Abstract
To determine whether baseline characteristics had an impact on clinical outcomes in the LixiLan‐O trial (N = 1170), we compared the efficacy and safety of iGlarLixi, a titratable fixed‐ratio combination of insulin glargine 100 U (iGlar) and lixisenatide (Lixi) with iGlar or Lixi alone in patients with uncontrolled type 2 diabetes mellitus (T2DM) on oral therapy. Subgroups according to baseline glycated haemoglobin (HbA1c; <8% or ≥8% [<64 or ≥64 mmol/mol]), T2DM disease duration (<7 or ≥7 years) and body mass index (BMI; <30 or ≥30 kg/m2) were investigated. In all subpopulations, iGlarLixi was consistently statistically superior to iGlar and Lixi alone in reducing HbA1c from baseline to week 30; higher proportions of patients achieved HbA1c <7% (<53 mmol/mol) with iGlarLixi vs iGlar and Lixi alone. Compared with iGlar, iGlarLixi resulted in a substantial decrease in 2‐hour postprandial plasma glucose levels, and mitigation of weight gain, with no differences among subpopulations in incidence of symptomatic hypoglycaemia. iGlarLixi consistently improved glycaemic control compared with iGlar and Lixi alone, without weight gain or increase in hypoglycaemic risk compared with iGlar in the subpopulations tested, regardless of baseline HbA1c, disease duration and BMI.
Keywords: GLP‐1, glycaemic control, insulin therapy, type 2 diabetes mellitus
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are a potential treatment option in patients with type 2 diabetes mellitus (T2DM) who, despite basal insulin treatment, have uncontrolled postprandial plasma glucose (PPG) excursions. The short‐acting GLP‐1RA lixisenatide (Lixi; Lyxumia [Sanofi, Paris, France]; Adlyxin [Sanofi, Bridgewater, New Jersey]) has a prominent effect on substantially lowering PPG levels immediately after administration, mainly via delaying gastric emptying.1
iGlarLixi is a titratable fixed‐ratio combination of insulin glargine 100 U (iGlar) and Lixi. Owing to the complementary mechanism of actions of Lixi and basal insulin, reducing PPG and fasting plasma glucose (FPG), respectively, there is a clinical rationale for this combination. In phase III studies, iGlarLixi was administered via two pen injectors, which allowed flexibility for the dose to be titrated according to a patient's insulin requirement (up to 60 U/d) and for Lixi to be administered up to the maximum approved dose (20 µg/d).2, 3
In the LixiLan‐O trial, in a population of insulin‐naïve patients with T2DM inadequately controlled with metformin ± a second oral antidiabetic (glucose‐lowering) drug (OAD), which was discontinued at the start of the 4‐week lead‐in period, progressive titration of iGlarLixi added to metformin robustly improved glycaemic control compared with each of its components alone, mitigating weight gain, and without additional risk of hypoglycaemia compared with iGlar and with lower gastrointestinal side effects compared with Lixi alone.2
The ability to show that a treatment strategy is more or less equally effective according to the initial characteristics of the patients can be of benefit to clinicians and patients. Furthermore, understanding the underlying factors that may influence response to new treatment options such as iGlarLixi, may be of particular value.4, 5, 6 The objective of the present exploratory subpopulation analysis was to examine the efficacy in subpopulations of patients based on baseline glycated haemoglobin (HbA1c), disease duration and body mass index (BMI) in the LixiLan‐O trial.
2. METHODS
2.1. Trial design
The full methodology of the LixiLan‐O trial (NCT02058147) has been published previously.2 Adults (age ≥18 years) with T2DM diagnosed ≥1 year before the screening visit were eligible for inclusion if they had T2DM inadequately controlled (HbA1c ≥7.5% and ≤10% [≥58 and ≤86 mmol/mol] with metformin alone or ≥7% and ≤9% [≥53 and ≤75 mmol/mol] for patients with metformin and a second OAD) for at least 3 months with metformin ± a second OAD. Patients whose HbA1c was ≥7% and ≤10% after a run‐in period during which only metformin was continued were randomized in a 2:2:1 ratio to receive open‐label, once‐daily iGlarLixi or iGlar (both titrated to FPG <5.6 mmol/L [<100 mg/dL] up to a maximum insulin dose of 60 U/d), or once‐daily Lixi (20 µg/d) continuing with metformin, for 30 weeks. The use of 2 pens, as described previously,2 allowed doses of iGlar between 10 and 60 U/d, while limiting the Lixi dose to a maximum of 20 µg/d.
