Abstract
Cimaglermin alfa (GGF2) is a recombinant human protein growth factor in development for heart failure. Phase I trials were suspended when two cimaglermin alfa‐treated subjects experienced concomitant elevations in serum aminotransferases and total bilirubin, meeting current US Food and Drug Administration criteria for a serious liver safety signal (i.e., “Hy's Law”). We assayed mechanistic biomarkers in archived clinical trial serum samples which confirmed the hepatic origin of the aminotransferase elevations in these two subjects and identified apoptosis as the major mode of hepatocyte death. Using a mathematical model of drug‐induced liver injury (DILIsym) and a simulated population, we estimated that the maximum hepatocyte loss in these two subjects was <13%, which would not result in liver dysfunction sufficient to significantly increase serum bilirubin levels. We conclude that the two subjects should not be considered Hy's Law cases and that mechanistic biomarkers and modeling can aid in refining liver safety risk assessment in clinical trials.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ In clinical trials, subjects experiencing rises in serum aminotransferases and bilirubin above certain thresholds are considered “Hy's Law cases”—the current gold standard liver safety concern. The assumption is that serum bilirubin rises due to global liver dysfunction secondary to hepatocyte death.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Can mechanistic biomarkers and mathematical modeling determine the extent of hepatocyte loss and resultant impact on serum bilirubin in two cimaglermin alfa‐treated subjects who met Hy's Law Criteria?
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ Mechanistic biomarkers and mathematical modeling determined the maximum hepatocyte loss experienced by the two subjects would not cause a loss of global liver function sufficient to account for a significant rise in serum bilirubin. Hence, these subjects shouldn't be considered typical Hy's Law cases.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ This study illustrates the capability of mechanistic biomarkers and mathematical modeling to improve liver safety risk assessment by differentiating a toxic event from a more complex interference with liver function that may be reversible.
Cimaglermin alfa is a recombinant version of naturally occurring glial growth factor 2 (GGF2). Cimaglermin alfa acts at the cellular level on cardiomyocytes and neurons by interacting with the epidermal growth factor receptor family members ERBB3 and ERBB4. In phase I clinical trials, dose‐related improvements in cardiac function were observed in patients with heart failure following a single administration of cimaglermin alfa. However, clinical trials were suspended when two cimaglermin alfa‐treated subjects experienced concomitant elevations in serum aminotransferases and bilirubin, meeting the stopping criteria outlined in the 2009 US Food and Drug Administration (FDA) Guidance on Drug‐Induced Liver Injury (DILI).1
The elevated liver chemistries in both subjects returned to near baseline within 2 weeks and long‐term follow‐up demonstrated no subsequent evidence of hepatic dysfunction. However, both subjects experienced aminotransferase levels >3× upper limits of normal (ULN) and total bilirubin >2× ULN. Because there was no other evident cause for these events, these subjects fit the criteria for “Hy's Law,” a term coined by Dr. Bob Temple of the FDA based on Dr. Hy Zimmerman's observation that hepatocellular injuries sufficient to impair bilirubin excretion are capable of causing potentially lethal consequences, i.e., at least a 10% rate of liver failure. Even a single “Hy's Law case” in a clinical trial database can result in termination of drug development programs or failure to receive regulatory approval for marketing.2
Hy's Law cases typically present with increases in serum aminotransferases (due to release during hepatocyte death) preceding and rising to a much greater extent than elevations in total serum bilirubin. This is consistent with the assumption that a large fraction of total hepatocytes must die before there is sufficient reduction in global liver function to result in a rise in serum bilirubin. The two putative Hy's Law cases observed in the cimaglermin alfa clinical trials did not fit this profile, as total (and conjugated) bilirubin rose concurrently with, and to a similar fold change as, serum aminotransferases. Thus, the two putative cases differed from typical Hy's Law cases in which aminotransferase elevations occur first and are followed by a smaller elevation in serum bilirubin.
Novel hepatic safety biomarkers such as microRNA‐122 (miR‐122), glutamate dehydrogenase (GLDH), caspase‐cleaved cytokeratin‐18 (cc‐K18), and full length keratin‐18 (FL‐K18) have potential clinical utility, alongside traditional serum biomarkers, in assessing hepatotoxicity.3 miR‐122 and GLDH are liver‐specific biomarkers released during hepatocyte death. Keratin 18 (K18) is abundantly expressed in epithelial cells including hepatocytes. FL‐K18 is passively released from necrotic cells. In contrast, during apoptosis, caspase cleavage of K18 occurs resulting in cc‐K18 release from apoptotic cells after loss of membrane integrity. The ratio of cc‐K18 to FL‐K18 (the “apoptotic index”) has been proposed to estimate the relative contributions of necrosis and apoptosis to cell death.4 Macrophage colony‐stimulating factor (CSF‐1) represents a proregenerative biomarker that has been shown to increase following partial hepatectomy in mice and humans and to correlate with survival in acetaminophen‐induced hepatotoxicity.5
Recently, the FDA identified the development and use of computational models as an important tool for assessing product safety.6 Mechanistic, mathematical modeling can facilitate the interpretation of liver safety biomarker data by incorporating biomarker release and clearance kinetics and linking back the results to mechanisms underlying DILI. Previous work demonstrated the successful application of DILIsym, a mechanistic model of DILI, to aid in the interpretation of serum aminotransferase (but not bilirubin) elevations observed in healthy volunteers in a phase I clinical study.7
In the current study, serial serum samples collected from phase I clinical trial subjects were assayed for miR‐122, cc‐K18, FL‐K18, GLDH, and CSF‐1. DILIsym software8, 9, 10, 11 was used to interpret the kinetics of these biomarkers along with the traditional liver chemistries that had been measured in the trials. Findings from this study indicate that the amount of hepatocyte loss that likely occurred during treatment with cimaglermin alfa (<13%) would be significantly less than that required to cause the observed elevations in serum bilirubin. Therefore, factors other than hepatocyte death likely underlie the rise in serum bilirubin in cimaglermin alfa‐treated subjects. Our data suggest that the concomitant elevations in serum aminotransferases and total bilirubin observed in the two subjects treated with cimaglermin alfa were not indicative of typical Hy's Law cases that would identify severe liver injury.
