Abstract
Current reactive pest management methods have serious drawbacks such as the heavy reliance on chemicals, emerging genetic rodenticide resistance and high secondary exposure risks. Rodent control needs to be based on pest species ecology and ethology to facilitate the development of ecologically based rodent management (EBRM). An important aspect of EBRM is a strong understanding of rodent pest species ecology, behaviour and spatiotemporal factors. Gaining insight into the behaviour of pest species is a key aspect of EBRM. The landscape of fear (LOF) is a mapping of the spatial variation in the foraging cost arising from the risk of predation, and reflects the levels of fear a prey species perceives at different locations within its home range. In practice, the LOF maps habitat use as a result of perceived fear, which shows where bait or traps are most likely to be encountered and used by rodents. Several studies have linked perceived predation risk of foraging animals with quitting‐harvest rates or giving‐up densities (GUDs). GUDs have been used to reflect foraging behaviour strategies of predator avoidance, but to our knowledge very few papers have directly used GUDs in relation to pest management strategies. An opportunity for rodent control strategies lies in the integration of the LOF of rodents in EBRM methodologies. Rodent management could be more efficient and effective by concentrating on those areas where rodents perceive the least levels of predation risk. © 2017 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: rodent ecology, ecologically based rodent management, GUD, IPM, predation risk, rodent control, landscape of fear
1. INTRODUCTION
Putting integrated pest management (IPM) into practice with respect to rodents has often failed to recognize that rodent control needs to be based on a solid understanding of species‐specific behaviours, biology and the phenology of damage caused by different rodent species affecting agricultural production. In the past, there has been more attention paid to insect pests compared with rodent pests and, especially in developing countries, it is often thought that the ‘I’ in IPM stands for ‘insect’.1 A result of this is that IPM strategies for rodent pests still lag seriously behind those for insect pests. Effective rodent management in an agricultural landscape consists of four general elements: prevention, monitoring, implementation of a combination of control methods; and community involvement in management.1, 2
1.1. Ecologically based rodent management
Ecologically based rodent management (EBRM) builds on IPM; it is the reduction of the impact of rodent pests using specific knowledge about rodent species behaviour, ecology, biology and damage to sustainably manage them. EBRM proceeds on the basis that integrated rodent management strategies can be developed from a sound ecological basis (e.g. rodent pest species' habitat use and population dynamics) in order to reduce the economic and social impact of rodent pests in cost‐beneficial ways that do not adversely affect the environment.3, 4 EBRM was promoted due to a growing demand for more effective and species‐specific rodent control strategies that were not entirely recognized by early IPM practitioners who overly relied on chemical rodenticides.3 Moreover, rodenticide use has become less acceptable because of increased genetic resistance5, 6 and heightened animal welfare concerns.7
Generally, traditional forms of pest management are reactive; rodent control is mostly practised once damage to crops or stored produce becomes visible.8 Several Asian studies have shown EBRM to be highly effective in diminishing rodent damage9, 10, 11, 12 and have reduced farmers' reliance on rodenticides.10, 11, 13, 14 For EBRM to be effective it is also important to recognize that <10% of all rodent species are pest species, and many current rodent control methods do not sufficiently discriminate between pest and non‐pest species.15 Moreover, it is often not known what proportion of the population of a pest species needs to be culled for a significant reduction in economic damage.8, 15 Thus more knowledge (i.e. monitoring) on the species present, their behaviour and the consequences of their presence is essential for effective control.