2.2. Subpopulation analysis
To evaluate the potential impact of well‐recognized disease characteristics on the LixiLan‐O clinical outcomes, participants were split into the following categories according to baseline characteristics: HbA1c <8% or ≥8% (<64 or ≥64 mmol/mol; randomization strata of HbA1c at visit 4/week −1 in the trial protocol; prespecified thresholds in the trial population and also the approximate baseline mean for the study population), duration of T2DM (<7 or ≥7 years; the approximate median duration for the trial population, allowing a more even distribution among the groups [categories determined post hoc]) and BMI (<30 or ≥30 kg/m2; prespecified thresholds in the trial protocol). The following clinical endpoints, changes from baseline to week 30, were analysed post hoc by the above subpopulation categories: HbA1c (analysis prespecified for HbA1c and BMI categories); FPG level; 2‐hour PPG level after a standardized breakfast meal test; and body weight. The proportion of patients who achieved HbA1c <7% (53 mmol/mol; responders) was also analysed for each subpopulation.
Documented symptomatic hypoglycaemia, defined as an event with typical symptoms of hypoglycaemia that were accompanied by measured plasma glucose concentration of ≤3.9 mmol/L (≤70 mg/dL), was also analysed in the safety population in the on‐treatment period in these subpopulations.
2.3. Statistical analyses
A mixed‐effect model with repeated‐measures or two‐way analysis of variance (ANOVA) with last observation carried forward to impute missing data, was used to assess comparisons between treatment groups within subpopulations for continuous data and heterogeneity P values (ANOVA). Variation in baseline values was accounted for by the large sample sizes and by using change from baseline. Heterogeneity was tested using the one degree of freedom contrast corresponding to the test of treatment by subgroup interaction. Comparisons between treatment groups within subpopulations for categorical data were based on Cochran–Mantel–Haenszel tests.
3. RESULTS
A total of 1170 patients were randomized; 469 to iGlarLixi, 467 to iGlar and 234 to Lixi. Baseline demographics and characteristics were similar across treatment groups.2 Each subgroup of patients, based on baseline HbA1c, diabetes duration and BMI, from the iGlarLixi, iGlar and Lixi treatment groups had similar numbers (Table S1).
3.1. Subpopulation analyses according to baseline HbA1c
In the analysis according to baseline HbA1c (<8% or ≥8% [<64 or ≥64 mmol/mol]), mean baseline HbA1c in the subgroups ranged from 7.5% to 8.7% (58‐72 mmol/mol; Table 1). In these subpopulations, iGlarLixi was shown to be statistically superior to both iGlar and Lixi in reducing HbA1c from baseline to week 30, irrespective of the baseline HbA1c (P ≤ .0001; Table 1). The proportion of patients who achieved HbA1c <7% (<53 mmol/mol; responders) was significantly higher with iGlarLixi than with iGlar and Lixi alone for both HbA1c subgroups, with greater differences between iGlarLixi and Lixi in those with HbA1c ≥8% (≥64 mmol/mol) at baseline (Figure 1A).
Table 1.
