Abstract
The fungus Cryptococcus neoformans is a major cause of morbidity and mortality in patients with impaired CD4+ T cell function, particularly those with AIDS. To identify cryptococcal antigens that could serve as vaccine candidates by stimulating T cell responses, C. neoformans-reactive CD4+ T cell hybridomas were generated by immunization of C57BL/6 mice and fusion of splenocytes with thymoma cells. The antigen that stimulated one of the hybridomas, designated P1D6, to produce IL-2 was purified to homogeneity by sequential anion exchange chromatography, hydrophobic interaction chromatography, and SDS/PAGE. Based on its apparent molecular mass of 98 kDa and mannosylation, the antigen of interest was named MP98. MP98 was N terminal-sequenced, and the gene encoding the protein was cloned and sequenced. Recombinant MP98, expressed in Saccharomyces cerevisiae, stimulated P1D6 to produce IL-2. Analysis of the derived 458-aa sequence of MP98 reveals an N-terminal cleavable signal sequence, a polysaccharide deacetylase domain found in fungal chitin deacetylases, and a serine/threonine-rich C-terminal region. Overall, there were 103 serine/threonine residues serving as potential O-linked glycosylation sites as well as 12 possible N-linked glycosylation sites. Thus, a C. neoformans mannoprotein has been characterized that stimulates T cell responses and has molecular properties of a chitin deacetylase.
The fungus Cryptococcus neoformans has a worldwide distribution in nature (1, 2). Exposure of people to C. neoformans is thought to commonly occur yet overt cryptococcosis is rare in those with intact immune systems. In contrast, cryptococcosis occurs at greatly increased frequency in persons with impaired cell-mediated immunity, particularly those with AIDS, and lymphoreticular malignancy, and recipients of immunosuppressive therapy (1–3). Despite advances in therapy, cryptococcosis can be a difficult infection to successfully treat; morbidity and mortality commonly occur (4). Similar to the situation in humans, in animal models of cryptococcosis, impairment of cell-mediated immunity results in a more deleterious course of infection (5–7). Thus, it is clear that intact T cell function is critical for effective defenses against cryptococcosis. Identification of immunoprotective protein antigens may be critical for development of an effective cryptococcal vaccine, a task assigned a high priority by the National Institutes of Health (8).
Despite much effort from many laboratories, the antigen(s) on C. neoformans that stimulate a protective cell-mediated immunity response against cryptococcosis remain poorly characterized. Murphy and colleagues (9) found that the greater than 50,000 molecular weight fraction of a C. neoformans culture filtrate stimulated delayed-type hypersensitivity responses in immunized mice (9). Further work established that activity resided in the mannoprotein (MP) fraction (9, 10). In humans, lymphocytes from patients recovered from cryptococcosis proliferate in response to C. neoformans culture filtrate and MPs (11, 12).
The T cell receptor (TCR) on CD4+ cells recognizes processed antigen fragments generally consisting of peptides of ≈15 to 24-aa residues presented by class II MHC molecules on an antigen-presenting cell (APC; e.g., dendritic cell, macrophage, or B cell) (13). When CD4+ T cells recognize foreign antigen bound to class II MHC molecules, they secrete cytokines and proliferate. To define the specific antigen reactivity of T cells, T cell clones of defined specificity can be generated. Compared with a mixed population of T cells where cells specific for the antigen of interest may occur at a low frequency, each T cell clone has a unique TCR reactive with a specific epitope. T cell clones can be generated by clonally expanding normal immune T cells by repetitive stimulation with appropriate antigens and APCs. Alternatively, T cells can be immortalized by fusion with a tumor cell line to make hybridomas (14). In the presence of an APC, the antigen of interest will stimulate the hybridoma to make IL-2 or IL-4.
In the studies reported herein, a panel of murine T cell hybridomas reactive with cryptococcal proteins was created. The antigen that stimulated one of the hybridomas was purified to homogeneity, its gene was cloned, and recombinant protein was engineered. The protein of interest was named MP98 owing to its mannosylation and its apparent molecular mass of 98 kDa. Based on homologs in sequence databases, MP98 is likely to be a chitin deacetylase.
Experimental Procedures
Materials.
