Abstract
Introduction:
Nicotine is a key biologically active compound of cigarettes. Although nicotine is a risk factor for various health issues, it may also be beneficial when treated at moderate concentrations. Nicotine has been shown to bidirectionally regulate stem cell proliferation and differentiation depending on the doses applied. It is not clear whether or how nicotine regulates mouse embryonic stem cell (mESC) survival and proliferation.
Methods:
Mouse embryonic stem cells were cultured in the presence of 0.01, 0.1, 1, or 10 μM nicotine. The effects of nicotine on cell survival and proliferation were examined. The signaling pathway that mediated these effects was analyzed.
Results:
Cell viability was not affected by nicotine at all 4 concentrations examined. The proliferation of mESCs was promoted by 0.01 and 0.1 μM nicotine and suppressed by 1 and 10 μM. This dose-dependent regulation was mediated through the Wnt/β-catenin pathway. Modulation of Wnt/β-catenin activity either worsens or reverses the effects of nicotine.
Conclusions:
We have identified a bidirectional function of nicotine on mESC proliferation through regulation of the Wnt/β-catenin pathway and this is associated with different doses. This study suggests that concentration of nicotine is a crucial aspect for consideration when designing research or therapeutic strategies.
Keywords: mouse embryonic stem cell, nicotine, proliferation, cell cycle, WNT
Introduction
Smoking is a risk factor for various diseases and a leading cause for premature deaths.1 Nicotine is a key compound found in cigarettes that may lead to various adverse health issues in addition to addiction.2 Despite all the adverse effects, recent studies have also found that nicotine may be beneficial to some physiological processes. Reduced incidence of Parkinson disease has been shown to be associated with smoking.3 Nicotine administration in mouse models of Parkinson disease and Alzheimer disease is also neuroprotective.4,5 Similarly, nicotine suppresses neuroinflammation by inhibiting microglia production and protects neurons against brain injury.6
Nicotine has been shown to exhibit dose-dependent bimodal effects. Prolonged exposure of high-dose nicotine inhibits proliferation of mouse neural stem cells (mNSCs),7 while low concentration of nicotine protects mNSCs against neurotoxicity of microglial-derived factors.8 In cultured human alveolar bone marrow–derived mesenchymal stem cells, 1 to 2 mM nicotine treatment increases cell proliferation and 5 mM nicotine decreases cell proliferation.9 The effects of nicotine on cell survival and proliferation are not consistent, with some studies suggesting inhibiting while others suggesting promoting proliferation depending on the types of cells studied and the concentrations of nicotine used. For example, exposure of nicotine in adolescent mice leads to apoptosis and loss of both neurons and glia.10 In human Wharton’s jelly mesenchymal stem cell culture, 5 μM nicotine treatment impairs cell proliferation.11 At concentrations between 1.8 and 3.7 μM, nicotine leads to multiple adverse effects to human embryonic stem cells (hESCs), including increased cell death.12 On the other hand, nicotine has been shown to suppress apoptosis of some cancer cells and it also induces cell proliferation and invasion in a variety of cancer cell lines.13,14
Mouse embryonic stem cells (mESCs) are pluripotent cells used by basic and clinical research such as development of stem cell–based therapeutic strategies and genetic engineering.15 The canonical Wnt/β-catenin pathway is a master regulator of cell proliferation and cell cycle progression.16 It is critically involved in stem cell self-renewal and differentiation.17 Previously, nicotine has been shown to regulate the Wnt/β-catenin pathway in various cell types.8,18–20 It is not clear whether and how nicotine regulates mESC proliferation and survival. We hypothesized that nicotine regulates mESC proliferation through Wnt/β-catenin-induced cell cycle progression. In this study, we tested this hypothesis by exploring the effects of nicotine on mESC viability, proliferation, and cell cycle progression and whether Wnt/β-catenin signaling pathway is involved in these processes.