RESULTS
Liver function test abnormalities in cimaglermin alfa‐treated subjects
Two phase I studies of cimaglermin alfa were conducted in patients with heart failure. In the first study, an elevation in serum aminotransferases and total and conjugated bilirubin was encountered in the 40th subject who received the highest planned dose of 1.5 mg/kg (Table 1). In the second study, the 22nd of 28 planned subjects who received the highest planned dose of 0.38 mg/kg also experienced elevations in serum aminotransferases and total and conjugated serum bilirubin (Table 2). In both cases, the biochemical abnormalities returned to near baseline within 2 weeks; long‐term follow‐up demonstrated no subsequent evidence of hepatic injury or dysfunction. However, both subjects experienced aminotransferase levels >3× ULN and total bilirubin >2× ULN with no other obvious cause than cimaglermin alfa, thus satisfying Hy's Law criteria, and further development of cimaglermin alfa was put on hold.
Table 1.
Liver chemistries for subject meeting Hy's Law criteria in phase Ia trial
| Study day | Actual values | Multiples of ULNa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
AST (U/L) |
ALT (U/L) |
DBIL (mg/dL) |
TBIL (mg/dL) |
ALP (U/L) |
INR |
AST (41) |
ALT (63) |
DBIL (0.3) |
TBIL (1.2) |
ALP (126) |
|
| −16b | 42 | 40 | 0.3 | 1.1 | 50 | 1 | 1.0 | 0.6 | 1.0 | 0.9 | 0.4 |
| −1c | 42 | 30 | 0.4 | 1.2 | 53 | 1 | 1.0 | 0.5 | 1.3 | 1.0 | 0.4 |
| 0 | 57 | 33 | 0.5 | 1.0 | 50 | ND | 1.4 | 0.5 | 1.7 | 0.8 | 0.4 |
| 1 | 32 | 27 | 0.4 | 1.1 | 49 | 1 | 0.8 | 0.4 | 1.3 | 0.9 | 0.4 |
| 2 | 227 | 220 | ND | 4.6 | 94 | ND | 5.5 | 3.5 | ND | 3.8 | 0.7 |
| 3 | 243 | 233 | ND | 4.8 | 103 | ND | 5.9 | 3.7 | ND | 4.0 | 0.8 |
| 4 | 184 | 216 | 3.8 | 6.3 | 143 | ND | 4.5 | 3.4 | 12.7 | 5.2 | 1.1 |
| 8 | 62 | 130 | 0.8 | 2.0 | 146 | 1.1 | 1.5 | 2.1 | 2.7 | 1.7 | 1.2 |
| 15 | 45 | 77 | 0.4 | 1.3 | 108 | ND | 1.1 | 1.2 | 1.3 | 1.1 | 0.9 |
| 21 | 49 | 60 | 0.3 | 1.3 | 73 | 1.2 | 1.2 | 1.0 | 1.0 | 1.1 | 0.6 |
| 117 | 32 | 30 | 0.2 | 0.8 | 50 | ND | 0.8 | 0.5 | 0.7 | 0.7 | 0.4 |
AST, aspartate transaminase; ALT, alanine transaminase; DBIL, direct bilirubin; TBIL, total bilirubin; ALP, alkaline phosphatase; INR, international normalized ratio; ULN, upper limit of normal; ND, not determined.
Numbers in parentheses are the upper limits of normal (ULN).
Screening.
Baseline.
Table 2.
Liver chemistries for subject meeting Hy's Law criteria in phase Ib trial
| Study day | Actual values | Multiples of ULNa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
AST (U/L) |
ALT (U/L) |
DBIL (mg/dL) |
TBIL (mg/dL) |
ALP (U/L) |
INR |
AST (35) |
ALT (60) |
DBIL (0.5) |
TBIL (1.2) |
ALP (125) |
|
| −14b | 48 | 56 | 0.3 | 1.5 | 110 | 1.4 | 1.4 | 0.9 | 0.6 | 1.3 | 0.9 |
| −2c | 55 | 69 | 0.3 | 1.3 | 114 | 1.3 | 1.6 | 1.2 | 0.6 | 1.1 | 0.9 |
| −1c | 53 | 65 | 0.2 | 1.1 | 113 | 1.3 | 1.5 | 1.1 | 0.4 | 0.9 | 0.9 |
| 0Ad | 49 | 64 | 0.3 | 1.2 | 111 | 1.3 | 1.4 | 1.1 | 0.6 | 1.0 | 0.9 |
| 0Bd | 48 | 64 | 0.4 | 1.6 | 114 | 1.3 | 1.4 | 1.1 | 0.8 | 1.3 | 0.9 |
| 1Ad | 142 | 106 | 1.2 | 3.2 | 137 | 1.5 | 4.1 | 1.8 | 2.4 | 2.7 | 1.1 |
| 1Bd | 150 | 112 | 2.2 | 4.8 | 139 | ND | 4.3 | 1.9 | 4.4 | 4.0 | 1.1 |
| 2Ad | 169 | 130 | 3.5 | 6.4 | 160 | 1.6 | 4.8 | 2.2 | 7.0 | 5.3 | 1.3 |
| 2Bd | 181 | 142 | 2.7 | 5.2 | 176 | 1.5 | 5.2 | 2.4 | 5.4 | 4.3 | 1.4 |
| 3 | 170 | 145 | 1.9 | 3.9 | 201 | 1.3 | 4.9 | 2.4 | 3.8 | 3.3 | 1.6 |
| 4 | 113 | 132 | 1.0 | 2.6 | 209 | 1.2 | 3.2 | 2.2 | 2.0 | 2.2 | 1.7 |
| 5 | 90 | 122 | 0.8 | 2.3 | 239 | 1.1 | 2.6 | 2.0 | 1.6 | 1.9 | 1.9 |
| 9 | 80 | 92 | 0.3 | 1.3 | 117 | 1.1 | 2.3 | 1.5 | 0.6 | 1.1 | 1.4 |
| 16 | 90 | 117 | 0.2 | 0.8 | 161 | 1 | 2.6 | 2.0 | 0.4 | 0.7 | 1.3 |
| 23 | 61 | 93 | 0.1 | 0.6 | 131 | 1 | 1.7 | 1.6 | 0.2 | 0.5 | 1.0 |
| 42 | 47 | 71 | 0.1 | 0.7 | 97 | 1.1 | 1.3 | 1.2 | 0.2 | 0.6 | 0.8 |
| 86 | 41 | 58 | 0.2 | 0.9 | 104 | 1.1 | 1.2 | 1.0 | 0.4 | 0.8 | 0.8 |
AST, aspartate transaminase; ALT, alanine transaminase; DBIL, direct bilirubin; TBIL, total bilirubin; ALP, alkaline phosphatase; INR, international normalized ratio; ULN, upper limit of normal; ND, not determined.