Reagents were purchased from Sigma unless otherwise stated. PBS, RPMI 1640, and FBS were purchased from Life Technologies (Rockville, MD). Complete media is defined as RPMI 1640 with 10% FBS supplemented with penicillin, streptomycin, ciprofloxacin, and l-glutamine. All incubations were at 37°C in humidified air supplemented with 5% CO2, except where noted.
Fungi.
Acapsular C. neoformans strain cap67 (ATCC 52817) was used for protein purification procedures. This strain was derived from serotype D strain B3501 (ATCC 34873) by UV mutagenesis (15). The rationale for using cap67 was 3-fold. First, glucuronoxylomannan, the major capsular polysaccharide of C. neoformans, suppresses T cell responses (16). Second, use of acapsular organisms avoids the potential problem of glucuronoxylomannan interfering with glycoprotein purification. Third, the parent strain B3501 is relatively easy to manipulate genetically and is closely related to strain JEC 21 (ATCC 96910) (17), which was chosen for sequencing by the Stanford University Technology Center C. neoformans genome project (http://www-sequence.stanford.edu/group/C.neoformans/index.html) and the University of Oklahoma's Advanced Center for Genome Technology (http://www.genome.ou.edu/cneo.html). Saccharomyces cerevisiae strain INVSc1 (auxotrophic for histidine, leucine, tryptophan, and uracil) was purchased from Invitrogen.
Mice.
Male C57BL/6 mice were purchased (The Jackson Laboratory) and maintained under pathogen-free conditions. C57BL/6 mice are moderately susceptible to experimental cryptococcosis and require a CD4+ T cell-dependent immune response for optimal host defenses (6).
Generation of T Cell Hybridomas.
Supernatants of glass bead-disrupted C. neoformans were suspended in Ribi Adjuvant System (Ribi Immunochem) at a final concentration of 0.25 mg protein/ml, and 0.2 ml was injected i.p. into mice on days 0 and 21. For immunizations with live organisms, mice received 107 C. neoformans strain cap67 i.p. on days 0 and 21. T cell hybridomas were generated by using standard methodology (14, 18) with the BW5147 TCR-α−β− thymoma cell line (a gift of George Deepe, Univ. of Cincinnati) as the fusion partner. This cell line lacks CD3-δ, CD3-ξ/η, and FcɛRIγ transcripts as well as functional TCR α and β chain genes (19). Mice were killed on day 28, spleens were removed, and splenocytes were isolated and stimulated in 24-well plates with the same antigenic preparation used for in vivo immunization. For experiments with live C. neoformans, amphotericin B (0.25 μg/ml) was added to prevent fungal overgrowth. On day 31, the stimulated splenocytes were collected and fused with BW5147 TCR-α−β− cells at a 1:1 ratio using 50% polyethylene glycol, molecular weight 1500 (14). Fused cells were then added to 96-well plates (at 104 cells per well) containing complete medium and 6 × 103 resident peritoneal cells as feeder cells. The next day, hypoxanthine/aminopterin/thymidine was added to kill unfused BW5147 cells. Growing hybridomas were identified by microscopy, expanded, and cloned by limiting dilution.
Screening of Hybridomas for Reactivity with C. neoformans Antigens.
The screening procedure took advantage of the fact that each hybridoma expresses a unique TCR with antigen specificity. In the presence of an APC and the appropriate antigen, the hybridomas make IL-2 or IL-4. In 96-well plates, irradiated (3,000 rads) C57BL/6 mouse splenocytes were incubated 24 h with hybridoma cells and an antigen preparation. Supernatants were collected, freeze-thawed, and screened by bioassay using the IL-2/IL-4-sensitive CTLL-2 cell line (20). Briefly, supernatants were coincubated with CTLL-2 cells for 24 h, after which 10 μl of alamarBlue (BioSource International, Camarillo, CA) was added to each well. Plates were incubated an additional 18 h and A570-A600 was determined on a plate reader. Results were compared with a standard curve generated with known amounts of IL-2.
Purification of Antigens that Stimulate Hybridomas.
