Abstract
Background
Over 150 000 cases of suspected transient ischaemic attack (TIA) are referred to outpatient clinics in England each year. The majority of referrals are made by GPs.
Aim
This study aimed to identify how many patients referred to a TIA clinic actually have TIA (that is, calculate the positive predictive value [PPV] of first-contact healthcare referral) and to record the alternative diagnoses in patients without TIA, in order to determine the optimal service model for patients with suspected TIA.
Design and setting
A systematic review of TIA clinic referrals from first-contact health professionals (GPs and emergency department [ED] doctors) was undertaken.
Method
Four databases were searched using terms for TIA and diagnostic accuracy. Data on the number of patients referred to a TIA clinic who actually had a TIA (PPVs) were extracted. Frequencies of differential diagnoses were recorded, where reported. Study quality was assessed using the QUADAS-2 tool.
Results
Nineteen studies were included and reported sufficient information on referrals from GPs and ED doctors to derive PPVs (n = 15 935 referrals). PPVs for TIA ranged from 12.9% to 72.5%. A formal meta-analysis was not conducted due to heterogeneity across studies. Of those not diagnosed with TIA, approximately half of the final diagnoses were of neurological or cardiovascular conditions.
Conclusion
This study highlights the variation in prevalence of true vascular events in patients referred to TIA clinics. For patients without a cerebrovascular diagnosis, the high prevalence of conditions that also require specialist investigations and management are an additional burden on a care pathway that is primarily designed to prevent recurrent stroke. Service commissioners need to assess whether the existing outpatient provision is optimal for people with pathologies other than cerebrovascular disease.
Keywords: diagnosis; general practitioners; ischaemic attack, transient; predictive value of tests; primary health care; stroke
INTRODUCTION
A transient ischaemic attack (TIA) is a temporary focal neurological disturbance due to an interruption in the blood supply to an area of the brain.1 The term ‘transient neurological symptoms’ is used to describe the broad range of symptoms that may occur following a TIA or another condition that may mimic TIA. There is no gold-standard clinical test that can be used to diagnose a TIA or stroke based on symptomology; diagnosis of TIA is based on the assessment of symptoms and adequate investigation by a clinician. Historically, TIA symptoms would need to resolve within 24 hours to be classified as TIA and not a minor stroke; however, in 2009 a tissue-based definition of TIA was proposed:
‘Transient ischemic attack (TIA): a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.’ 1
In practice, the time-based definition may be the more-operable, working definition, because identification of infarcts requires imaging and not all TIA clinic attendees are imaged.
The incidence of transient neurological symptoms is high — estimated at 190 cases per 100 000 population2 — and clinic referral rates amount to approximately 16 per 10 000 patients every year.3 Outpatient TIA clinics are well equipped to identify and treat TIA and minor stroke, but only a proportion of suspected TIA cases will be confirmed.4 Those patients not presenting with a TIA may, nevertheless, experience adverse health consequences.5
The clinical assessment for TIA can be complex because the symptoms are transient and there are no persisting signs on examination to guide the referring clinician. Previously, a brief review of predictive values in TIA was undertaken,6 but an update is timely as more data have been published to inform estimates of accuracy. Richer data now also exists on alternative diagnoses in this complex clinic population. The objective of this study was to evaluate the positive predictive values (PPVs) associated with first-contact healthcare referral — that is, those made by GPs or emergency department (ED) doctors — to a TIA clinic, and to describe the alternative diagnoses in referred patients.
METHOD
Data sources
Four databases (MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects) were searched from 1989 to week 28 of 2016, using terms for TIA combined with a diagnostic filter (available from the authors on request). The Bachmann filter (adapted to run on each database) was used — this has been identified as one of the most sensitive diagnostic filters available, with acceptable precision.7 Additional papers were sought by screening the citations of retrieved studies. All data screening, extraction, and full-text assessment were done by a single reviewer and checked in detail by a second.
How this fits in
The positive predictive value of a referral to a transient ischaemic attack (TIA) clinic has previously been described in selected populations in single studies. The systematic review presented here found that 12.9–72.5% of clinic referrals had a confirmed TIA; this was usually ≥50% when a composite (TIA or minor stroke) reference standard was used. Alternative diagnoses suggest that the total population with transient neurological symptoms may represent a susceptible population for further investigations and treatment that is not presently discussed in UK stroke and TIA guidance. Commissioners should ensure that TIA services can meet the needs of a heterogeneous patient group.
Inclusion criteria
Primary studies of any design, conference abstracts, and systematic reviews reporting information necessary to derive PPVs of TIA diagnosis from first-contact health professionals (primarily GPs or ED doctors) were included. If PPVs were reported in more than one study, duplicate values were not reported. When there was a duplication of reporting, preference was given to full-text studies that report the most detail with respect to the application of the index test and reference standard.
Data extraction and study quality assessment
Data were extracted on the:
type of study;
geographical location;
method of patient selection;
age of population; and
number of patients included in the study.
Information was also collected on:
positive and negative diagnoses;
frequencies of unverified diagnoses;
which reference standard the study applied (TIA alone or TIA and minor stroke); and
what definition of TIA was used (tissue- or time-based definition).
Systematic reviews were identified as a source of relevant studies. QUADAS-2 was used to assess the risk of bias and applicability of included studies.8 The frequencies of differential diagnoses for false-positive TIAs were also recorded, and the details of all non-TIA/stroke diagnoses tabulated.
Statistical analysis and synthesis
For each study, the PPV was calculated as the number of true positives divided by the sum of true and false positives (that is, the total number of patients referred to the clinic). The binomial exact standard errors were calculated if the standard error of the PPV was not reported. Due to high unexplained variation in the underlying prevalence of TIA, it was decided that a summary PPV would not be estimated. Forest plots were used to display the individual study estimates of PPV, with 95% confidence intervals (CIs) analysing the target conditions (TIA and the composite outcome of TIA and minor stroke) separately. Although the main analysis reports results for full texts only, a sensitivity analysis including conference abstracts was carried out to examine the robustness of the results.