Materials and Methods
Chemicals
Dickkopf-1 (DKK-1) was purchased from Sigma (St Louis, Missouri), and Wnt-3a was purchased from R&D System (Minneapolis, Minnesota).
Mouse Embryonic Stem Cell Culture and Nicotine Treatment
Mouse embryonic stem cells (line D3) were purchased from ATCC (Manassas, Virginia) and maintained in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, California) supplemented with 1000 U/mL leukemia inhibitory factor (Chemicon, Billerica, MA), 15% fetal bovine serum (Gibco, Grand Island, New York), 0.1 mM nonessential amino acids, 1% penicillin/streptomycin (Invitrogen), and 0.1 Mm β-mercaptoethanol (Sigma, St. Louis, MO). Cells were passaged every 48 hours and cultured at 37°C with 5% CO2. Freshly plated mESCs were treated with nicotine (Sigma) at different concentrations (0.01, 0.1, 1, and 10 μM) for 48 hours before further experiments.
Cell Viability Measurement
Forty-eight hours after nicotine treatment, mESCs were stained with recombinant annexin V conjugated to fluorescein isothiocyanate and propidium iodide (PI) for apoptotic and dead cells according to the manufacturer’s instruction (Abcam, Cambridge, Massachusetts). Cells were then analyzed using a BD FACScanto II flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Percentage of cells with low or no fluorescent signal represents the ratio of viable cells.
Bromodeoxyuridine (BrdU) Incorporation Assay
BrdU incorporation assay was used to determine the effects of nicotine on cell proliferation. This assay was performed using a BrdU incorporation assay kit (Cell Signaling, Danvers, Massachusetts) as described previously.21 Briefly, 48 hours after nicotine treatment, mESCs were treated with 10 mM BrdU for 4 hours and incubated with anti-BrdU peroxidase conjugate for 90 minutes before adding a tetramethylbenzidine substrate. Incorporated BrdU was measured by an enzyme-linked immunosorbent assay reader, and data were represented as fold change compared to control group.
Cell Proliferation Analysis
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was used to measure the proliferation rate of mESCs post nicotine treatment according to the manufacturer’s instruction. Briefly, mESCs were plated onto 96-well plates and treated with nicotine at indicated concentrations for 48 hours. After adding 10 μL/well of kit solution to the culture, absorbance was measured at 450 nm with a microplate reader (Packard Instrument Co, Downers Grove, IL). Data were represented as relative optical density to control group.
Tritium Update Assay
DNA synthesis rate was measured using tritium update assay as described previously.21 Briefly, 48 hours after nicotine treatment, 0.5 mCi of [methyl-3H] thymidine deoxyribose (TdR; Amersham Pharmacia Biotech Inc, Piscataway, New Jersey) was added to each well of 96-well plates for 16 hours. The 3H-TdR-incorporated cells were analyzed with a liquid scintillation counter (Packard Instrument Co) by measuring β emission for 1 minute. Data were represented as ratio of TdR uptake to control group.
Western Blot Analysis
After indicated treatment, cells were harvested and lysed in NP-40 lysis buffer containing protease inhibitor mixture (Roche, Penzberg, Upper Bavaria, Germany) and phosphatase inhibitors. Protein concentrations were measured using Pierce BCA protein assay kit. Equal amount of proteins were separated by 4% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were subsequently transferred to a polyvinylidene fluoride membrane. Membrane was blocked with 10% skim milk in Tris-buffered saline (TBS), incubated with primary antibodies diluted in 1% skim milk in TBS at 4°C overnight before incubation of secondary antibodies. Proteins were visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) and quantified using ImageJ version 1.48.] Normalized values were expressed as a ratio of the mean expression level of controls. The following antibodies were used in this study: proliferating cell nuclear antigen (PCNA; 1:200; Abcam), cyclin A (1:200; Abcam), cyclin B (1:100; Abcam), β-catenin (1-400; Sigma), β-tubulin (1:1000; Sigma).