Subpopulation analyses according to baseline HbA1c (<8% or ≥8% [<64 or ≥64 mmol/mol]), diabetes duration and BMI
| Subpopulation analyses according to baseline HbA1c | ||||||
|---|---|---|---|---|---|---|
| Variable | iGlarLixi | iGlar | Lixi | |||
| <8% | ≥8% | <8% | ≥8% | <8% | ≥8% | |
| HbA1c, % | ||||||
| Baseline (n) | 7.5 ± 0.4 (222) | 8.6 ± 0.5 (245) | 7.5 ± 0.3 (223) | 8.6 ± 0.5 (241) | 7.5 ± 0.3 (109) | 8.7 ± 0.5 (124) |
| Week 30 | 6.3 ± 0.7 | 6.7 ± 0.8 | 6.7 ± 0.7 | 7.0 ± 0.8 | 7.0 ± 0.9 | 7.6 ± 0.8 |
| Week‐30 changea | −1.2 ± 0.7 | −1.9 ± 0.9b | −0.8 ± 0.7 | −1.6 ± 0.8b | −0.5 ± 0.9 | −1.1 ± 0.8b |
| P valuec | <.0001 | .0001 | <.0001 | <.0001 | ||
| FPG, mmol/L | ||||||
| Baseline (n) | 9.1 ± 2.1 (221) | 10.6 ± 2.3 (245) | 8.8 ± 1.8 (223) | 10.6 ± 2.4 (242) | 8.9 ± 1.8 (109) | 10.6 ± 2.2 (123) |
| Week‐30 changea | −2.8 ± 2.3 | −4.0 ± 2.7b | −2.2 ± 2.3 | −4.0 ± 2.9b | −0.8 ± 2.5 | −2.0 ± 2.7b |
| P valuec | .009 | .961 | <.0001 | <.0001 | ||
| 2‐hour PPG, mmol/L | ||||||
| Baseline (n) | 14.0 ± 3.3 (206) | 16.3 ± 3.5 (224) | 13.4 ± 3.4 (210) | 15.8 ± 3.5 (220) | 13.7 ± 3.0 (90) | 15.6 ± 3.3 (106) |
| Week‐30 changea | −5.1 ± 3.8 | −6.9 ± 4.5b | −2.3 ± 3.2 | −4.2 ± 3.6b | −4.4 ± 3.9 | −5.1 ± 4.3 |
| P valuec | <.0001 | <.0001 | .147 | <.0001 | ||
| Body weight, kg | ||||||
| Baseline (n) | 89.7 ± 17.9 (222) | 89.2 ± 16.5 (245) | 90.3 ± 16.2 (223) | 89.2 ± 16.5 (242) | 92.0 ± 17.1 (109) | 89.8 ± 15.5 (124) |
| Week‐30 changea | −0.7 ± 3.8 | 0.1 ± 3.5 | 0.5 ± 4.3 | 1.6 ± 3.8 | −2.6 ± 3.6 | −2.1 ± 3.2 |
| P valuec | <.0001 | <.0001 | <.0001 | <.0001 | ||
| Hypoglycaemiad, % | ||||||
| Events/patient‐year (n) | 1.3 (222) | 1.6 (247) | 1.4 (223) | 1.1 (244) | 0.2 (110) | 0.5 (123) |
| % Patients | 23.0 | 27.9 | 22.4 | 24.6 | 5.5 | 7.3 |
| P valuec | .851 | .361 | <.0001 | <.0001 | ||
| Subpopulation analyses according to diabetes duration | ||||||
|---|---|---|---|---|---|---|
| iGlarLixi | iGlar | Lixi | ||||
| <7 years | ≥7 years | <7 years | ≥7 years | <7 years | ≥7 years | |
| Diabetes duration, years | ||||||