C. neoformans cap67 was grown in yeast nitrogen broth for 3 days at 30°C with shaking. After centrifugation and washing in PBS, the yeast cells were disrupted by using 400- to 600-μm diameter glass beads in an ice-chilled bead-beater (Biospec Products, Bartlesville, OK). The slurry of disrupted organisms was subjected to sequential low-speed (200 g × 10 min) and high-speed (80,000 g × 30 min) centrifugations, with the supernatant collected after each centrifugation. The final supernatant was passed through a 0.22-μm filter.
Column chromatography was used to purify proteins based on their physicochemical properties. To separate on the basis of size, Sephacryl S-200 (Amersham Pharmacia) gel filtration chromatography was performed. To separate based on charge, protein was diluted in 50 ml of 20 mM Tris⋅HCl buffer, applied to a anion exchange (Mono-Q) column, and eluted with a salt gradient. Sequential runs were performed at pH 6.0 and 7.0. Mono-Q chromatography was carried out by FPLC using an ÄKTAFPLC machine (Amersham Pharmacia) equipped with UV, pH, and conductivity detectors. Final separation was on the basis of hydrophobicity using RP-HPLC (Hewlett–Packard 1090 series II) (21). Briefly, the proteins were applied to a C18 column (catalog 218TP54, Vydac, Heperia, CA) equilibrated in solvent A (aqueous 0.1% triflouroacetic acid) and eluted with a gradient of solvent B (0.09% triflouroacetic acid in acetonitrile) at a flow rate of 1 ml/min. Fractions (0.5 ml) were dried by using a speed vacuum apparatus, reconstituted in 100 μl of PBS, and tested for ability to stimulate hybridoma IL-2 production. Active fractions were then applied to a C4 column (catalog 214TP54, Vydac) and further fractionated in an identical manner as for the C18 column.
Con A Affinity Chromatography to Purify MPs.
MPs were purified from C. neoformans Cap67 culture supernatants as described (22) with slight modifications. Briefly, supernatants were dialyzed and concentrated by using tangential flow filtration (Prep/Scale-TFF-1 Spiral Wound Filter Module with a 10-kDa molecular mass cutoff, Millipore) and subjected to Con A affinity chromatography. MPs were eluted with 0.2 M methyl-α-d-mannopyranoside. Flow-through and eluted fractions were collected, boiled for 5 min to inactivate any Con A leached from the column, and tested for capacity to stimulate IL-2 production from the hybridomas.
T Cell Western Blots.
Supernatants from glass bead-disrupted C. neoformans cap67 were resolved on SDS/PAGE and transferred to nitrocellulose. The nitrocellulose then was cut into six pieces (fractions) so that each fraction contained ng quantities of antigen of a defined molecular size range based on concurrently run molecular weight standards. The apparent molecular weight ranges of the fractions were: fraction 1, >140 K; fraction 2, 80 K–140 K; fraction 3, 40 K–80 K; fraction 4, 30 K–40 K; fraction 5, 25 K–30 K; and fraction 6, <25 K. The pieces then were dissolved in DMSO and fed to irradiated splenocytes in the presence of hybridoma cells (23).
Protein Sequencing.
Samples obtained after HPLC purification were run on a 1-mm thick 7.5% SDS/PAGE gel, lightly stained with Coomassie blue to identify the protein of interest, and destained, and the heart of the band was excised. The gel fragment was washed with HPLC grade 50% acetonitrile/50% water. N-terminal sequence analysis was performed by the Harvard Microchemistry Facility (Cambridge, MA) by Edman degradation.
Construction of a cDNA Library.
To isolate mRNA from C. neoformans strain B3501, an equal number of stationary and logarithmic growth-phase yeast cells incubated at 37°C in yeast nitrogen broth was harvested. The yeast cells were suspended in denaturing solution (4 M guanidium thiocyanate, 25 mM sodium citrate, and 0.1 M 2-β-mercaptoethanol), and an equal volume of phenol-chloroform-isoamyl alcohol was added. The cells then were disrupted with RNase-free glass beads in a bead beater, and the RNA was purified and precipitated by using standard methods (2). mRNA was reverse-transcribed, and a cDNA library was constructed in the Uni-Zap XR vector using a kit (Stratagene). More than 106 original colonies were obtained. The library was amplified once and titered at >1010 plaque-forming units/ml.
PCR.