Quantitative Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (RT-PCR) was performed to measure the messenger RNA (mRNA) levels of relevant genes. Total RNA was isolated using TRIzol RNA isolation reagents (Valencia, California). An equivalent amount of RNA from each sample was used as template to make complementary DNA (cDNA) using the iScript cDNA synthesis kit. Quantitative RT-PCR was performed according to the standard protocol using SsoAdvanced Universal SYBR Green Supermix (Biorad, Hercules, CA). Data were analyzed by using the 7500 Software v2.0.1. The relative expression level (fold changes) of Ctnnb1 and Ccnd1 to those of control cells was calculated. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control.
Statistical Analysis
Results presented in the figures are representative of at least 3 different repetitions. Data were analyzed by SPSS 21.0 (SPSS Inc, Chicago, Illinois) and presented as mean (standard derivation). Comparisons between groups are evaluated by 1-way analysis of variance. The difference was regarded statistical significant when P value < .05.
Results
Effects of Nicotine on mESC Viability
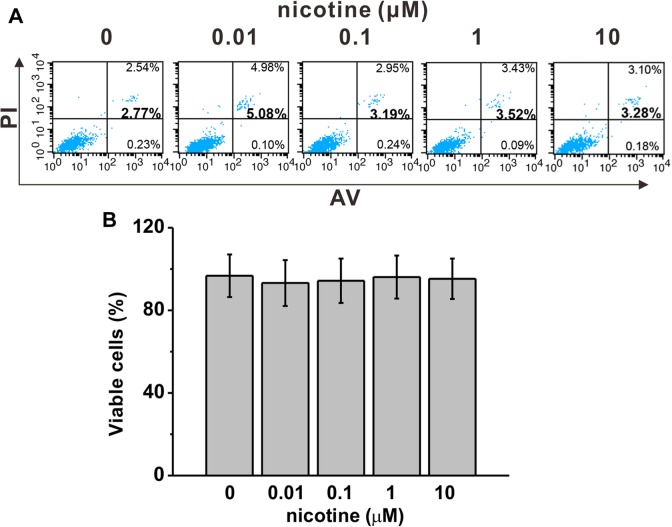
We first investigated whether nicotine at doses of our interest (0, 0.01, 0.1, 1, 10 μM) had any effect on cell viability. Forty-eight hours after nicotine exposure, we measured the ratio of cells undergoing apoptosis and necrosis by annexin V and PI (Figure 1A) and calculated the percentage of living cells (Figure 1B). We found that nicotine treatment at 10 μM or lower did not significantly alter the viability of mESCs.
Figure 1.
Nicotine exposure did not affect cell survival in mouse embryonic stem cells (mESCs) at indicated concentrations for 48 hours. A, Cell survival was characterized by annexin V/propidium iodide (PI) staining and further analyzed by flow cytometry. B, There is no statistical difference in percentage of viable cells among the experimental groups. Data were represented as mean (standard deviation)
Nicotine Regulates mESC Proliferation in a Dose-Dependent Manner
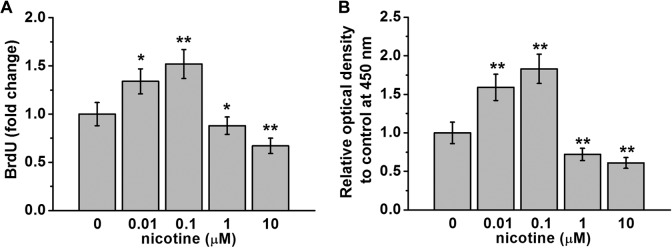
Previously, it has been shown that 5 μM nicotine treatment decreased proliferation of human Wharton’s jelly mesenchymal stem cells after 3-day exposure.11 We investigated whether nicotine treatment had an effect on mESC proliferation. First, we examined BrdU incorporation into DNA as a result of nicotine treatment. We found that at doses of 0.01 and 0.1 μM, nicotine treatment significantly increased BrdU incorporation to 1.3- and 1.5-folds that of control cells (Figure 2A). On the other hand, at doses of 1 and 10 μM, nicotine treatment significantly reduced BrdU incorporation to approximately 0.8- and 0.6-folds that of control cells (Figure 2A). To further confirm this bidirectional regulation by nicotine, we quantified cell proliferation using the CCK-8. We found that the number of live cells as indicated by the optical absorbance of reagent WST-8 at a wavelength of 450 nm significantly increased after 48-hour nicotine exposure at doses of 0.01 and 0.1 μM compared to control treatment, and this number was significantly reduced when cells were treated with 1 or 10 μM nicotine (Figure 2B).