| Mean baseline (n) | 4.2 ± 1.6 (202) | 12.4 ± 4.7 (266) | 4.0 ± 1.7 (210) | 12.4 ± 4.8 (256) | 4.1 ± 1.8 (96) | 12.2 ± 6.1 (137) |
| HbA1c, % | ||||||
| Baseline (n) | 8.0 ± 0.8 (202) | 8.1 ± 0.7 (265) | 8.0 ± 0.7 (210) | 8.1 ± 0.7 (254) | 8.0 ± 0.8 (96) | 8.2 ± 0.7 (137) |
| Week‐30 changea | −1.5 ± 0.9 | −1.6 ± 0.9 | −1.2 ± 0.9 | −1.3 ± 0.8 | −0.8 ± 0.8 | −0.8 ± 0.9 |
| P valuec | .0004 | .0002 | <.0001 | <.0001 | ||
| FPG, mmol/L | ||||||
| Baseline (n) | 9.5 ± 2.2 (201) | 10.2 ± 2.4 (265) | 9.5 ± 2.4 (210) | 10.0 ± 2.3 (255) | 9.3 ± 2.3 (95) | 10.1 ± 2.0 (137) |
| Week‐30 changea | −3.0 ± 2.4 | −3.8 ± 2.7 | −2.7 ± 2.6 | −3.5 ± 2.8 | −1.1 ± 2.0 | −1.7 ± 3.0 |
| P valuec | .222 | .248 | <.0001 | <.0001 | ||
| 2‐hour PPG, mmol/L | ||||||
| Baseline (n) | 14.5 ± 3.4 (185) | 15.7 ± 3.7 (245) | 14.1 ± 3.5 (195) | 15.0 ± 3.7 (235) | 13.9 ± 3.4 (82) | 15.3 ± 3.1 (114) |
| Week‐30 changea | −5.5 ± 4.0 | −6.5 ± 4.5 | −2.9 ± 3.2 | −3.6 ± 3.7 | −3.8 ± 3.9 | −5.4 ± 4.1 |
| P valuec | <.0001 | <.0001 | .001 | .017 | ||
| Body weight, kg | ||||||
| Baseline (n) | 91.2 ± 18.0 (202) | 88.1 ± 16.4 (265) | 93.4 ± 16.1 (210) | 86.8 ± 16.0 (255) | 92.6 ± 16.4 (96) | 89.5 ± 16.1 (137) |
| Week‐30 changea | −0.3 ± 3.6 | −0.4 ± 3.7 | 1.0 ± 4.5 | 1.2 ± 3.7 | −2.1 ± 2.8 | −2.4 ± 3.8 |
| P valuec | .0007 | <.0001 | <.0001 | <.0001 | ||
| Hypoglycaemiad, % | ||||||
| Events/patient‐year (n) | 1.3 (202) | 1.6 (267) | 0.8 (210) | 1.6e (257) | 0.3 (96) | 0.4 (137) |
| % Patients | 21.3 | 28.8 | 19.0 | 27.2 | 7.3 | 5.8 |
| P valuec | .668 | .664 | .001 | <.0001 | ||
| Subpopulation analyses according to BMI | ||||||
|---|---|---|---|---|---|---|
| iGlarLixi | iGlar | Lixi | ||||
| <30 kg/m2 | ≥30 kg/m2 | <30 kg/m2 | ≥30 kg/m2 | <30 kg/m2 | ≥30 kg/m2 | |
| BMI, kg/m2 | ||||||
| Mean baseline (n) | 27.1 ± 2.3 (173) | 34.3 ± 2.9 (295) | 27.1 ± 2.0 (178) | 34.5 ± 3.0 (288) | 26.9 ± 2.2 (74) | 34.4 ± 2.9 (159) |
| HbA1c, % | ||||||
| Baseline (n) | 8.1 ± 0.7 (173) | 8.1 ± 0.7 (294) | 8.1 ± 0.7 (178) | 8.1 ± 0.7 (286) | 8.1 ± 0.7 (74) | 8.1 ± 0.7 (159) |
| Week‐30 changea | −1.6 ± 0.9 | −1.5 ± 0.9 | −1.2 ± 0.9 | −1.3 ± 0.9 | −0.7 ± 0.8 | −0.8 ± 0.9 |