Numbers in parentheses are the upper limits of normal (ULN).
Screening.
Baseline.
A and B designate two samples taken at different times on the same study day.
Hy's Law cases typically present as an increase in serum aminotransferases (due to hepatocyte necrosis) preceding and rising to a greater extent than elevations of total serum bilirubin (due to liver dysfunction). However, the pattern of enzyme elevations observed in the two subjects described above did not fit this profile, as total (and conjugated) bilirubin was elevated concurrently and to a similar extent as serum aminotransferases (Tables 1, 2).
Hepatic safety biomarker analysis
Five investigational safety biomarkers were analyzed in serial serum samples archived from 12 subjects included in the phase I cimaglermin alfa clinical studies (see Supplementary Table S1 for subject demographics). The subjects whose samples were analyzed include the two putative Hy's Law cases as well as others with elevated serum aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels, and two placebo‐treated subjects with no AST/ALT elevations. Time‐dependent elevations in levels of serum biomarkers specifically associated with liver injury, miR‐122 (Figure 1 a) and GLDH (Figure 1 b), were observed in four subjects, including the two subjects meeting Hy's Law criteria (Subjects 8 and 12 in Figure 1). The maximal observed values for miR‐122 and GLDH were comparable to those previously reported in healthy adults receiving therapeutic doses of acetaminophen12 and heparins13 and are, on average, 25× lower than elevations seen for serious DILI induced by acetaminophen overdose.14 In all subjects, the increase in miR‐122 returned to baseline values and GLDH appeared to be returning to baseline levels at the final measurement on Day 28.
Figure 1.
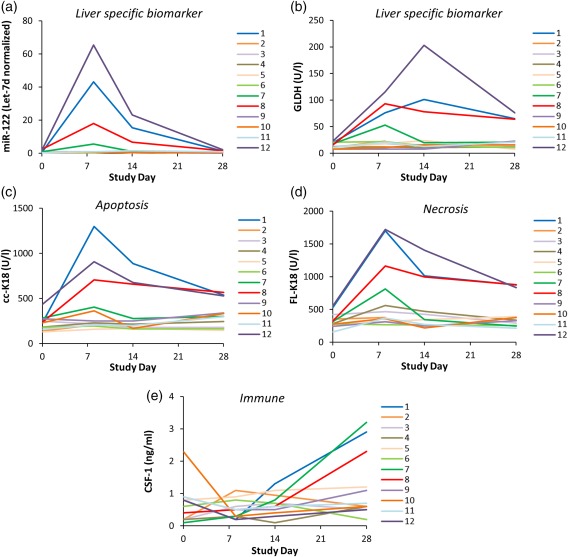
Serum levels of liver safety biomarkers (a) microRNA‐122 (miR‐122), (b) glutamate dehydrogenase (GLDH), (c) caspase‐cleaved fragment of K18 (cc‐K18), (d) full length keratin‐18 (FL‐K18), and (e) macrophage colony stimulating factor (CSF‐1). Serum samples were collected at four different timepoints relative to cimaglermin alfa (or placebo) administration from 12 patients who participated in phase I clinical trials including two patients which met FDA drug‐induced liver injury (DILI) guidance stopping criteria (Subjects 8 and 12), as well as others with elevated serum aminotransferases that fell short of meeting FDA DILI guidance stopping criteria (Subjects 1, 2, 3, 4, 7, 9, 10, 11), and two placebo‐dosed individuals (Subjects 5 and 6).
Elevated levels of cc‐K18 (Figure 1 c) and FL‐K18 (Figure 1 d) were also observed in the same subjects that experienced elevations in miR‐122 and GLDH. The time course of the elevations was similar to that of miR‐122 and appeared to be returning to baseline levels at the final measurement. The absolute values for the increase in both M30 (cc‐K18) and M65 (cc‐K18 and FL‐K18) were higher than previously reported in healthy adult volunteers receiving recurrent therapeutic doses of acetaminophen12 but were significantly lower than those observed in patients with serious DILI due to acetaminophen overdose.15 The mathematical ratio of M30 to M65 is termed the “apoptotic index” and has been recently proposed to estimate the relative proportions of cell death by apoptosis and necrosis.4 Across all subjects with elevated cc‐K18 and FL‐K18, the apoptotic index (calculated using peak cc‐K18 and peak FL‐K18 values for each subject) ranged from 0.53–0.76, suggesting that apoptosis was the major form of cell death.