Figure 2.
Bidirectional regulation of mouse embryonic stem cell (mESC) proliferation by nicotine exposure at indicated concentrations for 48 hours. Cell proliferation was analyzed by BrdU incorporation assay (A) and cell proliferation assay kit (CCK-8 assay; B). Data were represented as mean (standard deviation). *P < .05, **P < .01 compared to control (0 μM group, first column).
Nicotine Regulates mESC Cell Cycle Progression in a Dose-Dependent Manner
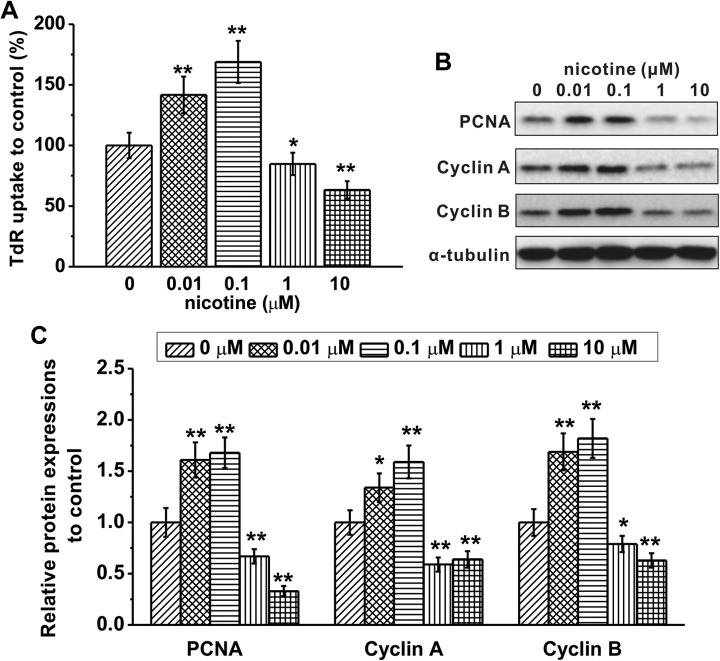
To determine how nicotine controls cell proliferation, we first assessed DNA synthesis by measuring the levels of 3H-TdR incorporation. We found that the ratio of 3H-TdR uptake was significantly increased in cells treated with 0.01 or 0.1 μM nicotine and decreased in cells treated with 1 or 10 μM nicotine compared to that of control cells (Figure 3A). We then examined the levels of proteins involved in cell cycle progression, including PCNA, cyclin A, and cyclin B (Figure 3B). We found that 0.01 or 0.1 μM nicotine significantly increased while 1 or 10 μM nicotine significantly decreased the levels of PCNA, cyclin A, and cyclin B (Figure 3C). These results suggest that nicotine exerts a bidirectional regulation of DNA synthesis and cell cycle progression in a dose-dependent manner, with low doses promoting and high doses suppressing cell cycle progression.
Figure 3.
Effects of nicotine exposure at indicated concentrations for 48 hours on cell cycle progression in mouse embryonic stem cells (mESCs). A, DNA synthesis rate was analyzed by 3H-TdR incorporation assay. B and C, Protein expressions of proliferating cell nuclear antigen (PCNA), cyclin A, and cyclin B were analyzed by Western blotting. α-Tubulin was used as a loading control. Data were represented as mean (standard deviation) *P < .05, **P < .01 compared to control (0 μM group, first column).