RESULTS
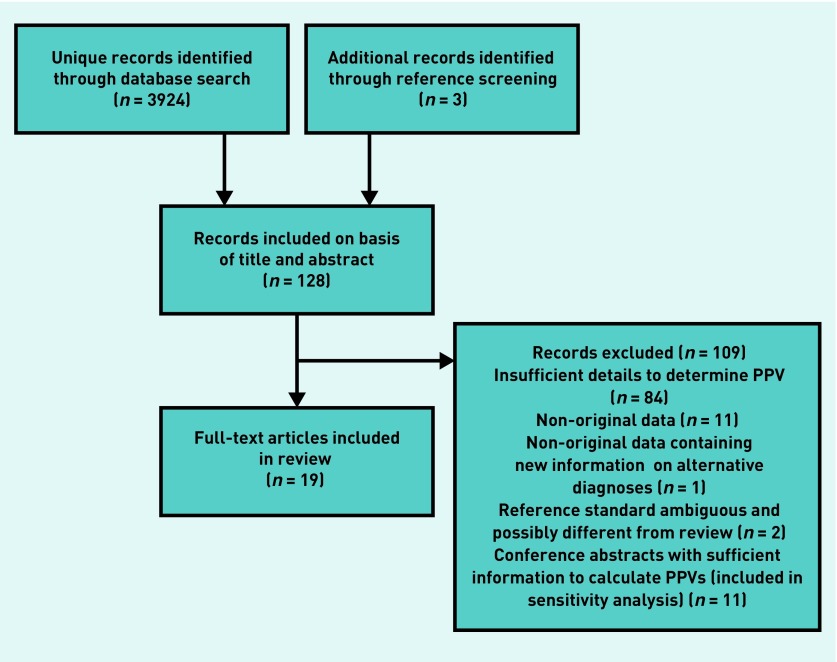
The search identified 3924 unique records. Of these, and a further three records identified by screening the references, 19 full texts met the eligibility criteria (Figure 1). Eleven conference abstracts also met the inclusion criteria and were included in the sensitivity analysis. Study characteristics are presented in Table 1.
Figure 1.
Study selection process. PPV = positive predictive value.
Table 1.
Study characteristics and extracted data (stratified by referral route) for clinician accuracya
| Study | Location | Type | Referral source(s) | Mean age, years (SD) | TIA | TIA or stroke | Diagnosis unknown | Total number of cases of suspected TIA, n | PPV for reference Dx of TIA | PPV for reference Dx of TIA and stroke |
|---|---|---|---|---|---|---|---|---|---|---|
| Banerjee et al, 200911 | London, UK | Prospective cohortb | GP and ED | 68 (13.5) | 133 | 213 | 2 (15 unavailable) | 282 | 47.2 | 75.5 |
| Bradley et al, 201312 | Dublin, Ireland | Prospective cohort | GP and ED | 60 (14.3) | 49 | 56 | None | 101 | 48.5 | 55.4 |
| Cameron et al, 20119 | Glasgow, UK | Prospective cohort | GP and ED | 65 (13.6) | – | 1890 | None | 3533 | – | 53.5 |
| Dawson et al, 200913 | Glasgow, UK | Prospective cohort | GP and ED | 65 (12.8) | – | 2358 | None | 3467 | – | 68.3 |
| Dutta, 201615 | Gloucester, UK | Prospective cohort — DOT validation cohort | GP and ED | 71 (14.0) | 160 | 236 | None | 525 | 30.5 | 45.0 |
| Dutta et al, 201514 | Gloucester, UK | Prospective cohort | GP and ED | 72c (IQR: 60–80) | 337 | 529 | None | 1067 | 31.6 | 49.6 |
| Fallon et al, 200616 | Dublin, Ireland | Prospective cohort | ED (primarily) and other — not specified | 75.5 (−) | 56 | 72 | 18 | 117 | 47.9 | 61.5 |
| Ferro et al, 199610 | Central & southern Portugal | Prospective cohort | GP | – | 10 | 36 | None | 52 | 19.2 | 69.2 |
| Ferro et al, 199610 | Central & southern Portugal | Prospective cohort | ED | – | 4 | 18 | None | 31 | 12.9 | 58.1l |
| Fonseca and Canhão, 200926 | Lisbon, Portugal | Prospective cohort | GP and ED | 65 (−) | 259 definite TIA | – | 109 cases recorded as ‘possible’ TIA | 458 | −56.6 | – |
| Karunaratne et al, 199917 | Scottish Borders, UK | Prospective cohort | GP and ED | 67 (14) | 31 | 64 | 7 non-TIA with no clear diagnosis | 128 | 24.2 | 50.0 |
| Lasserson et al, 201527 | Oxford, UK | Prospective cohort | GP | 73 (12.8) | 209 | – | None | 513 | 40.7 | – |
| Lavallée et al, 200718 | Paris, France | Prospective cohort | GP and ED | Median ages reported by final diagnosis alone | 643 definite TIA | 701 | 144 cases recorded as ‘possible TIA’ | 1085 | 72.5 | 77.9 |
| Lee and Frayne, 201519 | Melbourne, Australia | Prospective cohort | GP and ED | 67 (16.9) | 13 | 18 | 4 non-TIA with no clear diagnosis | 82 | 15.9 | 22.0 |
| Magin et al, 201320 | Hunter New England, Australia | Prospective cohort | GP | 65 (15) | 29 | 50 | 13 unclassified | 127 | 22.8 | 39.4 |
| Magin et al, 201320 | Hunter New England, Australia | Prospective cohort | ED | 65 (15) | 46 | 66 | 9 unclassified | 104 | 44.2 | 63.5 |
| Martin et al, 199725 | Liverpool, UK | Prospective cohort | GP and ED | 62c (IQR: 23–94) | 200 | – | Unclear | 332 | 60.2 | – |
| Murray et al, 20073 | Glasgow, UK | Retrospective cohort | GP and ED | Age ranges given | 217 | 283 | None | 811 | 26.8 | 34.9 |
| Palomeras Soler et al, 201521 | Barcelona, Spain | Prospective cohort | GP and ED | – | 282 | 310 | None | 411 | 68.6 | 75.4 |
| Sheehan et al, 200922 | Dublin, Ireland | Prospective cohort | GP and ED | 69 (13) | 292 | 337 | None | 257 | 49.2 | 56.7 |
| Walker et al, 201223 | Leicester, UK | Prospective cohort | GP and ED | – | – | 1273 | None | 2452 | – | 51.9 |
Where there is more than one data entry for a single study this reflects that the study provided sufficient information to calculate PPVs by different referral sources.
