); the local Primary Care Research Networks’ Comprehensive Local Research Networks; the National Institute for Social Care and Health Research — Coordinating Research Centre; the clinicians who implemented the study in their practices; and all of the patients who participated.
Funding
The research leading to these results has received funding from the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement number 282512. This work was part of the EU funded ‘Resistance in Gram-Negative Organisms: Studying Intervention Strategies (R-GNOSIS)’, FP7 HEALTH.2011.2.3.1-3, #282512. The funders had no role in determining the study design, data collection, analysis, writing the report, or in the decision to submit the paper for publication.
Ethical approval
A Research Ethics Committee recognised by the United Kingdom Ethics Committee Authority (REC reference: 12/WA/0111) and relevant European committees in the Netherlands: Utrecht Medical Ethics Testing Committee (Ref: 12/356) and Spain Jordi Gol i Gurina Ethics Committee, Barcelona (Ref: P12/64) approved the study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Philips H, Huibers L, Holm Hansen E, et al. Guidelines adherence to lower urinary tract infection treatment in out-of-hours primary care in European countries. Qual Prim Care. 2014;22(4):221–231. [PubMed] [Google Scholar]
- 2.Hawker JI, Smith S, Smith GE, et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995–2011: analysis of a large database of primary care consultations. J Antimicrob Chemother. 2014;69(12):3423–3430. doi: 10.1093/jac/dku291. [DOI] [PubMed] [Google Scholar]
- 3.Ironmonger D, Edeghere O, Bains A, et al. Surveillance of antibiotic susceptibility of urinary tract pathogens for a population of 5.6 million over 4 years. J Antimicrob Chemother. 2015;70(6):1744–1750. doi: 10.1093/jac/dkv043. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler CC, Hood K, Verheij T, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wennberg JE. Forty years of unwarranted variation — and still counting. Health Policy. 2014;114(1):1–2. doi: 10.1016/j.healthpol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluroquinolone antibiotics. Fam Pract. 2007;24(5):427–434. doi: 10.1093/fampra/cmm040. [DOI] [PubMed] [Google Scholar]
- 8.Wood F, Phillips C, Brookes-Howell L, et al. Primary care clinicians’ perceptions of antibiotic resistance: a multi-country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243. doi: 10.1093/jac/dks338. [DOI] [PubMed] [Google Scholar]
- 9.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 10.Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract. 2015. DOI: https://doi.org/10.3399/bjgp15X686965. [DOI] [PMC free article] [PubMed]
- 11.O’Brien K, Hillier S, Simpson S, et al. An observational study of empirical antibiotics for adult women with uncomplicated UTI in general practice. J Antimicrob Chemother. 2007;59(6):1200–1203. doi: 10.1093/jac/dkm108. [DOI] [PubMed] [Google Scholar]
- 12.Little P, Merriman R, Turner S, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ. 2010;340:b5633. doi: 10.1136/bmj.b5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIsaac WJ, Low DE, Biringer A, et al. The impact of empirical management of acute cystitis on unnecessary antibiotic use. Arch Intern Med. 2002;162(5):600–605. doi: 10.1001/archinte.162.5.600. [DOI] [PubMed] [Google Scholar]
- 14.Nazareth I, King M. Decision making by general practitioners in diagnosis and management of lower urinary tract symptoms in women. BMJ. 1993;306(6885):1103–1106. doi: 10.1136/bmj.306.6885.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vellinga A, Cormican M, Hanahoe B, et al. Antimicrobial management and appropriateness of treatment of urinary tract infection in general practice in Ireland. BMC Fam Pract. 2011;12(1):108. doi: 10.1186/1471-2296-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolle LE, AMMI Canada Guidelines Committee Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349–360. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flottorp S, Oxman AD, Havelsrud K, et al. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002;325(7360):367. doi: 10.1136/bmj.325.7360.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little P, Turner S, Rumsby K, et al. Validating the prediction of lower urinary tract infection in primary care: sensitivity and specificity of urinary dipsticks and clinical scores in women. Br J Gen Pract. 2010. DOI: https://doi.org/10.3399/bjgp10X514747. [DOI] [PMC free article] [PubMed]