| P valuec | .0002 | .0002 | <.0001 | <.0001 | ||
| FPG, mmol/L | ||||||
| Baseline (n) | 9.8 ± 2.5 (173) | 9.9 ± 2.3 (293) | 9.7 ± 2.3 (178) | 9.8 ± 2.4 (287) | 9.8 ± 2.1 (74) | 9.8 ± 2.2 (158) |
| Week‐30 changea | −3.6 ± 2.7 | −3.3 ± 2.5 | −3.3 ± 2.8 | −3.0 ± 2.8 | −1.6 ± 2.6 | −1.3 ± 2.7 |
| P valuec | .295 | .154 | <.0001 | <.0001 | ||
| 2‐hour PPG, mmol/L | ||||||
| Baseline (n) | 15.4 ± 3.8 (164) | 15.1 ± 3.5 (266) | 15.0 ± 3.9 (165) | 14.4 ± 3.4 (265) | 14.9 ± 3.4 (61) | 14.6 ± 3.3 (135) |
| Week‐30 changea | −6.3 ± 4.7 | −5.9 ± 4.0 | −3.4 ± 3.9 | −3.2 ± 3.3 | −4.7 ± 4.3 | −4.7 ± 4.1 |
| P valuec | <.0001 | <.0001 | .011 | .005 | ||
| Body weight, kg | ||||||
| Baseline (n) | 75.3 ± 11.2 (173) | 97.7 ± 14.4 (294) | 77.1 ± 11.0 (178) | 97.6 ± 14.0 (287) | 76.0 ± 11.8 (74) | 97.7 ± 13.1 (159) |
| Week‐30 changea | 0.3 ± 2.8 | −0.6 ± 4.0 | 2.0 ± 3.4 | 0.6 ± 4.4e | −1.8 ± 2.7 | −2.6 ± 3.6 |
| P valuec | <.0001 | <.0001 | <.0001 | <.0001 | ||
| Hypoglycaemiad, % | ||||||
| Events/patient‐year (n) | 2.2 (174) | 1.0e (295) | 1.8 (179) | 0.9e (288) | 0.8 (75) | 0.1e (158) |
| % Patients | 31.6 | 22.0 | 29.1 | 20.1 | 12.0 | 3.8 |
| P valuec | .535 | .617 | .0001 | <.0001 | ||
All data are mean ± standard deviation, unless stated otherwise. Treatment comparison P values based on 2‐factor ANOVA compared with iGlarLixi. Heterogeneity was assessed and all P values for heterogeneity were not significant, with the exception of hypoglycaemic events/patient‐year by baseline HbA1c for iGlarLixi vs iGlar (P = .004).
Mean change at week 30.
Between‐subpopulation comparison P < .001.
iGlarLixi vs iGlar or Lixi.
Incidence of documented symptomatic hypoglycaemia, defined as event with typical symptoms accompanied by measured plasma glucose concentration of ≤3.9 mmol/L (≤70 mg/dL).
Mean difference <30 kg/m2 vs ≥30 kg/m2.
Between‐subpopulation comparison P < .0001.
Figure 1.
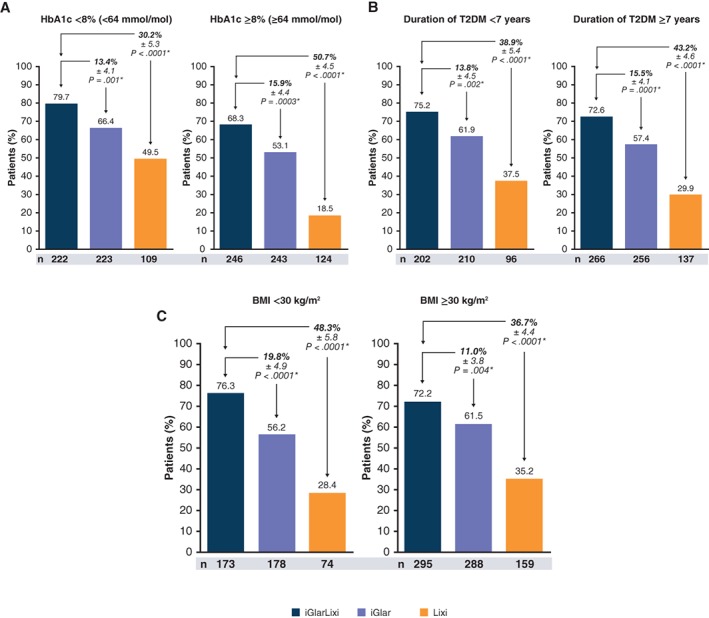
Patients who achieved HbA1c target <7% (<53 mmol/mol; responders) by A, baseline HbA1c; B, diabetes duration; and C, BMI.*Treatment comparisons are based on 2‐factor Cochran–Mantel‐Haenszel.