PPVs only reported for suspected TIAs that were seen at an anterior circulation TIA clinic.
Median not mean reported. Dx = diagnosis. ED = emergency department. IQR = interquartile range. PPV = positive predictive value. SD = standard deviation. TIA = transient ischaemic attack.
Ten studies were conducted in the UK, three in Ireland, two in Australia, two in Portugal, one in Spain, and one in France. All patients identified were TIA clinic referrals/attendees, using consecutive or all referrals within a given timeframe. In total, 19 studies provided sufficient information to calculate the PPV for at least one of the reference diagnoses (TIA and/or the composite reference diagnosis of TIA and minor stroke). The number of suspected cases of TIA referred from, or including, GPs (18 of 19 studies) ranged from 52 to 3533 (Table 1).9,10
Specialist diagnosis (reference standard)
In all cases, the reference standard was the clinical diagnosis of the stroke physician in clinic. Several studies reported that the assessment of TIA was standardised at their clinic and/or of additional retrospective notes review to confirm the diagnosis made by a senior stroke/vascular specialist. Studies dichotomised diagnoses into two outcomes:
TIA, which sometimes included minor stroke; and
not TIA.
Almost all studies used the time-based definition of TIA, even when the later tissue-based definition was available, but there was one exception: one conference abstract included in the sensitivity analysis24 used the tissue-based definition of TIA.
Differential diagnoses
Twelve of the included studies reported on the final diagnoses received by patients, although one study did not report sufficient information to determine frequencies for all alternative diagnoses.25 The frequency of alternative diagnoses, where reported, are shown in Table 2.
Table 2.
Clinical diagnoses in suspected TIA and stroke cases identified by first-contact health professionals: frequency and percentage of all suspected TIA clinic referrals
| Clinical diagnosis | Banerjee et al, 200911 | Bradley et al, 201312 | Cameron et al, 20119 | Dutta et al, 201514 | Fallon et al, 200616 | Ferro et al, 199610 | Fonseca and Canhão, 201126 | Karunaratne et al, 199917 | Lee and Frayne, 201519 | Murray et al, 20073 | Palomeras Soler et al, 201521 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Accidental fall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arrythmia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.6 | 0 | 0 |
| Asymptomatic stenosis carotid artery | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.4 | 0 | 0 |
| Brain tumour/brain metastases/CNS lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1.9 | 1 | 0.9 | 1 | 1.2 | 0 | 0 | 4 | 3.1 | 0 | 0 | 9 | 1.1 | 0 | 0 |
| Carotid sinus hypersensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cataract related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Central retinal vein occlusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cervical myelopathy/spondylosis and spinal cord stenosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2.3 | 0 | 0 | 14 | 1.7 | 0 | 0 |
| Dehydration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Delirium/confusional episode | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 4.6 |
| Dementia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 1.4 | 0 | 0 |
| Epilepsy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.2 | 0 | 0 |
| Gastroenteritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperventilation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 1 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoglycaemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isolated nerve palsy including Bell’s palsy | 1 | 0.4 | 0 | 0 | 0 | 0 | –a | –a | 0 | 0 | 1 | 1.2 | 0 | 0 | 4 | 3.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Labyrinthitis/vestibular neuronitis | 0 | 0 | 3 | 3.0 | 0 | 0 | –a | –a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lipoedema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medication side effects | 0 | 0 | 1 | 1.0 | 0 | 0 | 0 | 0 | 1 | 0.9 | 0 | 0 | 1 | 0.2 | 0 | 0 | 2 | 2.4 | 1 | 0.1 | 13 | 3.2 |
| Middle-ear disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 1.4 | 0 | 0 |
| Migraine/tension headache | 11 | 3.9 | 15 | 14.9 | 63 | 1.8 | 139 | 26.5 | 2 | 1.7 | 3 | 3.6 | 4 | 0.9 | 6 | 4.7 | 17 | 20.7 | 35 | 4.3 | 5 | 1.2 |
| Motor neurone disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.4 | 0 | 0 |
| Movement disorder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.9 | 0 | 0 | 1 | 0.2 | 0 | 0 | 0 | 0 | 3 | 0.4 | 0 | 0 |
| Musculoskeletal | 0 | 0 | 2 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Multiple sclerosis/demyelination | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.8 | 0 | 0 | 3 | 0.4 | 0 | 0 |
| Myasthenia | 0 | 0 | 0 | 0 | 0 | 0 | –a | –a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular event (amaurosis fugax/occluded retinal artery) | 29 | 10.3 | 3 | 3.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.9 | 0 | 0 |
| Overdose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral nerve lesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral neuropathy/radiculopathy | 5 | 1.8 | 1 | 1.0 | 0 | 0 | –a | –a | 1 | 0.9 | 0 | 0 | 2 | 0.4 | 1 | 0.8 | 0 | 0 | 17 | 2.1 | 6 | 1.5 |
| Pneumonia and erysipelas | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Previous stroke/previous neurological deficit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.4 | 0 | 0 | 1 | 1.2 | 4 | 0.5 | 0 | 0 |
| Psychological/psychiatric | 4 | 1.4 | 3 | 3.0 | 56 | 1.6 | –a | –a | 0 | 0 | 2 | 2.4 | 18 | 3.9 | 1 | 0.8 | 7 | 8.5 | 14 | 1.7 | 7 | 1.7 |
| Radial nerve palsy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Secondary to traffic accident | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Seizure/convulsion | 1 | 0.4 | 3 | 3.0 | 48 | 1.4 | –a | –a | 3 | 2.6 | 3 | 3.6 | 19 | 4.1 | 12 | 9.4 | 0 | 0 | 16 | 2 | 11 | 2.7 |
| Subdural haematoma | 0 | 0 | 0 | 0 | 0 | 0 | –a | –a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.2 | 0 | 0 | 0 | 0 |
| Syncope/pre-syncope/postural hypotension/ vasovagal attack | 1 | 0.4 | 10 | 9.9 | 0 | 0 | –a | –a | 12 | 10.3 | 8 | 9.6 | 22 | 4.8 | 5 | 3.9 | 13 | 15.9 | 44 | 5.4 | 23 | 5.6 |
| Temporal arteritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Transient global amnesia | 0 | 0 | 0 | 0 | 35 | 1.0 | 17 | 3.2 | 3 | 2.6 | 3 | 3.6 | 6 | 1.3 | 3 | 2.3 | 0 | 0 | 8 | 1.0 | 0 | 0 |
| Trigeminal neuralgia | 0 | 0 | 1 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 0 |
| Unspecified cardiac disorder | 0 | 0 | 0 | 0 | 144 | 4.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unspecified metabolic disorder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.9 | 0 | 0 | 4 | 4.9 | 0 | 0 | 0 | 0 |
| Vertebrobasilar insufficiency | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vertigo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.9 | 3 | 3.6 | 6 | 1.3 | 1 | 0.8 | 0 | 0 | 2 | 0.2 | 0 | 0 |
| Diagnosis stated as ‘other’ | 0 | 0 | 3 | 3.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diagnosis stated as ‘unknown’ | 2 | 0.7 | 0 | 0 | 0 | 0 | 373 | 71 | 18 | 15.4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4.9 | 179 | 22.1 | 0 | 0 |