In three subjects (including one of the putative Hy's law cases and another subject with elevated miR‐122 and GLDH), an increase in the proregenerative biomarker CSF‐1 was observed (Figure 1 e). The absolute values for the increase in CSF‐1 was lower than previously reported postoperative increases in serum CSF‐1 in partial hepatectomy patients.5
Simulations in the baseline human in DILIsym
The baseline human in DILIsym represents a typical normal, healthy volunteer. We performed simulations in the baseline human, using the hepatocyte life cycle and liver injury biomarker submodels in DILIsym (Figure 2), to investigate the percent hepatocyte loss associated with plasma ALT elevations in the four subjects with the highest elevations in serum aminotransferases, including the two Hy's Law cases. As described above, mechanistic biomarkers supported apoptosis as the major mode of cimaglermin alfa‐induced hepatocyte death. With apoptosis as the mode of cell death, the simulated and observed serum ALT, AST, and total bilirubin profiles for the four cimaglermin alfa subjects are presented in Figure 3. Clinical data and simulation results are also shown for the placebo‐treated subject (Figure 3). For comparison, the results with necrosis as the mode of simulated cell death are shown in Supplementary Figure S1. There was good agreement between simulated ALT profiles and clinical data; this was by design, as plasma ALT data from the clinical trial subjects were employed for optimization. Plasma AST levels, which were not used for optimization, were also simulated and compared favorably with the clinical data (Figure 3 b, Supplementary Figure S1). These results confirmed that the representation of biomarkers (i.e., ALT and AST) and the hepatocyte life cycle within DILIsym reasonably recapitulated clinically observed data.
Figure 2.
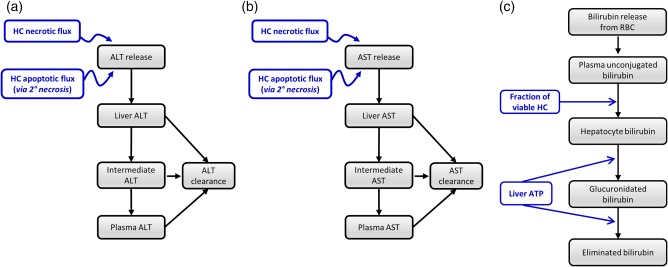
Diagrams of serum alanine transaminase (ALT) (a), serum aspartate transaminase (AST) (b), and bilirubin (c) submodels within DILIsym version 4B.
Figure 3.
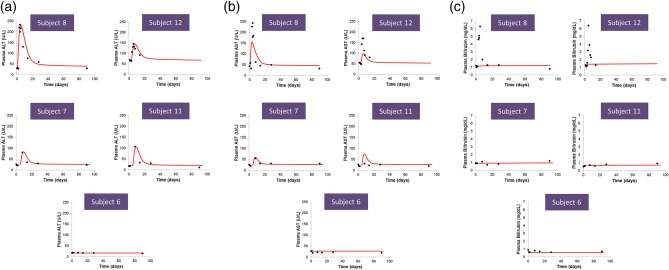
Observed and simulated plasma levels of (a) alanine transaminase (ALT), (b) aspartate transaminase (AST), and (c) bilirubin for the four cimaglermin alfa‐treated subjects and the placebo‐treated subject included in the DILIsym analyses. Subjects included in the DILIsym analyses consist of two subjects who met FDA DILI guidance stopping criteria (Subjects 8 and 12), as well as two subjects with elevated serum aminotransferases who fell short of meeting FDA DILI guidance stopping criteria (Subjects 7 and 11), and one placebo‐treated subject (Subject 6). Subjects 6, 7, 8, 11, and 12 were also included in the set of 12 subjects included in the serum biomarker analysis (Figure 1). Simulations on the baseline human in DILIsym were performed assuming 100% apoptosis as the cell death modality. (Analogous simulation results with 100% necrosis as the cell death modality are shown in Supplementary Figure S1.) The ALT model was optimized to the cimaglermin alfa ALT clinical data. AST and bilirubin data were not used for the optimization; plasma AST and bilirubin levels were simulated based on the amount of hepatocyte injury optimized to ALT dynamics. Closed black circles represent clinical data. Solid red lines represent simulated results due to 100% apoptosis.
The plasma bilirubin levels were simulated based on the predicted viable hepatocyte mass and compared to the clinical data (clinical bilirubin data was not used for the optimization). No significant rise in bilirubin was predicted in the baseline human simulations for the four cimaglermin alfa‐treated subjects with the highest serum aminotransferases levels (Figure 3 c). The lack of bilirubin elevations in the DILIsym simulations is not consistent with the marked rise in serum bilirubin observed in the two cimaglermin alfa‐treated subjects who met Hy's Law criteria. In the simulations, hepatocyte loss of ∼60% was necessary for total bilirubin to reach 6 mg/dL (i.e., the approximate bilirubin levels observed in both subjects who met Hy's Law criteria). While simulated bilirubin levels of 6 mg/dL could be achieved in simulations of such substantial increases in hepatocyte loss, the observed bilirubin kinetics could not be reproduced in the simulations (simulated time to peak bilirubin was several weeks, while observed peak bilirubin levels occurred within several days).
Predicted hepatocyte loss in the baseline human simulations for the cimaglermin alfa‐treated subjects ranged from 6–9% when apoptosis was the mechanism of hepatocyte death. Lower levels of cell loss were predicted when necrosis was the mode of hepatocyte death (1–5%). Predicted hepatocyte loss for the placebo‐treated subject was 0%.
Hepatocyte loss in a simulated population (SimPops)
To estimate what peak percent hepatocyte loss could have occurred if interpatient variability is considered, we explored a simulated patient population (SimPops) which included variation in parameters relevant to ALT dynamics and hepatocyte regeneration (Supplementary Table S2). Population analyses were restricted to plasma ALT <500 U/L, or 2‐fold higher than the maximum serum ALT observed to date in cimaglermin alfa‐treated patients (233 IU/L). Estimated ranges of hepatocyte loss at different plasma peak ALT levels over the representative time frame of ALT elevations are presented in Table 3 and Figure 4. For the representative cimaglermin alfa subject ALT dynamic profile, lower estimated cell loss was again predicted for 100% apoptosis (Table 3, Figure 4 a) vs. 100% necrosis (Table 3, Figure 4 b). At the maximum plasma peak ALT observed in cimaglermin alfa‐treated patients (233 IU/L), the estimated hepatocyte loss range was 6.6–12.4% with apoptosis as the cell death modality and 2.7–7.8% with necrosis as the cell death modality (Table 3). No bilirubin elevations were predicted for this range of estimated hepatocyte loss (i.e., up to 12.4%).