1.2. Progression from dominance of rodenticides to integrated rodent management
In 1944, anticoagulant rodenticides were accidently discovered in the USA following the detection of dicoumarin (warfarin) in spoiled sweet clover hay fed to cattle that subsequently suffered from internal bleeding.16, 17 Because rodents do not immediately feel ill after eating bait laced with warfarin, warfarin and its modern‐day anticoagulant analogues have become the definitive tool for controlling rodents. Until the late 1980s, their efficacy and relative safety certainly contributed to the stifling of other research avenues, such as developing more ecologically sound methods of rodent management.16 Rodent control practices in agricultural environments are still mostly based on the use of rodenticides.8, 18, 19, 20 However, incorrect application of such chemicals fast tracks the development of rodenticide resistance (reported from 1966 onwards for several rodent species) and increases the risk of both primary and secondary exposure of predators.21
1.3. State of the art of EBRM use on pest rodents
An important aspect of EBRM is the use of spatiotemporal factors in the context of the population dynamics of rodent pests and the agricultural resource to be protected. As an example, it is more effective to cull far fewer animals during the early stages of rice production than to kill many later in the season to reduce crop damage.15 The EBRM spatiotemporal aspect is often applied in cropping systems to reduce pre‐harvest losses, but there have been few studies on EBRM to reduce post‐harvest losses. Fluctuations in the population abundance of peri‐urban and urban rodent species (i.e. species that are continuously present in the neighbourhood of humans and cause losses to stored products and increased risks of disease transmission) may be less than those of field rodent species, but the spatiotemporal aspect of EBRM is still important. For example, if rodent numbers are managed before agricultural produce is put into a storage facility, the population growth of rodent pests and negative consequences to stored grain can be significantly curtailed. Particularly in the post‐harvest situation, rodent management should focus more on the behaviour of the pest rodent species than on the current reactive methods. A behaviour all animals have in common is the search for provisions. So, what happens when one focuses on species‐specific foraging behaviour to gain more knowledge to enable managing those pest species?
2. SEARCH FOR PROVISIONS
The optimization of foraging behaviour in animals addressing what food type should be included in the diet was first published by Pianka and MacArthur22 and Emlen.23 In 1976, Charnov developed the first optimal patch use model, which is known as the Marginal Value Theorem (MVT).24 This hypothesizes that foraging animals assume that nutritional products occur in clusters, and that food consumption decreases linearly (but not constantly) with time spent in that exact location. When making foraging decisions, animals balance the benefit of energy rewards and the price of predation.25 The MVT predicts that animals foraging in a particular food patch will decide whether to depart, not based on the depletion of the patch, but rather on the assessment of costs of foraging and the yield rate of the current patch versus the yield rate of another ‘new’ food patch.24, 26 By creating food patches and assessing the amount of food left after foraging, the giving‐up density (GUD)36 of a food source becomes a measurable unit.25, 27, 28 The GUD reflects the perceived costs of foraging on that location. The more food left in a patch after the departure of an animal, the higher the GUD, indicating high costs.25 GUDs provide insights into the feeding behaviour and habitat preferences of animals.25, 29 Furthermore, GUDs also reveal the balance between food and safety; the metabolic costs of a foraging animal, its perceived predation risk during foraging, and the missed opportunity costs (MOC) of the forager by not engaging in activities other than foraging.25, 30 With feeding rate being a direct function to food density, GUDs can be used as an index of the forager's quitting harvest rate.31, 32
2.1. Perceived predation risks
Because rodents serve as prey for many different species of reptiles, birds and mammals, they avoid places where the relative risk of predation is high. Both indirect cues (e.g. vegetation cover, weather conditions, light intensity) and direct cues (e.g. sound, odours, urine or other excrements from potential predators) enable rodents to assess predation risk during foraging.33 A study on the effect of owl predation on rodents' search for provisions in America showed that adjustments in foraging behaviour as a response to perceived predation risk are based predominantly on an awareness of the presence of a predator, rather than on the actual capture or killing of prey by the predator.25, 34 Brown25 postulates that prey animals ‘manage risk’ according to H = C + P + MOC, where H is harvest rate, C is the metabolic cost and P is the cost of risk of predation. Research on foraging and predation risk trade‐off has been used in many different animal contexts, from aquatic to terrestrial systems.35 A review in 2013 on GUD methodologies discussed its use, practical benefits and drawbacks, and gave insight into the many species that have been studied [mule deer (Odocoileus hemionus), red fox (Vulpes vulpes ), voles (Microtus spp. and Myodes spp., gerbils (Gerbillus allenbyi), goldfish (Carassius auratus), squirrels (Tamiasciurus hudsonicus, Callospermophilus lateralis and Sciurus niger), mice (Rhabdomys pumilio, Baeolophus bicolor, Acomys russatus, Acomys cahirinus and Peromyscus maniculatus), possums (Trichosurus vulpecula), rats (Rattus fuscipes) and chipmunks (Tamias minimus)].36 For all foraging animal species, the perception of safety of feeding activities includes the encounter rate with predators, the lethality of the predator and the chance of surviving predation.30, 37, 38, 39 Prey animals have to continuously balance between demand for food and safety, e.g. reduced predation risk.40 With the costs of risk of predation (P) varying across the landscape, so does the intensity of patch exploitation. The way in which animals use their habitat during their foraging behaviour41 as a result of fear for predation is called the landscape of fear (LOF). Such a landscape is strongly based on the ecology of a particular prey species, and on the ecology and hunting techniques of their predators.3, 42 In our opinion, the LOF is wider than the concept introduced by Laundré et al.,41 and should include both the way foraging animals use their habitat as result of perceived fear, and the actual landscape. Thus, in addition to predator–prey relations, the LOF can also be constructed on perceived fear of intraspecific relations. An intruder (e.g. rat from a different colony) will also be able to provoke fear among the rats in a resident colony,43 however, intruders can also be in fear of residents. In this case, risk of injury from interference and aggression from conspecifics will affect the LOF.