From baseline to week 30, treatment with iGlarLixi lowered FPG to a similar extent to treatment with iGlar, while the difference vs Lixi alone was more pronounced (P < .0001 for both subpopulations). iGlarLixi had greater reductions than iGlar in 2‐hour PPG from baseline to week 30 for both HbA1c subgroups (P < .0001). Moreover, iGlarLixi was more effective (P < .0001) at lowering 2‐hour PPG levels than Lixi alone in the subpopulation of patients with HbA1c ≥8% (≥64 mmol/mol). Compared with iGlar alone, iGlarLixi also showed significant mitigation of weight gain (P < .0001; Table 1).
3.2. Subpopulation analyses according to duration of T2DM
When participants were divided into categories according to duration of T2DM (<7 or ≥7 years), the mean baseline duration in the subgroups ranged from 4.0 to 12.4 years. iGlarLixi vs both iGlar and Lixi was shown to be consistently better in reducing HbA1c from baseline to week 30 (P < .001; Table 1), with the percentage of responders achieving HbA1c <7% at week 30 being significantly higher, regardless of diabetes duration (Figure 1B).
As expected, based on the prandial mechanism of action of Lixi, the reduction from baseline to week 30 in FPG was significantly smaller in the Lixi group (P < .0001), but was similar for iGlarLixi vs iGlar for both subgroups. Compared with iGlar and Lixi alone, iGlarLixi resulted in greater reductions in 2‐hour PPG at week 30 (P < .0001 and P < .05, respectively), and iGlarLixi showed significant mitigation of weight gain at week 30 compared with iGlar alone (P < .001; Table 1).
3.3. Subpopulation analyses according to BMI
When divided into subgroups according to BMI (<30 or ≥30 kg/m2), baseline BMI in the subgroups ranged from 26.9 to 34.5 kg/m2 (Table 1). iGlarLixi vs both iGlar and Lixi was shown to lead to consistently greater reductions in HbA1c (P < .001; Table 1) and an increased percentage of patients who achieved HbA1c <7% (<53 mmol/mol) at week 30, irrespective of baseline BMI (P < .01; Figure 1C).
iGlarLixi showed similar changes in FPG compared with iGlar alone but greater reductions compared with Lixi (P < .0001). Although iGlarLixi was significantly superior to both iGlar and Lixi in 2‐hour PPG decreases from baseline to week 30 (Table 1), the mean difference vs iGlarLixi was bigger for iGlar than for Lixi. Compared with iGlar alone, iGlarLixi also showed significant mitigation of weight gain (P < .0001; Table 1).
3.4. Hypoglycaemia
No significant differences in the incidence of documented symptomatic hypoglycaemia, as defined previously (percentage and event rate), were noted between the iGlarLixi and the iGlar subgroups (Table 1); however, a slightly higher incidence was noted with iGlarLixi in the HbA1c ≥8% (≥64 mmol/mol) subgroup, which may be linked to the significantly higher (P < .05) mean iGlar dose (U) in this population compared with the HbA1c <8% (<64 mmol/mol) (Table S2). As expected across the subpopulations analysed, there were significantly fewer incidences of hypoglycaemia with Lixi alone than with iGlarLixi (P ≤ .001).
4. DISCUSSION
Understanding the relationships among different patient characteristics, and their impact on treatment efficacy for T2DM may help physicians to personalize therapeutic intervention.
By examining the impact of baseline HbA1c, disease duration and BMI on clinical outcomes, this exploratory, subpopulation analysis showed that in insulin‐naïve metformin‐treated patients evaluated in the LixiLan‐O trial, iGlarLixi was effective across all subpopulations tested, including those with a higher baseline HbA1c (≥8% [≥64 mmol/mol]), those with a longer duration of T2DM (≥7 years) and obese patients (BMI ≥30 kg/m2). Consistently better glycaemic control was evident in iGlarLixi‐ vs iGlar‐treated patients, with the iGlarLixi subpopulations achieving near‐normal HbA1c levels at the end of the trial (6.3%‐6.7% [45‐50 mmol/mol]). In the iGlarLixi group compared with iGlar alone, this was accompanied by a robust PPG‐lowering effect, with mitigated weight gain and generally no increase in hypoglycaemic risk (apart from those with baseline HbA1c ≥8% [≥64 mmol/mol] who had a modest increase in mild hypoglycaemic events and also had a significantly higher corresponding mean iGlar dose at study end). Furthermore, a significantly higher proportion of patients reached target HbA1c levels (<7% [<53 mmol/mol]) across all iGlarLixi subpopulations tested compared with either iGlar or Lixi alone. Across treatment groups, reductions in HbA1c, FPG and PPG were greater in the higher baseline HbA1c (≥8% [≥64 mmol/mol]) subpopulation compared with the respective baseline HbA1c <8% (<64 mmol/mol) group; however, the relative efficacy of iGlarLixi vs iGlar and Lixi alone remained constant.