Nicotine Regulates Wnt/β-Catenin Signaling Pathway in a Dose-Dependent Manner
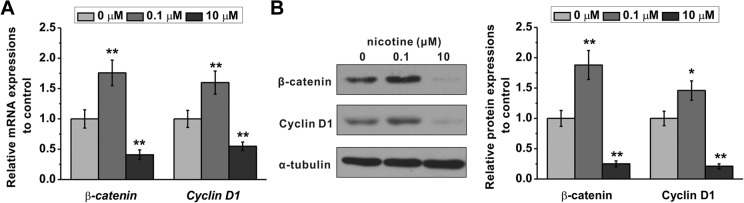
Wnt/β-catenin signaling pathway is critical in maintaining stem cell proliferation.22,23 Previously, it has been shown that nicotine regulates Wnt signaling in various cell types and tissues.18–20 We hypothesized that nicotine exerts a dose-dependent regulation of Wnt/β-catenin signaling pathway to control mESC proliferation. To test this hypothesis, we first examined the mRNA levels of Ctnnb1, the gene that encodes β-catenin protein, and Ccnd1, the gene that encodes cyclin D1 protein downstream of Wnt/β-catenin signaling, by RT-PCR. We found that the relative mRNA levels of both genes were significantly increased following 0.1 μM nicotine treatment, while 10 μM nicotine significantly decreased these levels compared to that of control cells (Figure 4A). We then accessed the protein levels of β-catenin and cyclin D1 by Western blot analysis and found that, similarly, 0.1 μM nicotine significantly increased while 10 μM nicotine significantly decreased these levels (Figure 4B).
Figure 4.
Bidirectional regulation of Wnt/β-catenin signaling pathway by different concentrations of nicotine treatment (0.1 and 10 μM). A, Relative messenger RNA (mRNA) expressions of Ctnnb1 (the gene that encodes β-catenin) and Ccnd1 (the gene that encodes cyclin D1) were quantified by real-time polymerase chain reaction (RT-PCR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. B, Protein expressions of β-catenin and cyclin D1 were analyzed by Western blotting. α-Tubulin was used as a loading control. Data were represented as mean (standard deviation). *P < .05, **P < .01 compared to control (0 μM group, first column).
The Bidirectional Effects of Nicotine on Cell Proliferation Are Reversed by Modulating the Activities of Wnt Signaling Pathway
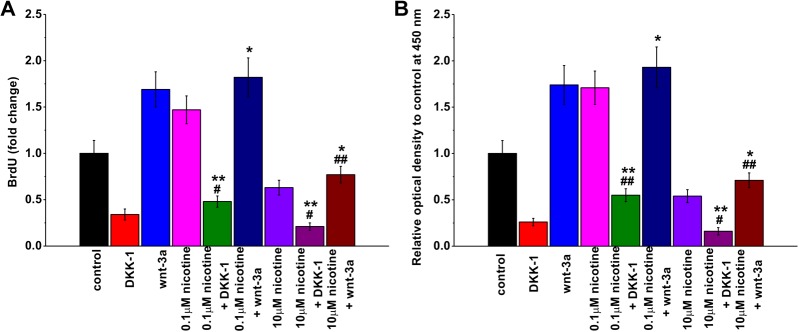
Finally, we determined whether modulating the activities of Wnt signaling pathway by antagonist or agonist is sufficient to ameliorate the effects of nicotine treatment on mESC proliferation. Dickkopf-1 is a potent inhibitor of Wnt signaling.24,25 When added to neural stem cell cultures, DKK-1 reduces neural stem cell proliferation and differentiation.26 Similar to this observation, we found that DKK-1 treatment alone in mESC culture significantly reduced mESC proliferation in both BrdU uptake experiment (Figure 5A) and CCK-8 cell proliferation assay (Figure 5B). Dickkopf-1 suppressed 0.1 μM nicotine-induced increase in mESC proliferation, although the proliferation rate was significantly higher than that of DKK-1 treatment alone. Dickkopf-1 further reduced 10 μM nicotine-induced suppression of mESC proliferation, which was lower than that of DKK-1 treatment alone. We then examined the effects of Wnt signaling agonist Wnt-3α on nicotine-induced regulation of mESC proliferation. We found that, contrary to DKK-1, Wnt-3α further increased mESC proliferation rate in the presence of 0.1 μM nicotine, and it also partially rescued the suppression of mESC proliferation induced by 10 μM nicotine. These results suggest that modulating the activities of Wnt signaling is sufficient to reverse nicotine-induced regulation of mESC proliferation.