3. MAKING BETTER USE OF RODENTS' NATURAL BEHAVIOUR
Several studies have linked perceived predation risk of foraging animals with their quitting harvest rates or GUDs (review by Brown and Kotler).30 The LOF reflects the levels of fear of predation perceived by a prey species at different locations within its home range.44 The LOF is species‐specific; our assumption is that a spatial LOF will look different for the grey squirrel (Sciurus carolinensis) than for the Norway rat (Rattus norvegicus ) because each species will perceive fear of predation via different cues. Furthermore, each prey species has different aptitudes (e.g. climbing ability, speed, agility) and thus each species is vulnerable to different degrees to different predators (e.g. terrestrial or/and aerial32), which leads to each species having different predation costs of foraging (i.e. fear). Knowledge of species‐specific short‐term temporal feeding patterns (e.g. night versus day activity) could be an effective guide for trap or bait placement, and offers possibilities to reduce risks for non‐target animals (e.g. by making the trap inactive during times the pest species is inactive). Knowledge of species‐specific behaviour could also improve trap/bait placement and trapping systems. By combining the perceived risk of predation with rodent behavioural responses, individual's spatial use patterns could be explained.44 In applying these concepts of rodent behaviour to rodent management, some rodent species, e.g. Norway rats (R. norvegicus), express a degree of neophobic behaviour that partly explains poor bait uptake when rodenticides are applied; other species, e.g. house mice, show neophilia and innate curiosity for what is new in their environment.45, 46
3.1. Landscape of fear as a component of rodent management
A recent study examined the relationship between the GUDs of Rattus tanezumi and the spatial heterogeneity of their damage to rice crops in the Philippines.47 It was concluded that bait or trap placement towards the centre of rice crops that are typically <0.1 ha, would be more likely to be visited by rats. Another study in wheat crops in Australia used GUDs to assess whether house mice modified their habitat selection based on perceived predation risk.29 Both studies highlighted that a better understanding of factors influencing habitat use by rodent pests could aid in decisions on their management. What is lacking is objective evidence on whether pest control strategies based on habitat use in pest rodents are more effective and have a more long‐term effect than reactive rodent management. We suggest that a better understanding of rodent behavioural ecology, especially the concept of the LOF, will result in more effective strategies for the management of rodent pests. To be able to use the LOF in management, it is essential to identify the possible advantages and disadvantages, and current knowledge gaps of the LOF methodology, which can point the way for further research.