| Diagnosis stated as ‘unavailable’ | 15 | 5.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total non-TIA | 69 | 24.5 | 45 | 44.6 | 0 | 0 | 539 | 102.7 | 45 | 38.5 | 32 | 38.6 | 90 | 19.7 | 64 | 2.9 | 49 | 59.8 | 392 | 48.3 | 84 | 20.4 |
Authors identify as positive cases, but do not provide information on frequencies. CNS = central nervous system. TIA = transient ischaemic attack.
The range of conditions diagnosed includes those for which guidance from the National Institute for Health and Care Excellence recommends assessment by an appropriately trained specialist, such as for multiple sclerosis, epilepsy, and cardiac arrhythmias. The most common diagnoses were seizure, syncope, transient global amnesia, and migraine/tension headache (Table 2).
Unexplained diagnoses
The majority — 14 out of 19 — of studies did not provide clear information on the number of patients for whom there was no clear diagnosis following referral to a TIA clinic (Table 2). Several studies had a ‘possible TIA’ category18,26 featuring symptoms that were broadly consistent with, but not clearly diagnostic for, TIA, as well as a ‘non-TIA’ category when this was not the case. As the diagnosis was essentially unconfirmed in these cases, the analysis in this review treated possible TIA as essentially unexplained, that is, negative cases in the analysis of PPV.
PPVs of TIA from first-contact health care
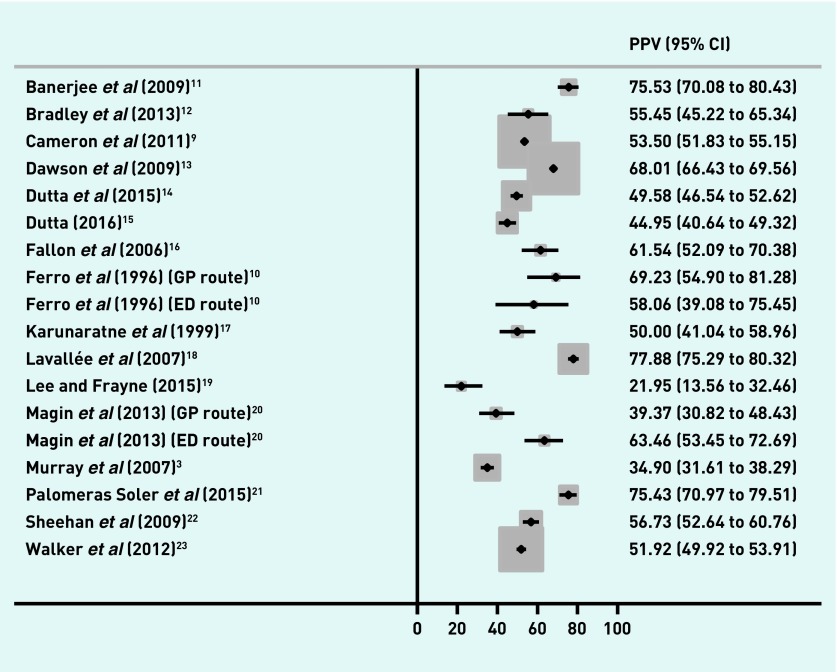
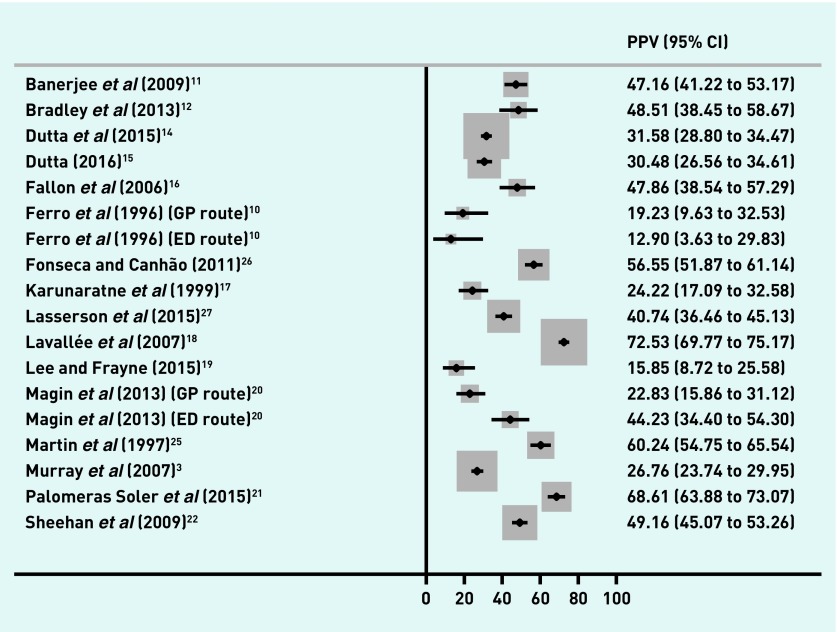
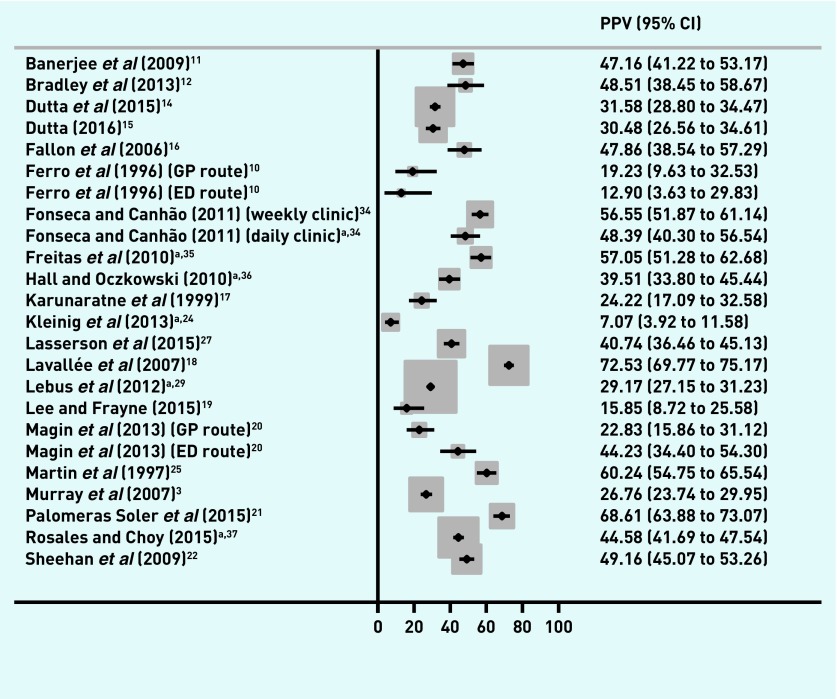
The proportion of referred patients with a final diagnosis of TIA and/or minor stroke ranged from 22.0% to 77.9% (Figure 2), and from 12.9% to 72.5% of patients with a final diagnosis of TIA (Figure 3). The distribution of PPV estimates appeared to differ depending on the reference standard — 13 out of 18 studies had a PPV of ≥50% for a combined TIA and minor stroke outcome, but only four of 18 studies had a PPV of ≥50% when the reference standard was just TIA.
Figure 2.
PPVs of first-contact healthcare diagnosis in TIA and stroke. CI = confidence interval. ED = emergency department. PPV = positive predictive value. TIA = transient ischaemic attack.
Figure 3.
PPVs of first-contact healthcare diagnosis in TIA. CI = confidence interval. ED = emergency department. PPV = positive predictive value. TIA = transient ischaemic attack.
Assessment of study quality
Application of the QUADAS-2 checklist yielded similar results across studies, with all having a high risk of bias in the reference-standard domain. The bias relates to the absence of a ‘gold-standard’ test and the diagnostician knowing that the patients were referred as having suspected TIA.
Influence of referral source and referral criterion
The majority of studies — 15 out of 19 — included all referrals and did not report on the composition of referrals (GP or ED doctor) and/or provide sufficient data to calculate PPVs by referral source. It is plausible that studies may have included referrals from other sources such as ophthalmology and secondary care, but reporting on this issue was scant.
Two studies10,20 provided sufficient information to calculate PPVs according to two referral routes (GP or ED doctor) and a further study provided information on PPVs for referrals purely from GPs.27 One study gave PPVs predominantly from an ED setting,16 whereas all other studies appeared to have largely comprised referrals from GPs. With the exception of one small study,10 the PPVs appear lower in GP referrals than in referrals from ED doctors (Table 1; Figures 2 and 3). Only one study, which restricted itself to suspected anterior circulation TIA events,11 described specific referral criteria.