Figure 5.
Effects of Wnt signaling pathway antagonist (DKK-1) and agonist (Wnt-3α) on mouse embryonic stem cell (mESC) proliferation with or without nicotine exposure. Cell proliferation was analyzed by BrdU incorporation assay (A) and cell proliferation assay kit (CCK-8 assay; B). Data were represented as mean (standard deviation). *P < .05, **P < .01 compared to the corresponding concentration of nicotine treatment group. #P < .05, ##P < .01 compared to Dickkopf-1 (DKK-1) or Wnt-3a alone group.
Discussion
In the current study, we identified a dual regulation of nicotine on mESC proliferation depending on the levels of nicotine that the cells were exposed to. We found that while nicotine did not affect overall cell survival, low concentrations of nicotine (0.01 and 0.1 μM) promoted mESC proliferation and high concentrations of nicotine (1 or 10 μM) suppressed mESC proliferation. We found that these bimodal effects of nicotine on mESC proliferation were at least partially mediated by Wnt/β-catenin signaling pathway, which eventually regulated cell cycle progression. Consistent with the idea that nicotine acts upstream of Wnt/β-catenin signaling pathway, modulations of Wnt activity by Wnt agonist or antagonist were sufficient to reverse or aggravate the effects of nicotine on mESC proliferation.
In the United States, smoking is the leading cause of premature deaths and is causally associated with various diseases involved in nearly all organs of the body including lung cancer.27 Nicotine is a key compound found in cigarettes that causes addiction as well as various adverse health issues. The level of toxicity caused by nicotine consumption is largely dose dependent and also is related to the duration of exposure.28 Low-level exposure of nicotine, such as by taking in secondhand smoke, is sufficient to induce cellular dysfunction and inflammation. Although there is no safe dose of tobacco smoke in healthy individuals, previous studies suggest that nicotine exposure is neuroprotective in some scenarios. Nicotine inhibits microglia proliferation and suppresses neuroinflammation during brain injury.6 Nicotine increases the number of neurons in hippocampus and alleviates cognitive impairment in a mouse model of Alzheimer disease.4 Nicotine is also beneficial to brain function in mouse models of Parkinson disease.5
Previous studies have also suggested that nicotine could intervene stem cell self-renewal.7–9 Stem cells provide a critical tool for studying genetic engineering and stem cell–based therapeutic strategies. They are able to self-renewal and can differentiate into various cell types.29 Since dysregulation of stem cell proliferation may lead to tumorigenesis, it is critical to study the signaling pathway involved in stem cell proliferation and cell cycle progression. Previously, it has been shown that 10 to 1000 nM nicotine exposure to mESCs significantly enhanced the expression levels of 2 genes critical for the development and differentiation of mESCs, Oct-4 and Rex-1.30
In this study, we explored the effects of nicotine on the survival and proliferation of mESCs. Since very high doses of nicotine have generally caused apoptosis of stem cells, we only analyzed 4 concentrations, 0.01, 0.1, 1, and 10 μM, and compared the effects to cells treated with vehicle alone. We found that nicotine treatment at these 4 doses had no adverse effect on cell survival. Interestingly, mESC proliferation was affected bidirectionally through regulation of proteins involved in cell cycle progression. We observed increased DNA synthesis and upregulated PCNA, cyclin A, and cyclin B in cells treated with 0.01 and 0.1 μM nicotine and a reverse effect in cells treated with 1 and 10 μM nicotine. Our results are consistent with the previous study that nicotine bidirectionally regulates the proliferation of human alveolar bone marrow–derived mesenchymal stem cells, although that study used much higher concentrations of nicotine and found that 1 to 2 mM nicotine treatment increases cell proliferation and 5 mM nicotine decreases cell proliferation.9 It is possible that this discrepancy is due to different cell types exhibiting different sensitivity to nicotine exposure. In fact, other studies using stem cells from other sources also found different effects of nicotine treatment. For example, 5 μM nicotine treatment impairs cell proliferation in human Wharton’s jelly mesenchymal stem cell culture.11 Similarly, increased cell death was observed in hESCs treated with nicotine at concentrations between 1.8 and 3.7 μM.12 These studies together with ours suggest that it is critical to optimize the dose of nicotine in order to reach the ideal outcome, especially in developing stem cell–based therapeutic strategies.