3.2. Gaps and opportunities for implementation of the landscape of fear as a rodent management tool
A classic paper by Rosenzweig48 provides prescient advice for pest managers to take habitat selection into account in order to improve the management results ‘Pest populations may be controlled most cheaply by concentrating on their cradle habitats (although natural selection might interfere)’48; this was also stated years later by Morris.49 As discussed earlier, not only does habitat use have a role when developing successful management methods, but foraging behaviours should be also taken into account as they provide reliable indicators of future situations (more reliable than use of ‘old’ cues indicating the past).50 We feel that GUDs are a valuable tool to measure an animal's decision making. Research on GUDs as a monitoring tool for rodent species habitat preferences in relation to population densities and food supply indicate that rodents take greater risks when foraging during periods of high animal densities and resource depletion.29, 51 Therefore, it is important to monitor the number of animals present; the perceived risk of an animal is lower when it lives in a large group, than when it is on its own. Moreover, competing species often create patterns in GUDs and habitat use that are convergent with predation risk.52 For example, two competing prey species using the same food patches could lead to the same effect as avoidance of predation risk; the feeding rates of both prey species will deteriorate as the species use up resource levels in shared food patches. A decrease in harvest yields will lead to more effort in foraging in a food patch which by GUDs would be indicated as ‘safe’.52 On the other hand, research from Australia showed that with high population densities of house mice, their spatial use became more opportunistic in some habitats where food is limited, which can also lead to a different result in the GUDs.29 These facts indicate the need to evaluate interspecific competition while measuring for predation risk behaviour in foraging animals when using GUDs.32, 52 A low GUD indicates a ‘safe place’, which might result in overconsumption there, whereas uptake of bait in riskier places (high GUD) will be lower. However, these dose rates might need to be adjusted to deal with the consumption rate in response to this LOF‐induced effect. This is only valid when: (i) there is no effect of density on GUDs; (ii) under‐consumption does not deliver the required dose or (iii) over‐consumption matters. Simple measures such as GUDs are generally cheap to conduct; however, Bedoya‐Perez et al.36 indicated seven important aspects that need careful consideration when using and interpreting GUDs: (i) the relationship between costs and benefits for the forager is linear, but not constant (e.g. curvilinear); (ii) the forager's physical condition; (iii) more than one forager can visit a food patch simultaneously and sequentially; (iv) the composition of the food‐patch (nutritional value of the food and properties of the substrate); (v) food patch predictability; (vi) the forager's behaviours to maximize fitness and overcome costs of searching for provisions; and (vii) non‐target species foraging from food patches.36 Based on these shortcomings, it can be stated that the use of GUDs to reflect foraging behaviour strategies of predator avoidance40 cannot be assumed to be completely sufficient. However, it is indisputably clear the GUDs are an effective tool to map a population's LOF, which could be beneficial for pest management by providing objective information on which to base decision making, collecting clear evidence of where rodents are more or less likely to forage and how to manipulate habitats to increase fear levels.
Current rodent management in agricultural and peri‐urban habitats have made little use of the LOF as an opportunity to strengthen pest management. For example, the intensity of rodenticide use and trapping could decrease significantly if an understanding of the LOF is applied in the spatial placement of such control interventions in agricultural landscapes.47 This is particularly the case in developing countries where there have been few reports of studies on the spatial and foraging behaviour of major rodent pest species. Current rodent trapping sometimes includes parts of the LOF implicitly, for example the placement of traps along walls because it is known that most commensal rodents prefer to move alongside walls. Trapping studies on microhabitat use have tried to reflect the concept of trap success depending on perceived predation risk. However, the most effective placement of rodent traps inside and around buildings or within agricultural fields is still generally based more on tacit knowledge of the pest controller rather than rigorous data on the behaviour of the targeted pest species in a landscape. Van der Merwe and Brown53 visualized the LOF of the cape ground squirrel via a physical map that showed the predation costs of foraging (Fig. 1a). A map of the LOF shows valleys representing relative safety, and peaks indicating perceived danger (Fig. 1b).44 In both graphics, the LOF was used as a model to visualize how fear could alter the area used by prey as it tries to reduce the risk of predation, specifically during foraging.41, 44, 54 Within the LOF, animals spend the most time in the valleys, where the perceived predation risk is the lowest. This information enables rodent management to place traps in those specific perceived low fear locations, which, we suggest, will increase trapping rates and thus pest management success.
Figure 1.