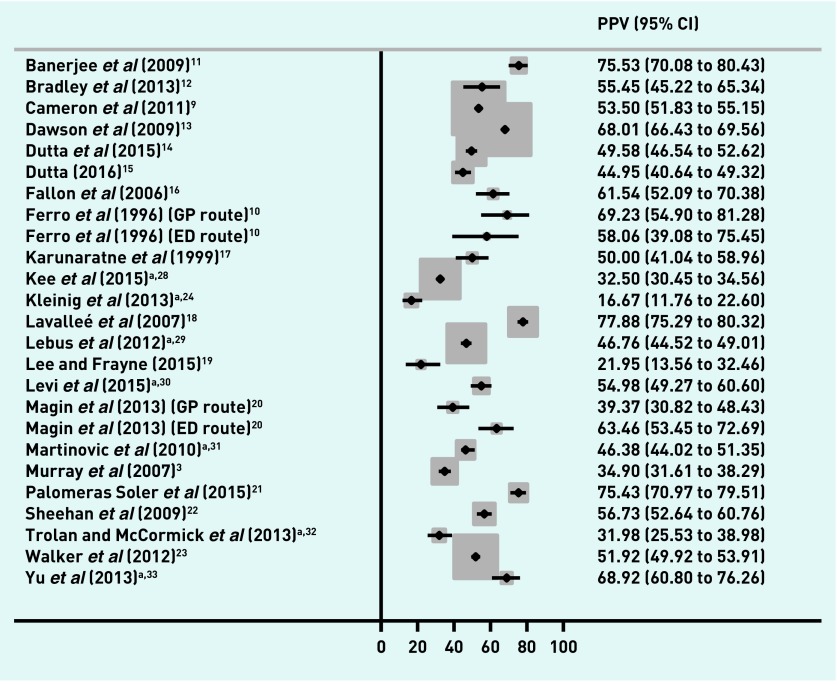
Impact of including conference abstracts
In general, interpretation of the results did not change when conference abstracts were included (Figures 4 and 5),24,28–37 although Kleinig et al 24 had much lower PPVs compared with the other studies for both the combined outcome (16.7%, 95% CI = 11.8 to 22.6, Figure 4) and TIA alone (7.1%, 95% CI = 3.9 to 11.6, Figure 5). This study was set in a magnetic resonance imaging-based referral clinic using the tissue-based reference standard.
Figure 4.
PPVs of first-contact healthcare diagnosis in TIA and stroke (sensitivity analysis including original data contained in conference abstracts). Letters
a Conference abstracts not included in main analysis. CI = confidence interval. ED = emergency department. PPV = positive predictive value. TIA = transient ischaemic attack.
Figure 5.
PPVs of first-contact healthcare diagnosis in TIA (sensitivity analysis including original data contained in conference abstracts).
a Conference abstracts not included in main analysis. CI = confidence interval. ED = emergency department. PPV = positive predictive value. TIA = transient ischaemic attack.
DISCUSSION
Summary
This review has identified considerable variability in PPVs for TIA across studies. The subset of studies identified, which report on alternative diagnoses, highlights the predominance of additional neurological and cardiological diseases that are TIA mimics and require specialist assessment, either within the TIA service or at a subsequent specialty clinic attendance.
Although the review demonstrates a variation in PPVs across studies, it could be that this is explained by a combination of referral source and diagnostic criteria, study age, and/or the cardiovascular event being diagnosed — for instance, some evidence was found to suggest that PPVs in primary care populations may be lower than in those whose referral was made by an ED doctor. However, inference about the possible influence of referral source and referral criterion is difficult because of other study differences.
PPVs also tended to be higher in studies conducted in recent years, which might reflect a change in operation of the diagnostic criteria and improving recognition of symptoms by doctors over time.
Studies that included stroke had higher PPVs; this might reflect the broader diagnostic criteria or that GPs and ED doctors may be more likely to correctly identify a stroke due to the persistent nature of the deficit.
Strengths and limitations
As there was no assessment of patients who were not thought by first-contact health professionals to have experienced a TIA, it was not possible for the authors to ascertain how many people with TIAs were missed. As such, it was not possible to compute sensitivity or specificity. This means that it was also not possible to interpret whether high predictive values were associated with higher referral thresholds (which are likely to be associated with lower sensitivity — that is, more TIAs missed by first-contact health professionals). It also means the prevalence of TIA in the population seen by first-contact health professionals cannot be computed, which is a key determinant of predictive value.
Although the authors believed the reference standard to be acceptable — in all cases it was analogous to how diagnoses are made in practice — specialists were not blind to the index GP/ED doctor diagnosis; this might have led to more non-TIAs being misclassified as TIAs.
Although not limitations as such, there are some caveats to inference on the basis of PPVs. The PPVs reported are at study level. Each study reflects the practice of multiple clinicians, which may vary considerably. In addition, a PPV does not indicate whether the referral to the TIA clinic was appropriate for the patient, and/or whether a more appropriate action should have been taken.
The decision to explore potential publication bias via a sensitivity analysis of conference abstracts is a strength. Predictive values for TIA were similar, suggesting that publication bias is unlikely to be a major issue.
Comparison with existing literature
This is the first review of predictive values from first-contact health care and as such it summarises the literature. However, the PPV of a stroke referral from a review of the literature is about 74%,4 which is higher than any individual PPV for TIA. This may be due to easier recognition of stroke with both persisting neurological deficit and a more severe clinical phenotype. It is interesting to note that the PPVs in this study are higher than those for cancer, where there is an accelerated referral process in order to expedite diagnosis and treatment.38–40 However, the complexity of diagnosis and ongoing management of the other conditions that are found among TIA referrals mandates that other specialties are involved in service delivery, an issue that has less of an impact on organ-specific cancer referral pathways.