In fact, a wide variety of chemicals and drugs have been reported to exhibit similar hormetic dose responses, with high dose inhibiting and low dose stimulating cellular processes.31–33 Hormesis or biphasic dose response is an important aspect to consider when designing drug treatment and determining cytotoxicity. A few common molecular mechanisms of drugs or chemicals with hermetic characteristics are their function toward receptors or intracellular signaling pathways.32
After identifying the dose-dependent regulation of nicotine on mESC proliferation, we determined the molecular mechanisms underlying this phenomenon. It has been established before that nicotine regulates Wnt/β-catenin signaling.8 In fact, the neuroprotective role of nicotine in a mouse model of Parkinson disease is mediated through activation of Wnt/β-catenin signaling.5 Since Wnt/β-catenin pathway is involved in cell cycle progression16 and it is critical for stem cell self-renewal and differentiation,23 we investigated whether nicotine regulated mESC proliferation and cell cycle progression through the Wnt/β-catenin pathway. We found this to be the case. In fact, suppression of this pathway by Wnt antagonist DKK-1 is sufficient to decrease mESC proliferation even in the presence of 0.1 μM nicotine. Similarly, activation of this pathway by Wnt agonist Wnt-3a increased mESC proliferation in the presence of 10 μM nicotine.
Conclusion
In the current study, we observed a bimodal effect of nicotine on mESC proliferation with lower concentrations promoting and higher concentrations suppressing cell proliferation, similar to drugs and chemicals exhibiting hermetic dose responses. No significant effect on the viability of mESCs was shown at the concentrations examined. We found that this biphasic effect of nicotine on cell proliferation could probably be mediated by the Wnt/β-catenin pathway.