Table 3.
Range of estimated (simulated) hepatocyte loss as a function of observed plasma peak alanine transaminase (ALT) range in phase I cimaglermin alfa‐treated subjects with 100% apoptosis or 100% necrosis as the cell death modality
| Peak ALT range (U/L) |
Hepatocyte loss (%) estimated with 100% apoptosis (lower bound – upper bound) |
Hepatocyte loss (%) estimated with 100% necrosis (lower bound – upper bound) |
|---|---|---|
| 1–100 | 0.0–7.0 | 0.0–2.8 |
| 101–200 | 4.9–9.9 | 2.7–6.2 |
| 201–300 | 6.6–12.4 | 2.7–7.8 |
Figure 4.
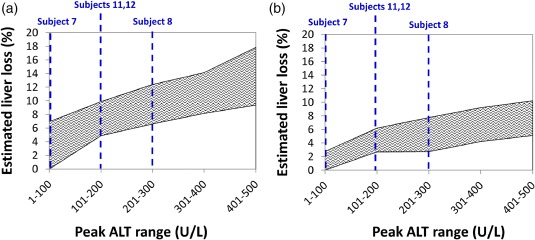
Range of estimated hepatocyte loss as a function of observed plasma peak alanine transaminase (ALT) range in cimaglermin alfa subjects for two different cell death modalities (100% apoptosis (a), 100% necrosis (b)). Dashed lines show the corresponding peak ALT bins for each of the four specific cimaglermin alfa phase I subjects. The five subjects included in the DILIsym analyses consist of two subjects whom met FDA DILI guidance stopping criteria (Subjects 8 and 12), as well as two subjects with elevated serum aminotransferases who fell short of meeting FDA DILI guidance stopping criteria (Subjects 7 and 11), and one placebo‐treated subject (Subject 6). Subjects 6, 7, 8, 11, and 12 were also included in the set of 12 subjects included in the serum biomarker analysis (Figure 2).
The time required to regenerate lost hepatocytes was also assessed in the SimPops for individuals losing up to 13% of hepatocytes (simulated peak ALT levels <300 U/L). Following liver loss of 13%, the recovery time to reach 95% viable liver mass ranged from 13–42 weeks (Supplementary Figure S2).
DISCUSSION
DILI can have a major impact on clinical drug development programs. When liver safety concerns arise for new drug candidates, development may be terminated or larger and longer clinical trials may be required, increasing costs and potentially delaying patient access to new medications.16 Cimaglermin alfa is in development for the treatment of chronic heart failure. The observation of two cimaglermin alfa‐treated subjects meeting current criteria for Hy's Law cases resulted in a hold placed on the cimaglermin alfa clinical development program due to liver safety concerns. Here we utilized investigational, mechanistic liver safety biomarkers in conjunction with mathematical modeling to refine the interpretation of clinical safety data from phase I clinical studies of cimaglermin alfa.
Our observation that elevations in serum aminotransferases were accompanied by elevations in miR122 and GLDH confirmed liver origin of the enzymes. The apoptotic index (cc‐K18/FL‐K18) supported apoptosis as the major mode of cell death. DILIsym modeling in a simulated population of patients encompassing variability in relevant parameters predicted that the maximum percent hepatocyte loss was <13% in patients completing the phase I clinical trials. The estimated peak values for hepatocyte loss are in the range of what has been estimated to occur as a result of asymptomatic and reversible elevations in serum aminotransferases observed in healthy adults during recurrent therapeutic doses of acetaminophen12 or heparins13 rather than clinically important hepatotoxicity. It is of note that both of the putative Hy's Law cases did experience symptoms at the time of the biochemical abnormalities; one experienced nausea and abdominal pain and the other experienced flu‐like symptoms.
Published data also suggest that minimal changes in liver function are expected at the level of hepatocyte loss we estimated for cimaglermin alfa patients. For example, in the early 1980s liver biopsies were performed acutely on ∼70 acetaminophen overdose patients.17 Substantial elevations in serum prothrombin time and peak bilirubin levels were observed only when reductions in the estimated viable hepatocyte volume fraction exceeded ∼25% (i.e., changes in function were not evident for cell loss less than ∼25%).17 Overall, these data suggest that the maximum hepatocyte loss estimated using serum ALT data from cimaglermin alfa phase I subjects was less than the extent necessary to detectably interfere with liver function.
Further, there were no increases in simulated serum bilirubin levels for the predicted hepatocyte loss in the two putative Hy's Law cases, while serum levels of bilirubin exceeding 5× ULN were observed. The inconsistencies between simulated bilirubin levels and bilirubin clinical data, and the observation that the clinical elevations were largely conjugated bilirubin, would be consistent with the hypothesis that a transient selective defect in bilirubin transport rather than global liver dysfunction was responsible for the rise in serum bilirubin in the cimaglermin alfa‐treated subjects who met Hy's Law biochemical criteria. This hypothesis is supported by recent in vitro data demonstrating transient cimaglermin alfa‐mediated effects on hepatic physiology related to bilirubin and bile acid transport.18 In that study, global gene expression profiling in primary human hepatocytes exposed to cimaglermin alfa identified ∼50% reductions in bilirubin transporters and bile acid conjugating enzymes that may impact the biliary clearance of conjugated bilirubin.