Primers were designed with the help of a software program (SCI-ED Software, Durham, NC) and manufactured by Life Technologies. Forward primers included: NF1, 5′-GGCGAAATGATCCCTTCCAC; NF5, 5′-TGGACCGGTGTCAACTACAA; NF9, 5′-GGCTGGTAACGGTACTTACA; and T7, 5′-GTAATACGACTCACTATAGGGC. Reverse primers included: NR1, 5′-CAGTCCGGGTCACTGTTAGA; NR3, 5′-GTTAGAGTAGTTGACACCGG; NR5, 5′-CTTCGTTCAAAGCACATCAC; and T3, 5′-AATTAACCCTACTAAAGGG. PCR (25 cycles) was run by using Taq DNA polymerase (Promega), except where indicated. Each cycle consisted of 45-sec denaturation at 95°C, 30-sec annealing at 55°C, and 45-sec extension at 72°C. For the first cycle, denaturation time was increased to 5 min. For the last cycle, extension time was increased to 7 min.
Cloning of MP98.
C. neoformans cDNA library was plated at 2–5 × 104 phage/plate, transferred to nylon membranes (Amersham Pharmacia), and probed with PCR amplimers labeled with 32P-dCTP (New England Nuclear) by using the RTS RadPrime DNA Labeling System (Life Technologies). Prehybridization and hybridization were performed at 65°C. Positive clones were identified by autoradiography. The pBluescript SK plasmid containing the insert of interest was excised from positive phage by using the ExAssist helper phage and transformed into Escherichia coli SOLR cells according to the protocol supplied by Stratagene. Plasmid was isolated from transformed SOLR cells by using a kit (Qiagen, Valencia, CA). Sequencing was performed on a ABI 377–96 automated DNA sequencer (Applied Biosystems).
Expression of MP98 in S. cerevisiae.
PCR was performed with primers NF1 and NR1 and the pBluescript SK plasmid containing full-length MP98 cDNA as the template by using BIO-X-ACT DNA polymerase (Bioline, Kenilworth, NJ). The amplimer was ligated into the pYES2.1/V5-His-TOPO vector (Invitrogen) and then transformed into competent E. coli. pYES2.1/V5-His/lacZ was used as a control vector. Colonies for screened for correct orientation and size by restriction enzyme digestion and PCR. Plasmid was purified from positive colonies containing the correct pYES2.1/V5-His-TOPO clone (Qiagen) and transformed into S. cerevisiae strain INVSc1. Colonies growing on SC minimal medium lacking uracil were collected, and recombinant MP98 was induced by growth on SC minimal medium containing 2% galactose as the carbon source.
Results
Creation of C. neoformans-Reactive T Cell Hybridomas.
In three independent experiments, mice were immunized with either live C. neoformans or glass bead-disrupted fungi and T cell hybridoma lines generated as described in Experimental Procedures. Of the 603 lines created, 108 (17.9%) were reactive with C. neoformans, as defined by their production of IL-2 or IL-4 after stimulation with cryptococcal antigens presented by irradiated splenocytes. As expected, by flow cytometric analysis, all reactive hybridomas expressed the TCR-CD3 complex and CD4 (data not shown). CD8+ hybridomas cannot be created by using BW5147 cells in this system.
Of the 108 hybridomas created, one hybridoma, designated P1D6, was chosen for further study. P1D6 was selected because it made large amounts of IL-2 in response to APCs stimulated with bead-disrupted and live C. neoformans (strains cap67, B3501, and 145) but did not respond to S. cerevisiae antigen preparations. Moreover, this hybridoma grew quickly and retained reactivity after multiple passages as well as after freezing and thawing. As demonstrated by using neutralizing antibodies, P1D6 made IL-2 when stimulated. The experiments described below focused on purification and molecular characterization of the cryptococcal protein that stimulates P1D6 and cloning of the gene encoding for the protein. The protein of interest was named MP98.
Stimulation of P1D6 by Cryptococcal MPs.