Implications for practice
PPVs are a key statistic used in predictive risk modelling and the planning of prevention services.41 The use of PPVs as statistics for planning TIA services has been contested42 because patients with transient symptoms that are not a result of TIA have been recognised as a similarly morbid population to those with true TIA.43–45 Clinical need is, therefore, not limited to confirmed TIAs but to the broader populace with transient symptoms. The dual findings of this review — relatively high but variable predictive values and a predominance of cardiovascular pathologies — suggest that active risk-factor management, including early initiation of antiplatelet agents, is still appropriate to mitigate early recurrent stroke risk after initial suspicion of TIA.46 This study shows that TIA specialist services need to handle a broad range of diagnoses, not only TIA. Many of the most common alternative diagnoses could benefit from appropriate specialty input — the challenge for service commissioners is how best to deliver comprehensive care for patients who present with transient neurological symptoms. Although the TIA clinic is well placed to manage the hyper-acute risk of recurrent stroke, it may not be the optimal configuration in terms of specialist assessment for the range of neurological, cardiological, and psychiatric conditions that also require ongoing care.
Acknowledgments
The authors would like to thank Professor Willie Hamilton for commenting on the study design.
Funding
This study was unfunded; however, Rebecca Kandiyali previously carried out a literature review on this topic as part of a University of Birmingham medical school studentship and Daniel Lasserson is supported by the NIHR Oxford Diagnostic Evidence Cooperative. Penny Whiting and Alison Richards were supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West at University Hospitals Bristol NHS Foundation Trust. The views are those of the authors and not necessarily those of the NIHR, NHS, or Department of Health. The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs RG, Newson R, Lawrenson R, et al. Diagnosis and initial management of stroke and transient ischemic attack across UK health regions from 1992 to 1996: experience of a national primary care database. Stroke. 2001;32(5):1085–1090. doi: 10.1161/01.str.32.5.1085. [DOI] [PubMed] [Google Scholar]
- 3.Murray S, Bashir K, Lees KR, et al. Epidemiological aspects of referral to TIA clinics in Glasgow. Scott Med J. 2007;52(1):4–8. doi: 10.1258/rsmsmj.52.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Gibson LM, Whiteley W. The differential diagnosis of suspected stroke: a systematic review. J R Coll Physicians Edinb. 2013;43(2):114–118. doi: 10.4997/JRCPE.2013.205. [DOI] [PubMed] [Google Scholar]
- 5.Bos MJ, van Rijn MJ, Witteman JC, et al. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877–2885. doi: 10.1001/jama.298.24.2877. [DOI] [PubMed] [Google Scholar]
- 6.Mant J, Ryan R, McManus R, et al. What is the optimum model of service delivery for transient ischaemic attack? Report for the National Co-ordinating Centre for NHS Service Delivery and Organization R&D (NCCSDO) 2008. http://www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1504-112_V01.pdf (accessed 19 Oct 2017)
- 7.Bachmann LM, Coray R, Estermann P, Ter Riet G. Identifying diagnostic studies in MEDLINE: reducing the number needed to read. J Am Med Inform Assoc. 2002;9(6):653–658. doi: 10.1197/jamia.M1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Cameron AC, Dawson J, Quinn TJ, et al. Long-term outcome following attendance at a transient ischemic attack clinic. Int J Stroke. 2011;6(4):306–311. doi: 10.1111/j.1747-4949.2011.00591.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist: a validation study. Stroke. 1996;27(12):2225–2229. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Natarajan I, Biram R, et al. FAST-TIA: a prospective evaluation of a nurse-led anterior circulation TIA clinic. Postgrad Med J. 2009;85(1010):637–642. doi: 10.1136/pgmj.2009.083162. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D, Cronin S, Kinsella JA, et al. Frequent inaccuracies in ABCD2 scoring in non-stroke specialists’ referrals to a daily Rapid Access Stroke Prevention service. J Neurol Sci. 2013;332(1–2):30–34. doi: 10.1016/j.jns.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Dawson J, Lamb KE, Quinn TJ, et al. A recognition tool for transient ischaemic attack. Q J Med. 2009;102(1):43–49. doi: 10.1093/qjmed/hcn139. [DOI] [PubMed] [Google Scholar]
- 14.Dutta D, Bowen E, Foy C. Four-year follow-up of transient ischemic attacks, strokes, and mimics: a retrospective transient ischemic attack clinic cohort study. Stroke. 2015;46(5):1227–1232. doi: 10.1161/STROKEAHA.114.008632. [DOI] [PubMed] [Google Scholar]
- 15.Dutta D. Diagnosis of TIA (DOT) score — design and validation of a new clinical diagnostic tool for transient ischaemic attack. BMC Neurol. 2016;16(1):20. doi: 10.1186/s12883-016-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon C, Noone I, Ryan J, et al. Assessment and management of transient ischaemic attack — the role of the TIA clinic. Ir J Med Sci. 2006;175(3):24–27. doi: 10.1007/BF03169168. [DOI] [PubMed] [Google Scholar]
- 17.Karunaratne PM, Norris CA, Syme PD. Analysis of six months’ referrals to a ‘one-stop’ neurovascular clinic in a district general hospital: implications for purchasers of a stroke service. Health Bull. 1999;57(1):17–28. [PubMed] [Google Scholar]