One limitation of the current study is that the percent hepatocyte loss estimated by DILIsym has not been validated experimentally. A second limitation is a lack of experimental data to assess potential impacts of cimaglermin alfa on hepatocellular ATP levels. Given the absence of such data, ATP levels were not varied in the simulations. Another limitation is that the patients included in the phase I studies received a single dose of cimaglermin alfa, while chronic therapy would be required in the target population. In addition, in the current study DILIsym was not parameterized for heart failure patients. While liver function test (LFT) abnormalities are common in heart failure patients, little is currently known about the effects of heart failure on hepatic function.19, 20, 21 In the current study the subjects had normal baseline LFTs. Reduced baseline hepatic function may be more likely in heart failure patients with abnormal baseline LFTs. Hepatocyte loss of 13% may be more clinically meaningful in patients with impaired liver function prior to treatment with cimaglermin alfa.
In conclusion, the mechanistic biomarker data and DILIsym analyses, suggest that less than 13%, primarily apoptotic, hepatocyte loss occurred in cimaglermin alfa‐treated subjects with serum liver enzyme elevations in phase I clinical trials. This level of hepatocyte loss is not consistent with global liver dysfunction leading to the rise in serum bilirubin. It therefore seems likely that the rise in serum bilirubin observed in the two subjects was due to transient defects in bilirubin transport rather than loss of functional hepatocytes. Therefore, these two cases do not represent typical Hy's Law cases and should not be interpreted as necessarily indicating that cimaglermin alfa has a serious liver injury potential. These findings suggest that mechanistic hepatic safety biomarkers, combined with mathematical modeling, can improve the interpretation of traditional liver safety signals. To fully understand both the value and limitations of these novel approaches, broad application to clinical trials is necessary. Efforts are currently under way by several groups, including the SAFE‐T (Safer and Faster Evidence‐based Translation) consortium, the IMI (Innovative Medicines Initiative), and the DILI‐sim Initiative, to understand how mechanistic biomarkers and modeling can improve the assessment of liver injury.
METHODS
Cimaglermin alfa phase I studies
Clinical development of cimaglermin alfa was initiated with Study NCT01258387: “A Phase 1, Double‐Blind Placebo‐Controlled Study of Single Ascending Doses of GGF2 in Patients with Left Ventricular Dysfunction and Symptomatic Heart Failure.” This study was designed to evaluate the safety, tolerability, pharmacokinetics, and immunogenicity of single administrations of cimaglermin alfa in patients with systolic left ventricular dysfunction and symptomatic heart failure. Cimaglermin alfa or placebo was administered as a single intravenous infusion to 40 subjects. Doses started at 0.007 mg/kg cimaglermin alfa and escalations were planned up to 1.512 mg/kg cimaglermin alfa. Subjects were hospitalized from Day –1 to Day 2 with safety tests and evaluations conducted at various timepoints throughout the study. The following serum liver chemistries were measured using standard assays on a clinical chemistry analyzer: ALT, AST, direct bilirubin, total bilirubin, and alkaline phosphatase. Prothrombin time was also measured and used to calculate international normalized ratio (INR). Subjects were discharged with instructions to return for their first postdose evaluation on Day 8 ± 2 days.
Study NCT01944683: “A Double‐Blind Pharmacokinetic Interaction Study Evaluating the Effect of a Single IV Infusion of GGF2 or Placebo on Midazolam Pharmacokinetics in Patients with Heart Failure” was designed to continue evaluating the safety and tolerability of cimaglermin alfa in patients with heart failure and to evaluate potential drug–drug interactions. The maximum of three dose levels selected for examination was 0.38 mg/kg cimaglermin alfa, a concentration 4‐fold less than the dose at which a limiting toxicity was observed previously (i.e., one subject met Hy's law criteria at the 1.512 mg/kg dose in the first study) and was selected with input from an external panel of cardiologists and hepatologists. Subjects were hospitalized from Day –1 to Day 8, and daily assessments with safety laboratory tests, including the liver chemistries described above, performed to assess for evidence of hepatotoxicity and other biochemical abnormalities. Subjects were discharged on Day 8 (5 days after receiving cimaglermin alfa) after all study procedures were completed.
Hepatic safety biomarker analyses
Hepatic safety biomarker analyses were performed on a total of 48 serum samples collected at four different timepoints (Days 0, 8, 14, 28) relative to cimaglermin alfa (or placebo) administration from 12 subjects who participated in the two phase I clinical trials. The subjects whose samples were analyzed include the two putative Hy's Law cases (Subjects 8 and 12) as well as others with elevated serum AST/ALT levels (Subjects 1, 2, 3, 4, 7, 9, 10, 11), and two placebo‐treated subjects who did not experience AST/ALT elevations (Subjects 5, 6).
Serum samples were analyzed for miR‐122, GLDH, FL‐K18, cc‐K18, and CSF‐1. miR‐122, GLDH, and K18 isoforms M30 (cc‐K18) and M65 (both cc‐K18 and FL‐K18) were quantified as previously described.22 CSF‐1 was analyzed as previously described.5
DILIsym version 4B
DILIsym is a mechanistic, mathematical model of DILI (DILIsym Services Inc., http://www.dilisym.com),8, 9, 10, 11 developed and maintained through the DILI‐sim Initiative, a public‐private partnership involving scientists in academia, industry, and the FDA. In the current study, DILIsym version 4B was utilized to estimate the amount of hepatocyte loss in cimaglermin alfa‐treated subjects with elevated ALT/AST, and to simulate circulating bilirubin levels for the inferred amount of hepatocyte loss (see details in Baseline human simulations, below). DILIsym analyses were performed on the four clinical trial subjects with the highest elevations in serum aminotransferases, including the two subjects meeting Hy's Law criteria.