These data are consistent with the primary analyses from the LixiLan‐O trial, which showed that the titratable fixed‐ratio combination of these two agents, iGlar and Lixi, with distinct but complementary mechanisms of action, was more effective in achieving meaningful improvements in glycaemic control than either component alone, with a mean HbA1c level of 6.5% (48 mmol/mol), mitigating weight gain and without increasing the risk of hypoglycaemia.2 By categorizing the LixiLan‐O trial population according to baseline characteristics, we were able to address the efficacy of iGlarLixi in insulin‐naïve T2DM patient groups, who can be difficult to treat. Importantly, these subpopulation analyses have shown a consistent impact on the efficacy of iGlarLixi in treating patients with T2DM who were inadequately controlled with metformin ± a second OAD and demonstrate that, despite discontinuing a second oral agent other than metformin in this trial population, a wide spectrum of patients can benefit from this titratable fixed‐ratio combination therapy.
A strength of the present exploratory analysis is that the data show that iGlarLixi not only consistently reduced HbA1c levels regardless of baseline characteristics but also affected outcomes such as body weight change, including in patients who were overweight rather than obese and who may be vulnerable to hypoglycaemia. These results support the rationale for combining the two components into one formulation, as iGlar improves FPG and Lixi decreases PPG levels and mitigates weight gain without increasing hypoglycaemia.1, 7, 8, 9, 10
Similar results have also been obtained with IDegLira, a fixed‐ratio combination of insulin degludec and the long‐acting GLP‐1RA liraglutide5, 6 when analysing subgroups of patients with baseline HbA1c and BMI, including a post hoc analysis of the DUAL I extension and DUAL II trials.11
The exploratory nature of these types of analyses, as with any such analysis, means that the results should be interpreted with caution. Additional limitations relating to the LixiLan‐O trial include its open‐label design and relatively short 30‐week duration.2
The present exploratory sub‐analysis supports the use of a titratable fixed‐ratio combination of iGlar and Lixi in a single injection, which takes advantage of their complementary mechanisms of action and consistently improves glycaemic efficacy, with generally no increases in hypoglycaemic risk vs iGlar, irrespective of baseline HbA1c, disease duration or BMI, as a suitable treatment option for patients with T2DM initiating basal insulin.
Supporting information
Table S1. Patients (n) in each subgroup for analysis.
Table S2. iGlar and Lixi doses at week 30 (end of study).
ACKNOWLEDGEMENTS
The authors would like to thank the LixiLan‐O trial investigators and their staff for participating in the study. Principal investigators at the clinical sites are listed in the primary LixiLan‐O publication. M. J. D. wishes to thank the National Institutes of Health Research Leicester‐Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit. The parent study (NCT02058147) for this analysis was funded by Sanofi. Medical writing support was provided by Debby Moss (Caudex, Oxford, UK) and was funded by Sanofi.
Conflict of interest
M. J. D. has acted as consultant, advisory board member and speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi, an advisory board member for Servier and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. She has also received grants in support of investigator and investigator‐initiated trials from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi. L. A. L. has received research funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Pfizer and Sanofi, has provided Continuing Medical Education on behalf of AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi, and has acted as an advisor to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, Servier and Takeda. B. G. has acted as an advisory panel/board member for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Novartis, Novo Nordisk, Roche and Sanofi. He has also acted as a clinical investigator for Abbott, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Medtronic, Merck, Roche and Sanofi, and received research support from Eli Lilly, Novo Nordisk, Medtronic, Sanofi and Vitalaire.