- 19.Grabe M, Bjerklund-Johansen TE, Botto H, et al. Guidelines on urological infections. European Association of Urology; 2013. http://uroweb.org/wp-content/uploads/18_Urological-infections_LR.pdf (accessed 9 Nov 2017) [Google Scholar]
- 20.Health Protection Agency Diagnosis of UTI: Quick reference guide for primary care. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/323398/UTI_guidelines_with_RCGP_logo.pdf (accessed 9 Nov 2017)
- 21.Snijders TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. London: Sage; 2012. [Google Scholar]
- 22.Hardy MA. Regression with dummy variables. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 23.André M, Mölstad S, Lundborg CS, et al. Management of urinary tract infections in primary care: a repeated 1-week diagnosis-prescribing study in five counties in Sweden in 2000 and 2002. Scand J Infect Dis. 2004;36(2):134–138. doi: 10.1080/00365540410019075. [DOI] [PubMed] [Google Scholar]
- 24.Canbaz S, Peksen Y, Tevfik Sunter A, et al. Antibiotic prescribing and urinary tract infection. Int J Antimicrob Agents. 2002;20(6):407–411. doi: 10.1016/s0924-8579(02)00252-2. [DOI] [PubMed] [Google Scholar]
- 25.Fahey T, Webb E, Montgomery AA, Heyderman RS. Clinical management of urinary tract infection in women: a prospective cohort study. Fam Pract. 2003;20(1):1–6. doi: 10.1093/fampra/20.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Galatti L, Sessa A, Mazzaglia G, et al. Antibiotic prescribing for acute and recurrent cystitis in primary care: a 4 year descriptive study. J Antimicrob Chemother. 2006;57(3):551–556. doi: 10.1093/jac/dkl008. [DOI] [PubMed] [Google Scholar]
- 27.Hummers-Pradier E, Ohse AM, Koch M, et al. Management of urinary tract infections in female general practice patients. Fam Pract. 2005;22(1):71–77. doi: 10.1093/fampra/cmh720. [DOI] [PubMed] [Google Scholar]
- 28.Martinez MA, Inglada L, Ochoa C, et al. Assessment of antibiotic prescription in acute urinary tract infections in adults. J Infect. 2007;54(3):235–244. doi: 10.1016/j.jinf.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Skerk V, Skerk V, Jaksic J, et al. Research of urinary tract infections in family medicine physicians’ offices — empiric antimicrobial therapy of urinary tract infections — Croatian experience. Coll Antropol. 2009;33(2):625–631. [PubMed] [Google Scholar]
- 30.Llor C, Rabanaque G, Lopez A, Cots JM. The adherence of GPs to guidelines for the diagnosis and treatment of lower urinary tract infections in women is poor. Fam Pract. 2011;28(3):294–299. doi: 10.1093/fampra/cmq107. [DOI] [PubMed] [Google Scholar]
- 31.Etienne M, Lefebvre E, Frebourg N, et al. Antibiotic treatment of acute uncomplicated cystitis based on rapid urine test and local epidemiology: lessons from a primary care series. BMC Infect Dis. 2014;14:137. [Google Scholar]
- 32.Willems CS, van den Broek D’Obrenan J, Numans ME, et al. Cystitis: antibiotic prescribing, consultation, attitudes and opinions. Fam Pract. 2014;31(2):149–155. doi: 10.1093/fampra/cmt077. [DOI] [PubMed] [Google Scholar]
- 33.Vellinga A, Galvin S, Duane S, et al. Intervention to improve the quality of antimicrobial prescribing for urinary tract infection: a cluster randomized trial. CMAJ. 2016;188(2):108–115. doi: 10.1503/cmaj.150601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gagyor I, Haasenritter J, Bleidorn J, et al. Predicting antibiotic prescription after symptomatic treatment for urinary tract infection: development of a model using data from an RCT in general practice. Br J Gen Pract. 2016. DOI: https://doi.org/10.3399/bjgp16X684361. [DOI] [PMC free article] [PubMed]
- 35.McLellan LK, Hunstad DA. Urinary tract infection: pathogenesis and outlook. Trends Mol Med. 2016;22(11):946–957. doi: 10.1016/j.molmed.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]