ALT, AST, and bilirubin submodels of DILIsym version 4B
The current work utilized the hepatocyte life cycle and liver injury biomarker submodels of DILIsym version 4B. The ALT submodel is shown in Figure 2 a. Plasma ALT levels in DILIsym are determined by the rates of hepatocyte injury and plasma ALT clearance. When hepatocyte death occurs via primary necrosis, cellular ALT is released and enters the circulation. ALT is also released during apoptosis, except when apoptosis occurs at very low levels (see details in the Supplementary Information). The ALT submodel consists of three compartments. The Liver compartment represents temporary association of ALT with cells as necrosis or apoptosis occurs. The Plasma compartment represents systemic circulation. Creation of the Intermediate compartment was required to optimize serum ALT dynamics observed in clinical response to several hepatotoxic compounds15, 23, 24, 25 and may represent the ALT in the interstitial space, the Space of Disse, or the liver sinusoids. The clearance of ALT is driven by a specified half‐life.26 Clearance of ALT occurs in all three compartments, as the exact mechanism and locations of liver enzyme clearance is not yet elucidated.27, 28 Hepatocyte levels of ALT taken from the literature were used to represent the amount of ALT released per necrotic or apoptotic cell.29, 30
The AST submodel structure within DILIsym version 4B is similar to the ALT submodel structure (Figure 2 b). AST is also cleared with a specified half‐life.26 Hepatocellular release of AST occurs with necrosis and apoptosis, as described above for ALT. Cellular levels of AST determined from the literature have been used to represent the amount of AST released per necrotic or apoptotic cell.31, 32
Changes in plasma bilirubin levels in DILIsym version 4B are determined by alterations in its hepatic clearance, as the formation of bilirubin from red blood cells is constant and dictated by homeostatic levels (Figure 2 c). In DILIsym version 4B, bilirubin clearance depends on the fraction of viable hepatocytes (as well as the level of hepatocellular ATP, which was not varied in the current study). Bilirubin model parameters were optimized using clinical data from the literature.17, 33, 34
Supplementary Table S3 shows the parameter values for the ALT, AST, and bilirubin submodels for the baseline human in DILIsym version 4B.
Baseline human simulations
DILIsym was used to approximate the ALT elevations observed in phase I clinical studies following treatment with cimaglermin alfa by simulating dynamic changes in liver necrosis or apoptosis in the baseline human. For each cell death modality (necrosis or apoptosis), the amount of cell death was optimized (individually for each subject) to achieve plasma ALT time‐course profiles that are similar to the observed ALT profiles for each subject. The hepatocellular mechanisms [drug exposure, hepatotoxicity mechanisms, adenosine triphosphate (ATP) depletion, etc.] leading up to cell loss were not considered herein. Instead, the method for simulating hepatocellular loss was an empirical, direct necrosis or direct apoptosis signal.
Assessing variability with a simulated human population (SimPops)
To account for the interindividual variability in ALT response and hepatocyte regeneration from a given level of hepatocyte loss, a simulated human population (SimPops) that consists of 300 healthy individuals was employed. In this SimPops, variability was introduced in the parameters relevant to ALT dynamics and hepatocyte regeneration. The range and variance of each parameter was obtained from the literature, and simulated hepatocyte loss and plasma ALT levels in the SimPops were validated using clinical data from the literature.17, 24 A list of parameters varied and their upper and lower bounds in the SimPops are presented in Supplementary Table S2. The SimPops was used for subsequent simulations of necrosis and apoptosis‐induced increases in circulating ALT.
CONFLICT OF INTEREST
D.M.L., G.T.G., B.A.H., and S.Q.S. are employees of DILIsym Services Inc., a company that licenses the DILIsym software for commercial use. D.M.L., G.T.G., B.A.H., S.Q.S., and P.B.W. have equity positions in DILIsym Services Inc. and M.M. and P.B.W. have served as a paid consultant for Acorda. D.B., A.C., A.E., T.P., J.I., and R.S. are employees of Acorda Therapeutics Inc. and have equity positions in Acorda Therapeutics Inc.
AUTHOR CONTRIBUTIONS
D.M.L., G.T.G., B.A.H., S.Q.S., M.M., and P.B.W. wrote the article; D.M.L., G.T.G., B.A.H., S.Q.S., D.J.A., D.B., A.C., A.E., J.I., R.S., T.P., M.M., and P.B.W. designed the research; D.M.L., G.T.G., and D.J.A. performed the research; D.M.L., G.T.G., and D.J.A. analyzed the data.
Supporting information
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4
Supporting Information S5
Supporting Information S6
ACKNOWLEDGMENTS
The authors thank the patients and clinical staff who participated in the cimaglermin alfa clinical studies.
References
- 1. Clinical, P. Guidance for Industry Drug‐Induced Liver Injury? Premarketing Clinical Evaluation Guidance for Industry Drug‐Induced Liver Injury? Premarketing Clinical Evaluation. (2009).
- 2. Temple, R. Hy's Law: predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf. 15, 241–243 (2006). [DOI] [PubMed] [Google Scholar]
- 3. Clarke, J.I. , Dear, J.W. & Antoine, D.J. Recent advances in biomarkers and therapeutic interventions for hepatic drug safety — false dawn or new horizon? Expert Opin. Drug Saf. 15, 625–634 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Kramer, G. et al Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18 differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 64, 1751–1756 (2004). [DOI] [PubMed] [Google Scholar]
- 5. Stutchfield, B.M. et al CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology 149, 1896–1909.e14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. U.S. Food and Drug Administration . Advancing Regulatory Science at FDA. Silver Spring, MD: FDA (2011).