G. G. has received research support from AstraZeneca, Eli Lilly, Lexicon, Medtronic, Merck, and Novo Nordisk, attended speakers’ bureaus for Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi. F. J. A.‐B. has received honoraria as a speaker and/or consultant from Abbott, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, LifeScan, Madaus, MannKind, Medtronic, Menarini, Merck, Merck Farma y Química, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Schering‐Plough and Solvay. C. Y., W. S., E. N. and E. S. are employees of, and own stock/are shareholders in, Sanofi. J. R. has served on scientific advisory boards and received honoraria or consulting fees from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Intarcia, Janssen, Lexicon, Novo Nordisk and Sanofi, and has received grants/research support from Asahi, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Hanmi, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Sanofi and Pfizer.
Author contributions
M. J. D. was involved in study conduct, data collection, interpretation of the results and drafting the manuscript. L. A. L. and G. G. were involved in data analysis and drafting the manuscript. B. G., F. J. A.‐B., C. Y. and W. S. were involved in data analysis, interpretation of the results and drafting the manuscript.
E. N. was involved in study conduct, data analysis, interpretation of results and drafting the manuscript. E. S. was involved in the development of the protocol, study conduct, data analysis, interpretation of results and drafting the manuscript. J. R. was involved in the design of the study, conduct, data collection, interpretation of the results and drafting the manuscript. All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship and that all authors have read, reviewed and agreed to the final version.
Davies MJ, Leiter LA, Guerci B, et al. Impact of baseline glycated haemoglobin, diabetes duration and body mass index on clinical outcomes in the LixiLan‐O trial testing a titratable fixed‐ratio combination of insulin glargine/lixisenatide (iGlarLixi) vs insulin glargine and lixisenatide monocomponents. Diabetes Obes Metab. 2017;19:1798–1804. https://doi.org/10.1111/dom.12980
Funding information The parent study (NCT02058147) for this analysis was funded by Sanofi. Medical writing support was provided by Debby Moss (Caudex, Oxford, UK) and was funded by Sanofi.
REFERENCES
- 1. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36:2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
- 3. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Shenouda SK, Bergenstal RM, et al. Baseline factors associated with glycemic control and weight loss when exenatide twice daily is added to optimized insulin glargine in patients with type 2 diabetes. Diabetes Care. 2012;35:955‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris S, Jaeckel E, Jodar E, et al. Impact of BMI on HbA1c reduction in response to IDegLira in subjects with type 2 diabetes (T2D) uncontrolled on SU, GLP‐1RA or insuline glargine: analyses from completed phase 3 b trials. Diabetes. 2016;65(suppl 1):938‐P. [Google Scholar]
- 6. Sorli C, Harris S, Jodar E, et al. IDegLira is efficacious across baseline HbA1c categories in subjects with type 2 diabetes uncontrolled on SU, GLP‐1RA or insulin glargine: analyses from completed phase 3b trials. Diabetes. 2016;65(suppl 1):925‐P. [Google Scholar]
- 7. Raccah D, Lin J, Wang E, et al. Once‐daily prandial lixisenatide versus once‐daily rapid‐acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications. 2014;28:40‐44. [DOI] [PubMed] [Google Scholar]
- 8. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36:2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal‐plus or basal‐bolus in type 2 diabetes: the GetGoal Duo‐2 trial. Diabetes Care. 2016;39:1318‐1328. [DOI] [PubMed] [Google Scholar]
- 10. Seino Y, Ikeda Y, Niemoeller E, et al. Efficacy and safety of lixisenatide in Japanese patients with type 2 diabetes insufficiently controlled with basal insulin +/− sulfonylurea: a subanalysis of the GetGoal‐L‐Asia study. Horm Metab Res. 2015;47:895‐900. [DOI] [PubMed] [Google Scholar]
- 11. Rodbard HW, Buse JB, Woo V, et al. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab. 2016;18:40‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients (n) in each subgroup for analysis.
Table S2. iGlar and Lixi doses at week 30 (end of study).