Footnotes
Authors’ Note: Qinglan Qu and Fengrong Zhang contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Checkoway H, Powers K, Smith-Weller T, et al. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155(8):732–738. [DOI] [PubMed] [Google Scholar]
- 4. Esteves IM, Lopes-Aguiar C, Rossignoli MT, et al. Chronic nicotine attenuates behavioral and synaptic plasticity impairments in a streptozotocin model of Alzheimer’s disease. Neuroscience. 2017;353:87–97. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Hao S, Yang B, et al. Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson’s disease model. Biochem Pharmacol. 2017;140:115–123. [DOI] [PubMed] [Google Scholar]
- 6. Guan YZ, Jin XD, Guan LX, et al. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol Neurobiol. 2015;51(3):1480–1488. [DOI] [PubMed] [Google Scholar]
- 7. Lee H, Park JR, Yang J, et al. Nicotine inhibits the proliferation by upregulation of nitric oxide and increased HDAC1 in mouse neural stem cells. In Vitro Cell Dev Biol Anim. 2014;50(8):731–739. [DOI] [PubMed] [Google Scholar]
- 8. Jiang DQ, Wei MD, Wang KW, et al. Nicotine contributes to the neural stem cells fate against toxicity of microglial-derived factors induced by Aβ via the Wnt/β-catenin pathway. Int J Neurosci. 2016;126(3):257–268. [DOI] [PubMed] [Google Scholar]
- 9. Kim BS, Kim SJ, Kim HJ, et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90(3-4):109–115. [DOI] [PubMed] [Google Scholar]
- 10. Oliveira-da-Silva A, Vieira FB, Cristina-Rodrigues F, et al. Increased apoptosis and reduced neuronal and glial densities in the hippocampus due to nicotine and ethanol exposure in adolescent mice. Int J Dev Neurosci. 2009;27(6):539–548. [DOI] [PubMed] [Google Scholar]
- 11. Yang X, Qi Y, Avercenc-Leger L, et al. Effect of nicotine on the proliferation and chondrogenic differentiation of the human Wharton’s jelly mesenchymal stem cells. Biomed Mater Eng. 2017;28(s1):S217–S228. [DOI] [PubMed] [Google Scholar]
- 12. Zdravkovic T, Genbacev O, LaRocque N, McMaster M, Fisher S. Human embryonic stem cells as a model system for studying the effects of smoke exposure on the embryo. Reprod Toxicol. 2008;26(2):86–93. [DOI] [PubMed] [Google Scholar]
- 13. Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guha P, Bandyopadhyaya G, Polumuri SK, et al. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, alpha9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res Treat. 2014;145(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Czechanski A, Byers C, Greenstein I, et al. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat Protoc. 2014;9(3):559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. [DOI] [PubMed] [Google Scholar]
- 17. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. [DOI] [PubMed] [Google Scholar]
- 18. Sakurai R, Cerny LM, Torday JS, Rehan VK. Mechanism for nicotine-induced up-regulation of Wnt signaling in human alveolar interstitial fibroblasts. Exp Lung Res. 2011;37(3):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang C, Xu X, Jin H, Liu G. Nicotine may promote tongue squamous cell carcinoma progression by activating the Wnt/β-catenin and Wnt/PCP signaling pathways. Oncol Lett. 2017;13(5):3479–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/beta-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;304(4):L199–L209. [DOI] [PubMed] [Google Scholar]
- 21. Kook SH, Jeon YM, Lim SS, et al. Fibroblast growth factor-4 enhances proliferation of mouse embryonic stem cells via activation of c-Jun signaling. PLoS One. 2013;8(8):e71641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu RM, Sun RG, Zhang LT, et al. Hyaluronic acid enhances proliferation of human amniotic mesenchymal stem cells through activation of Wnt/β-catenin signaling pathway. Exp Cell Res. 2016;345(2):218–229. [DOI] [PubMed] [Google Scholar]
- 23. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. [DOI] [PubMed] [Google Scholar]
- 24. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. [DOI] [PubMed] [Google Scholar]
- 25. Niida A, Hiroko T, Kasai M, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23(52):8520–8526. [DOI] [PubMed] [Google Scholar]
- 26. Tiwari SK, Agarwal S, Seth B, et al. Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-catenin pathway. Mol Neurobiol. 2015;52(3):1735–1757. [DOI] [PubMed] [Google Scholar]
- 27. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 28. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2010. [PubMed] [Google Scholar]
- 29. Daley GQ. Stem cells: roadmap to the clinic. J Clin Invest. 2010;120(1):8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Guo D, Wang L, et al. Effect of nicotine on Oct-4 and Rex-1 expression of mouse embryonic stem cells. Reprod Toxicol. 2005;19(4):473–478. [DOI] [PubMed] [Google Scholar]
- 31. Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27(7):1451–1474. [DOI] [PubMed] [Google Scholar]
- 32. Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. [DOI] [PubMed] [Google Scholar]
- 33. Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. [DOI] [PubMed] [Google Scholar]