- 7. Howell, B.A. et al A mechanistic model of drug‐induced liver injury AIDS the interpretation of elevated liver transaminase levels in a phase I clinical trial. CPT Pharmacomet. Syst. Pharmacol. 3, e98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Longo, D.M. , Yang, Y. , Watkins, P.B. , Howell, B.A. & Siler, S.Q. Elucidating differences in the hepatotoxic potential of tolcapone and entacapone with DILIsym(®), a mechanistic model of drug‐induced liver injury. CPT Pharmacomet. Syst. Pharmacol. 5, 31–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang, K. , Woodhead, J.L. , Watkins, P.B. , Howell, B.A. & Brouwer, K.L. Systems pharmacology modeling predicts delayed presentation and species differences in bile acid‐mediated troglitazone hepatotoxicity. Clin. Pharmacol. Ther. 589–598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shoda, L.K.M. , Woodhead, J.L. , Siler, S.Q. , Watkins, P.B. & Howell, B.A. Linking physiology to toxicity using DILIsym(®), a mechanistic mathematical model of drug‐induced liver injury. Biopharm. Drug Dispos. 35, 33–49 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Woodhead, J.L. et al Exploring BSEP inhibition‐mediated toxicity with a mechanistic model of drug‐induced liver injury. Front. Pharmacol. 5, 240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thulin, P. et al Keratin‐18 and microRNA‐122 complement alanine aminotransferase as novel safety biomarkers for drug‐induced liver injury in two human cohorts. Liver Int. 1–12 (2013). [DOI] [PubMed] [Google Scholar]
- 13. Harrill, A.H. et al The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin. Pharmacol. Ther. 92, 214–220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Starkey Lewis, P.J. et al Circulating microRNAs as potential markers of human drug‐induced liver injury. Hepatolology 54, 1767–1776 (2011). [DOI] [PubMed] [Google Scholar]
- 15. Antoine, D.J. et al Molecular forms of HMGB1 and keratin‐18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 56, 1070–1079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Watkins, P.B. Drug safety sciences and the bottleneck in drug development. Clin. Pharmacol. Ther. 89, 788–790 (2011). [DOI] [PubMed] [Google Scholar]
- 17. Portmann, B. et al Histopathological changes in the liver following a paracetamol overdose: correlation with clinical and biochemical parameters. J. Pathol. 117, 169–181 (1975). [DOI] [PubMed] [Google Scholar]
- 18. Mosedale, M. Transient changes in hepatic physiology impacting bilirubin and bile acid transport may help explain the elevations in liver chemistries observed in clinical trials of GGF2 (Cimaglermin alpha). Soc. Toxicol. Annu. Meet. (submitted) (2016). [DOI] [PubMed] [Google Scholar]
- 19. Vyskocilova, K. et al Prevalence and clinical significance of liver function abnormalities in patients with acute heart failure. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 159, 429–436 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Poelzl, G. et al Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur. J. Clin. Invest. 42, 153–163 (2012). [DOI] [PubMed] [Google Scholar]
- 21. Samsky, M.D. et al Cardiohepatic interactions in heart failure: an overview and clinical implications. J. Am. Coll. Cardiol. 61, 2397–2405 (2013). [DOI] [PubMed] [Google Scholar]
- 22. Antoine, D.J. et al Mechanistic biomarkers provide early and sensitive detection of acetaminophen‐induced acute liver injury at first presentation to hospital. Hepatolology 58, 777–787 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiødt, F.V. , Rochling, F.A. , Casey, D.L. & Lee, W.M. Acetaminophen toxicity in an urban county hospital. N. Engl. J. Med. 337, 1112–1117 (1997). [DOI] [PubMed] [Google Scholar]
- 24. Prescott, L.F. et al Intravenous N‐acetylcystine: the treatment of choice for paracetamol poisoning. Br. Med. J. 2, 1097–1100 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singer, A.J. , Carracio, T.R. & Mofenson, H.C. The temporal profile of increased transaminase levels in patients with acetaminophen‐induced liver dysfunction. Ann. Emerg. Med. 26, 49–53 (1995). [DOI] [PubMed] [Google Scholar]
- 26. Nicoll, D. , Lu, C.M. , Pignone, M. & McPhee, S.J. Pocket Guide to Diagnostic Tests, Sixth Edition (McGraw‐Hill Medical, New York; 2012). [Google Scholar]
- 27. Smit, M.J. , Duursma, A.M. , Bouma, J.M. & Gruber, M. Receptor‐mediated endocytosis of lactate dehydrogenase M4 by liver macrophages: a mechanism for elimination of enzymes from plasma. Evidence for competition by creatine kinase MM, adenylate kinase, malate, and alcohol dehydrogenase. J. Biol. Chem. 262, 13020–13026 (1987). [PubMed] [Google Scholar]
- 28. Murayama, H. , Ikemoto, M. , Fukuda, Y. , Tsunekawa, S. & Nagata, A. Serum level of ornithine carbamoyltransferase is influenced by the state of Kupffer cells. Clin. Chim. Acta Int. J. Clin. Chem. 380, 170–174 (2007). [DOI] [PubMed] [Google Scholar]
- 29. Remien, C.H. , Adler, F.R. , Waddoups, L. , Box, T.D. & Sussman, N.L. Mathematical modeling of liver injury and dysfunction after acetaminophen overdose: early discrimination between survival and death. Hepatolology 56, 727–734 (2012). [DOI] [PubMed] [Google Scholar]
- 30. Boyd, J.W. The mechanisms relating to increases in plasma enzymes and isoenzymes in diseases of animals. Vet. Clin. Pathol. Am. Soc. Vet. Clin. Pathol. 12, 9–24 (1983). [DOI] [PubMed] [Google Scholar]
- 31. Rej, R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin. Chem. 24, 1971–1979 (1978). [PubMed] [Google Scholar]
- 32. Sohlenius‐Sternbeck, A.‐K. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 20, 1582–1586 (2006). [DOI] [PubMed] [Google Scholar]
- 33. Schmidt, L.E. & Larsen, F.S. MELD score as a predictor of liver failure and death in patients with acetaminophen‐induced liver injury. Hepatolology 45, 789–796 (2007). [DOI] [PubMed] [Google Scholar]
- 34. Davidson, A.R. , Rojas‐Bueno, A. , Thompson, R.P. & Williams, R. Early unconjugated hyperbilirubinaemia after paracetamol overdosage. Scand. J. Gastroenterol. 11, 623–628 (1976). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4
Supporting Information S5
Supporting Information S6