MPs have been identified as major antigens in cryptococcal culture filtrates responsible for the delayed-type hypersensitivity reaction seen in animals immunized to C. neoformans (10, 24). Therefore, to determine whether the antigen of interest, MP98, was a secreted MP, culture filtrates were isolated and fractioned by Con A affinity chromatography. Crude culture filtrate and the eluted MP fraction stimulated IL-2 secretion by P1D6 (data not shown). In contrast, the flow-through fraction failed to stimulate even at protein concentrations up to 20 μg/ml). These data suggest that MP98 is mannosylated.
Molecular Sizing of MP98, the Cryptococcal Antigen that Stimulates P1D6.
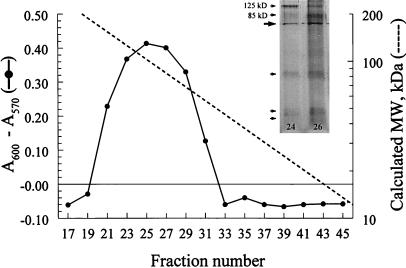
To determine the apparent molecular weight of MP98, two sets of experiments were performed—size-exclusion column chromatography and T cell Western blotting. The supernatant from disrupted cap67 was applied to a calibrated Sephacryl S-200 column, and fractions were screened for their capacity to stimulate P1D6 hybridomas to secrete IL-2 in the presence of irradiated splenocytes as APCs. Activity was seen over a broad range of column fractions, with peak activity at fraction 25, which corresponded to an apparent molecular mass of ≈102 kDa (Fig. 1). Column fractions 24 and 26 then were concentrated and run on SDS/PAGE followed by silver staining. Two bands were seen with apparent molecular masses of ≈85 and 125 kDa (Fig. 1 Inset). However, not seen well on the image of the gel, between the 85-kDa and 125-kDa bands is at least one band with negative staining. Negative staining bands can be seen with heavily glycosylated proteins. To further study the molecular weight of the antigen of interest, T cell Western blotting was performed. In agreement with the data from the Sephacryl S-200 column, P1D6 reacted with fraction 2 (data not shown), suggesting a molecular mass of between 80 and 140 kDa.
Figure 1.
Fractionation of cryptococcal proteins by size-exclusion chromatography. Supernatants from disrupted C. neoformans were applied to a calibrated Sephacryl S-200 column, and alternating fractions were tested for their capacity to stimulate the hybridomas to produce IL-2 in the presence of APC. A600–A570 represents reduction of alamarBlue by CTLL-2 cells (a direct measure of hybridoma IL-2 production). Absorbance (A600–A570) values for 0, 0.01, 0.1, and 1 units/ml of IL-2 standard were −0.073, 0.112, 0.234, and 0.385, respectively. Dotted line represents calculated molecular mass based on peaks obtained with molecular mass standards. Fraction 25, which had peak activity, had an estimated molecular mass of 102 kDa. Results are representative of five column runs. (Inset) Column fractions 24 and 26 were concentrated, separated by SDS/PAGE, and silver-stained. Two bands were seen with apparent molecular masses of ≈85 and 125 kDa. The large arrow points to a nonspecific band. The small arrows point to lower molecular mass bands.
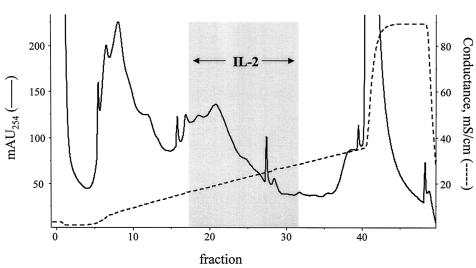
Separation of Cryptococcal Proteins by Ion-Exchange Chromatography.
The intracellular fraction of glass bead-disrupted cap67 was diluted in 20 mM Tris buffer, pH 6.0, applied to a Mono-Q column, and eluted with a sodium chloride gradient at pH 6.0. Three broad peaks were obtained. One-milliliter column fractions then were screened for their capacity to stimulate hybridomas to secrete IL-2 in the presence of irradiated splenocytes as APCs (Fig. 2). Peak IL-2 production was seen when P1D6 was stimulated with fractions eluted when the buffer conductivity was ≈20 mS. The three most stimulatory fractions were pooled, diluted in 20 mM Tris buffer, pH 7.0, applied to the Mono-Q column, and eluted with a salt gradient at pH 7.0. Peak bioactivity was seen in fractions corresponding to a conductivity of ≈19 mS (chromatogram not shown). Again, the three fractions with greatest bioactivity were pooled.