- 18.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 19.Lee W, Frayne J. Transient ischaemic attack clinic: an evaluation of diagnoses and clinical decision making. J Clin Neurosci. 2015;22(4):645–648. doi: 10.1016/j.jocn.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Magin P, Lasserson D, Parsons M, et al. Referral and triage of patients with transient ischemic attacks to an acute access clinic: risk stratification in an Australian setting. Int J Stroke. 2013;8(Suppl A100):81–89. doi: 10.1111/ijs.12014. [DOI] [PubMed] [Google Scholar]
- 21.Palomeras Soler E, Fossas Felip P, Cano Orgaz AT, et al. Rapid assessment of transient ischaemic attack in a hospital with no on-call neurologist. [Spanish] Neurologia. 2015;30(6):325–350. doi: 10.1016/j.nrl.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke. 2009;40(11):3449–3454. doi: 10.1161/STROKEAHA.109.557074. [DOI] [PubMed] [Google Scholar]
- 23.Walker J, Isherwood J, Eveson D, Naylor AR. Triaging TIA/minor stroke patients using the ABCD2 score does not predict those with significant carotid disease. Eur J Vasc Endovasc Surg. 2012;43(5):495–498. doi: 10.1016/j.ejvs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Kleinig T, Hall L, Jannes J, Dowie G. There’s (almost) no such thing as a TIA; high rates of TIA-mimics and minor stroke in a tertiary MRI- and emergency referral-based TIA service. Int J Stroke. 2013;8(Suppl 1):48. [Google Scholar]
- 25.Martin PJ, Young G, Enevoldson TP, Humphrey PRD. Overdiagnosis of TIA and minor stroke: experience at a regional neurovascular clinic. Q J Med. 1997;90(12):759–763. doi: 10.1093/qjmed/90.12.759. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca AC, Canhão P. Diagnostic difficulties in the classification of transient neurological attacks. Eur J Neurol. 2011;18(4):644–648. doi: 10.1111/j.1468-1331.2010.03241.x. [DOI] [PubMed] [Google Scholar]
- 27.Lasserson DS, Mant D, Hobbs FD, Rothwell PM. Validation of a TIA recognition tool in primary and secondary care: implications for generalizability. Int J Stroke. 2015;10(5):692–696. doi: 10.1111/ijs.12201. [DOI] [PubMed] [Google Scholar]
- 28.Kee Y, Negansan C, Mahmood S, Lawrence E. Fast +ve vs Fast −ve: an analysis of patients presenting to TIA clinic. Int J Stroke. 2015;10(Suppl 5):55. [Google Scholar]
- 29.Lebus C, Prabhakaran M, Mitchell J, et al. The ABCD2 score as a predictor of prognosis but also accurate diagnosis of TIA in a large teaching hospital service. Cerebrovasc Dis. 2012;33(Suppl 2):524–525. [Google Scholar]
- 30.Levi C, Davey A, Lasserson D, et al. Presentation patterns of patients with transient ischemic attack (TIA) and minor stroke, compared with those of stroke/TIA mimics. Int J Stroke. 2015;10(Suppl 2):362. [Google Scholar]
- 31.Martinovic O, Baht H, Balogun I, et al. An appointment based TIA service is unable to accommodate all high risk patients <24hrs. Cerebrovasc Dis. 2010;29(Suppl 2):238. [Google Scholar]
- 32.Trolan C, McCormick M. Prioritisation of referrals to a district general neurovascular clinic: role of ABCD2. Ir J Med Sci. 2013;182(Suppl 6):S281. [Google Scholar]
- 33.Yu CT, Tam YM, Tang SK, et al. A prospective evaluation of diagnostic yield of transient ischemic attack (TIA) in a nurse-led TIA clinic in Hong Kong. Cerebrovasc Dis. 2013;36(Suppl 1):29. [Google Scholar]
- 34.Fonseca M, Canhão P. Early vascular risk after TIA: comparison between a weekly and daily TIA clinic. J Neurol. 2011;258(1 Suppl):S34. [Google Scholar]
- 35.Freitas J, Damasio J, Magalhaes R, et al. Strokes ABCD2 score in the distinction between vascular and non-vascular transient neurologic attack. Cerebrovasc Dis. 2010;29(Suppl 2):238–239. [Google Scholar]
- 36.Hall C, Oczkowski W. Utility of the ABCD and ABCD2 scores in identifying true TIA events in the emergency department. CJEM. 2010;12(3):232. [Google Scholar]
- 37.Rosales CF, Choy LGY. Three year TIA clinic audit from a UK district general hospital. Cerebrovasc Dis. 2015;39(Suppl 2):173. [Google Scholar]
- 38.Hjertholm P, Moth G, Ingeman ML, Vedsted P. Predictive values of GPs’ suspicion of serious disease: a population-based follow-up study. Br J Gen Pract. 2014. DOI: https//doi.org/10.3399/bjgp14X680125. [DOI] [PMC free article] [PubMed]
- 39.Astin M, Griffin T, Neal RD, et al. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011. DOI: https//doi.org/10.3399/bjgp11X572427. [DOI] [PMC free article] [PubMed]
- 40.Shapley M, Mansell G, Jordan JL, Jordan KP. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010. DOI: https//doi.org/10.3399/bjgp10X515412. [DOI] [PMC free article] [PubMed]
- 41.Lewis G, Curry N, Bardsley M. Choosing a predictive risk model: a guide for commissioners in England. London: Nuffield Trust; 2011. https://www.nuffieldtrust.org.uk/files/2017-01/choosing-predictive-risk-model-guide-for-commissioners-web-final.pdf (accessed 19 Oct 2017) [Google Scholar]
- 42.Giles MF, Rothwell PM. Substantial underestimation of the need for outpatient services for TIA and minor stroke. Age Ageing. 2007;36(6):676–680. doi: 10.1093/ageing/afm088. [DOI] [PubMed] [Google Scholar]
- 43.Bos MJ, van Rijn ME, Witteman JM, et al. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877–2885. doi: 10.1001/jama.298.24.2877. [DOI] [PubMed] [Google Scholar]
- 44.Tuna MA, Li L, Tornada A, et al. The 12-year risk of stroke and coronary events after focal and non-focal transient neurological attacks: a population-based study. Eur Stroke J. 2016;1(1 Suppl):760. [Google Scholar]
- 45.Tuna MA, Tornada A, Li L, et al. Short and long-term risk of stroke after a specialist diagnosis of TIA/minor stroke mimic: a population-based study. Int J Stroke. 2015;10(Suppl 2):66. [Google Scholar]
- 46.Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388(10042):365–375. doi: 10.1016/S0140-6736(16)30468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]