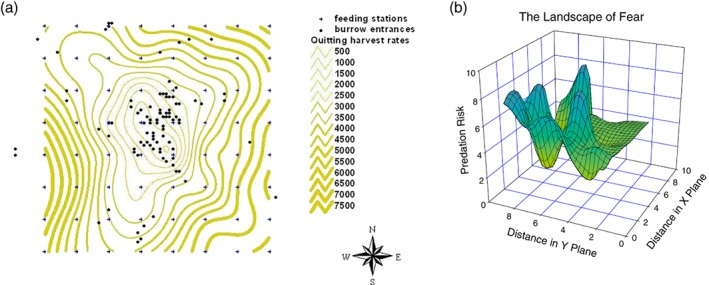
Two different ways of visualizing the landscape of fear. (a) 2D map of the cape ground squirrel, the thicker the grey line, the more ‘safe’ the squirrel feels to forage. Adapted from van der Merwe M and Brown JS, J Mammal 89:1162–1169 (2008). (b) 3D depiction of the landscape of fear, with highest giving up densities at the peaks. From Laundré JW, Hernández L and Ripple WJ, Open Ecol J 3:1–7 (2010).
Rodents can alter their risk management in several ways: by time allocation, e.g. shorten the exposure time and forage as fast and shortly as possible to reduce predatorily encounters; by vigilance, e.g. reduce the lethality of encounters with a predator; by safety in numbers by synchronized activity; and by night versus day activity to avoid encounters with predators. Again, trapping efficiency could be substantially improved if we had mapped the LOF of the specific rodent pest species and then placed the traps accordingly (where GUDs are lowest,47 i.e. peaks of the LOF). One option would be to conduct a systematic analysis of the behaviour of a pest species where their ethology may help clarify potential actors in response to GUDs for LOF and management actions for those species. Because LOF differs among species, it also differs between target and non‐target rodents, which could be used to minimize unwanted effects on non‐targets. In case of doubt, the LOF of the non‐target species should also be mapped to prevent trapping in overlapping perceived risk valleys. To date, however, no study has systematically mapped the spatial behaviour of rodent pest species where beneficial species would be at risk of non‐target poisoning. In our view, one should concentrate on the following four key points for the use of the LOF as basis for rodent management: (i) pest species with the lowest GUD will be most easiest to target; (ii) species are most susceptible during times of the year when their GUDs are lowest, and during these intervals management methods will be most effective; (iii) species are most likely to be trapped in (micro‐) habitats where their GUDs are lowest, thus concentrate rodent management where rodents perceive the least levels of predation risk; and (iv) management strategies that increase perceived risk of predation for the target pest species will lower pest damage. Measures to promote populations of appropriate predators should be taken, such as placing nest boxes for birds of prey (e.g. owls28) and educating local communities about the benefit of local biological predators (e.g. foxes55, 56). Research into the use of ‘biocontrol’ by domestic predators (e.g. cats, dogs) as rodent management method in Africa showed that the presence of these predators affected the foraging behaviour of pest rodents.57 The presence of both cats and dogs increased levels of fear (measured by increased GUDs) in local foraging rodent species, which led to diminished rodent activity.57 However, reliable scientific evidence that biocontrol via predation minimizes rodent population size below damage threshold levels is not yet available.
4. CONCLUSION
Connecting the LOF to rodent pest species is a novel approach with many opportunities to further enhance ecologically based rodent pest management. Implementing the LOF into rodent management may enable the development of preventive control rather than reactive methods through better timing and habitat targeting for the trapping or placement of rodenticides. It is extremely important to look continuously at alternatives for pest management. A recent study of Mul et al.58 developed a fully automated pest monitoring tool to implement IPM effectively. This was done by focusing on the behaviour of the pest species, after which monitoring was conducted to develop a model that predicts the location of and growth in the population.58, 59 In conclusion, for effective management, it is essential to align management methods with the pest species biology and behaviour. To date, there have been few studies on the behaviour of commensal and non‐commensal pest species over different habitats and environments (e.g. city versus countryside) which are a necessity for composing and using the LOF. It would be best to have an overview of all species present, and whether and when they compete with each other. Use of the LOF as an EBRM tool holds the promise of novel strategies and capacities for practical use as a unifying behavioural ecological concept. A study on the influence of domestic predators on pest rodent foraging behaviour by Mahlaba et al.57 suggests that integration of the LOF into EBRM will provide stronger insights into the ecology of rodent pest species. Use of the LOF is much stronger and broadly applicable than the use of tacit knowledge, because tacit knowledge is generally based on experience, can be highly subjective, and is difficult to transfer to another person by formal means The LOF concept is meant to provide a more evidence‐based approach, which, in turn, would enable the development of more efficient rodent management methods.
ACKNOWLEDGEMENTS
We thank all anonymous reviewers for their helpful insights during the development of this article.
REFERENCES
- 1. Singleton GR, Integrated management of rodents: a southeast Asian and Australian perspective. Belg J Zool 127(Suppl. 1):157–169 (1997). [Google Scholar]
- 2. Meerburg B, Bonde M, Brom F, Endepols S, Jensen A, Leirs H et al, Towards sustainable management of rodents in organic animal husbandry. NJAS‐Wageningen J Life Sci 52: 195–205 (2004). [Google Scholar]
- 3. Singleton GR, Leirs H, Hinds LA and Zhang Z, Ecologically‐based management of rodent pests–re‐evaluating our approach to an old problem. Ecologically‐based Management of Rodent Pests. Australian Centre for International Agricultural Research (ACIAR), Canberra, pp. 17–29 (1999). [Google Scholar]
- 4. Smith RF and van den Bosch R, Integrated control, in Pest Control, ed. by Kilgore WW. and Doutt RL. Academic Press; New York, pp. 295–340 (1967). [Google Scholar]
- 5. Rost S, Pelz H‐J, Menzel S, MacNicoll AD, León V, Song K‐J et al, Novel mutations in the VKORC1 gene of wild rats and mice–a response to 50 years of selection pressure by warfarin? BMC Genet 10:4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meerburg BG, van Gent‐Pelzer MP, Schoelitsz B and van der Lee TA, Distribution of anticoagulant rodenticide resistance in Rattus norvegicus in the Netherlands according to Vkorc1 mutations. Pest Manag Sci 70:1761–1766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meerburg BG, Brom FW and Kijlstra A, The ethics of rodent control. Pest Manag Sci 64:1205–1211 (2008). [DOI] [PubMed] [Google Scholar]
- 8. John A, Rodent outbreaks and rice pre‐harvest losses in Southeast Asia. Food Security 6:249–260 (2014). [Google Scholar]
- 9. Singleton GR and Brown PR, Comparison of different sizes of physical barriers for controlling the impact of the rice field rat, Rattus argentiventer, in rice crops in Indonesia. Crop Prot 22:7–13 (2003). [Google Scholar]
- 10. Singleton GR, Sudarmaji, Jacob J and Krebs CJ, Integrated management to reduce rodent damage to lowland rice crops in Indonesia. Agric Ecosyst Environ 107:75–82 (2005). [Google Scholar]
- 11. Palis FG, Singleton GR, Brown PR, Huan NH, Umali C and Nga NTD, Can humans outsmart rodents? Learning to work collectively and strategically. Wildlife Res 38:568–578 (2011). [Google Scholar]
- 12. Jacob J, Singleton GR, Herawati NA and Brown PR, Ecologically based management of rodents in lowland irrigated rice fields in Indonesia. Wildlife Res 37:418–427 (2010). [Google Scholar]
- 13. Brown PR, Tuan NP, Singleton GR, Ha PTT, Hoa PT, Hue DT et al, Ecologically based management of rodents in the real world: applied to a mixed agroecosystem in Vietnam. Ecol Appl 16:2000–2010 (2006). [DOI] [PubMed] [Google Scholar]
- 14. Brown PR and Khamphoukeo K, Changes in farmers' knowledge, attitudes and practices after implementation of ecologically‐based rodent management in the uplands of Lao PDR. Crop Prot 29:577–582 (2010). [Google Scholar]
- 15. Singleton GR, Brown PR, Jacob J and Aplin KP, Unwanted and unintended effects of culling: a case for ecologically‐based rodent management. Integr Zool 2:247–259 (2007). [DOI] [PubMed] [Google Scholar]
- 16. Hadler MR and Buckle AP, Forty five years of anticoagulant rodenticides—past, present and future trends. Proceedings of the Vertebrate Pest Conference 15:149–155 (1992). [Google Scholar]
- 17. Link KP, The Anticoagulant from Spoiled Sweet Clover Hay, The Harvey Lectures, New York, p. 162 (1944). [Google Scholar]
- 18. Arora K, Srivastava J and Pandey G, Evaluation of racumin (coumatetralyl) based anticoagulant against the black rat, Rattus rattus Linn. Pesticides 18:25–27 (1984). [Google Scholar]
- 19. Mathur R and Prakash I, Reduction in population of Indian desert rodents with anticoagulant rodenticides. Proc Animal Sci 93:585–589 (1984). [Google Scholar]
- 20. Parshad V and Malhi C, Comparative efficacy of two methods of delivering an anticoagulant rodenticide to three species of South Asian rodents. Int Biodeterior Biodegradation 36:89–102 (1995). [Google Scholar]
- 21. Jackson WB and Kaukeinen D, Resistance of wild Norway rats in North Carolina to warfarin rodenticide. Science 176:1343–1344 (1972). [DOI] [PubMed] [Google Scholar]
- 22. MacArthur R and Pianka E, On optimal use of a patchy environment. Am Nat 100:603–609 (1966). [Google Scholar]
- 23. Emlen JM, The role of time and energy in food preference. Am Nat 100:611–617 (1966). [Google Scholar]
- 24. Charnov EL, Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136 (1976). [DOI] [PubMed] [Google Scholar]
- 25. Brown JS, Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47 (1988). [Google Scholar]
- 26. Milinski M and Heller R, Influence of a predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeatus L.). Nature 275: 642–644 (1978). [Google Scholar]
- 27. Brown JS, Kotler BP and Mitchell WA, Competition between birds and mammals: a comparison of giving‐up densities between crested larks and gerbils. Evol Ecol 11:757–771 (1997). [Google Scholar]
- 28. Brown JS, Kotler BP, Smith RJ and Wirtz WO II, The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 76:408–415 (1988). [DOI] [PubMed] [Google Scholar]
- 29. Ylönen H, Jacob J, Davies MJ and Singleton GR, Predation risk and habitat selection of Australian house mice, Mus domesticus, during an incipient plague: desperate behaviour due to food depletion. Oikos 99:284–289 (2002). [Google Scholar]
- 30. Brown JS and Kotler BP, Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014 (2004). [Google Scholar]
- 31. Schmidt KA, Brown JS and Morgan RA, Plant defenses as complementary resources: a test with squirrels. Oikos 81:130–142 (1998). [Google Scholar]
- 32. Makin DF, Payne HF, Kerley GI and Shrader AM, Foraging in a 3‐D world: how does predation risk affect space use of vervet monkeys? J Mammal 93:422–428 (2012). [Google Scholar]
- 33. Orrock JL, Danielson BJ and Brinkerhoff R, Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437 (2004). [Google Scholar]
- 34. Verdolin JL, Meta‐analysis of foraging and predation risk trade‐offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464 (2006). [Google Scholar]
- 35. Werner EE and Hall DJ, Ontogenetic habitat shifts in bluegill: the foraging rate–predation risk trade‐off. Ecology 69:1352–1366 (1988). [Google Scholar]
- 36. Bedoya‐Perez MA, Carthey AJR, Mella VSA, McArthur C and Banks PB, A practical guide to avoid giving up on giving‐up densities. Behav Ecol Sociobiol 67:1541–1553 (2013). [Google Scholar]
- 37. Brown JS, Vigilance, patch use and habitat selection: foraging under predation risk. Evol Ecol Res 1:49–71 (1999). [Google Scholar]
- 38. Lima SL and Dill LM, Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640 (1990). [Google Scholar]
- 39. Abrams PA, Optimal traits when there are several costs: the interaction of mortality and energy costs in determining foraging behavior. Behav Ecol 4:246–259 (1993). [Google Scholar]
- 40. Jacob J and Brown JS, Microhabitat use, giving‐up densities and temporal activity as short‐ and long‐term anti‐predator behaviors in common voles. Oikos 91:131–138 (2000). [Google Scholar]
- 41. Laundré JW, Hernández L and Altendorf KB, Wolves, elk, and bison: reestablishing the ‘landscape of fear’ in Yellowstone National Park, USA. Can J Zool 79:1401–1409 (2001). [Google Scholar]
- 42. Matassa CM and Trussell GC, Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92:2258–2266 (2011). [DOI] [PubMed] [Google Scholar]
- 43. Davis DE, Emlen JT and Stokes AWCF, Studies on home range in the brown rat. J Mammal 29:207–225 (1948). [Google Scholar]
- 44. Laundré JW, Hernández L and Ripple WJ, The landscape of fear: ecological implications of being afraid. Open Ecol J 3:1–7 (2010). [Google Scholar]
- 45. Macdonald DW, Mathews F and Berdoy M, The behaviour and ecology of Rattus norvegicus: from opportunism to kamikaze tendencies, in Ecologically‐based Management of Rodent Pests, ed. by Singleton GR, Hinds LA, Leirsand H. and Zhang Z. Australian Centre for International Agriculture Research, Canberra, pp. 49–80 (1999). [Google Scholar]
- 46. Cowan P, Neophobia and neophilia: new‐object and new‐place reactions of three Rattus species. J Comp Physiol Psychol 91:63 (1977). [Google Scholar]
- 47. Jones CR, Lorica MRP, Villegas JM, Ramal AF, Horgan FG et al, The stadium effect: rodent damage patterns in rice fields explored using giving‐up densities. Integr Zool https://doi.org/10.1111/1749‐4877.12251 (2016). [DOI] [PubMed] [Google Scholar]
- 48. Rosenzweig ML, Density‐dependent habitat selection: a tool for more effective population management, in Modeling and Management of Resources Under Uncertainty: Proceedings of the Second US–Australia Workshop on Renewable Resource Management, ed. by Vincent TL, Cohen Y, Grantham WJ, Kirkwood GP, Skowronski JM. Springer, Berlin, pp. 98–111 (1987). [Google Scholar]
- 49. Morris DW, How can we apply theories of habitat selection to wildlife conservation and management? Wildlife Res 30:303–319 (2003). [Google Scholar]
- 50. Kotler BP, Morris DW and Brown JS, Direct behavioral indicators as a conservation and management tool. Conserv Behav 21:307 (2016). [Google Scholar]
- 51. Strauß A, Solmsdorff KY, Pech R and Jacob J, Rats on the run: removal of alien terrestrial predators affects bush rat behaviour. Behav Ecol Sociobiol 62:1551–1558 (2008). [Google Scholar]
- 52. Morris DW, Apparent predation risk: tests of habitat selection theory reveal unexpected effects of competition. Evol Ecol Res 11:209–225 (2009). [Google Scholar]
- 53. van der Merwe M and Brown JS, Mapping the landscape of fear of the cape ground squirrel (Xerus inauris). J Mammal 89:1162–1169 (2008). [Google Scholar]
- 54. Altendorf KB, Laundré JW, López González CA and Brown JS, Assessing effects of predation risk on foraging behavior of mule deer. J Mammal 82:430–439 (2001). [Google Scholar]
- 55. Saunders G, Coman B, Kinnear J and Braysher M, Managing Vertebrate Pests: Foxes. Australian Government Publications Service, Canberra: (1995). [Google Scholar]
- 56. Lindström ER, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L et al, Disease reveals the predator: sarcoptic mange, red fox predation, and prey populations. Ecology 75:1042–1049 (1994). [Google Scholar]
- 57. TaA Mahlabla, Monadjem A, McCleery R and Belmain SR, Domestic cats and dogs create a landscape of fear for pest rodents around rural homesteads. PloS one 12(2):e0171593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mul MF, Ploegaert JPM, George DR, Meerburg BG, Dicke M and Groot Koerkamp PWG, Structured design of an automated monitoring tool for pest species. Biosystems Engineering 151:126‐40 (2016). [Google Scholar]
- 59. Mul MF, JWv Riel, Roy L, Zoons J, André G, George DR et al, Development of a model to forecast population dynamics of Dermanyssus gallinae in laying hen houses to facilitate Integrated Pest Management. in press. [DOI] [PubMed]