Figure 2.
Separation of cryptococcal proteins by anion-exchange chromatography. Supernatants from glass bead-disrupted C. neoformans were separated on a Mono-Q column at pH 6 by using a NaCl gradient. One-milliliter fractions were collected and tested for their capacity to stimulate hybridoma P1D6 to produce IL-2 in the presence of APCs. Gray box demonstrates the fractions that stimulated IL-2 production. Chromatogram is representative of 10 experiments.
Separation of Cryptococcal Proteins by Hydrophobic Interaction Chromatography.
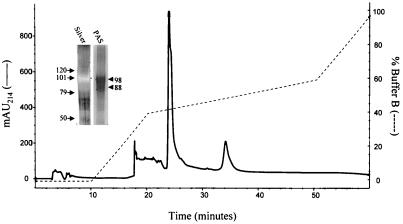
Pooled active fractions eluted as described above after ion-exchange chromatography were separated by RP-HPLC using a C18 column (Fig. 3). One large peak was seen along with several smaller peaks. Based on capacity to stimulate P1D6 to produce IL-2, the large peak contained the antigen of interest. However, the peak also contained a “right shoulder.” Resolution of the peak on SDS/PAGE followed by silver staining revealed two bands of estimated molecular mass of 98 and 88 kDa, which exhibited “negative” staining (Fig. 3 Inset). These bands were stained with periodic acid/Schiff reagent. On both silver staining and periodic acid/Schiff reagent staining, the 98-kDa band predominated. As a final “polishing” step, the large peak fraction from the C18 column was applied to a C4 column. A single large peak was obtained as well as three smaller peaks which appeared in later fractions (data not shown). The fractions representing the large peak stimulated P1D6 to produce IL-2.
Figure 3.
Purification of the cryptococcal protein that stimulates P1D6 by RP-HPLC. Active fractions from anion-exchange chromatography were applied to a C18 column and eluted with a gradient of acetonitrile in 0.1% triflouroacetic acid (buffer B). Bioactivity (as measured by the ability to stimulate P1D6 to produce IL-2 in the presence of APCs) was associated with the large peak seen at 24-min elution time. Results are representative of four column runs. (Inset) The large peak was resolved by 7.5% SDS/PAGE. Silver staining revealed two “negative” bands. These bands stained with periodic acid/Schiff reagent at apparent molecular masses of ≈98 and 88 kDa. The locations of molecular mass markers of 120, 101, 79, and 50 kDa are marked by arrows.
N-Terminal Sequencing of the Protein of Interest.
The fractions containing the protein of interest isolated after elution on the C4 column were combined, concentrated, and run on SDS/PAGE. A single band of apparent molecular mass of 98 kDa was seen after Coomassie blue staining. The band was excised and subjected to N-terminal sequencing. The sequence ASTDVNTEATAYGYAPVTEI was obtained. A tblastn search of the Stanford C. neoformans genome project database revealed several traces with 16/17 predicted identity (for amino acids 4–20) as well as one contig. A tblastn search of the University of Oklahoma C. neoformans strain H99 blast server database revealed one expressed sequence tag (EST) with a 15/19 identity (for amino acids 2–20). Of note, that EST was partially sequenced in both directions, thus revealing both the 5′ and 3′ ends of the mRNA.
Cloning of MP98.
Based on the sequences obtained from the two databases, the sequence of MP98 was predicted. PCR primers NF1 and NR1 were used to amplify cDNA obtained from the phagemid library. An amplimer of the expected size, 397 bp, was obtained and its identity was confirmed by DNA sequencing. This amplimer was 32P-labeled. Northern blotting of C. neoformans RNA revealed a single band migrating at ≈1.4–1.6 kB (data not shown). Labeled amplimer then was used to probe the C. neoformans cDNA phagemid library. After two rounds of screening, six positive plaques were obtained with the expected insert size, restriction endonuclease pattern, and size after PCR. The plasmid was excised from one positive colony and transformed into E. coli, and the insert containing MP98 cDNA was sequenced in both directions with a minimum of 2-fold redundancy using the primer sets described in Experimental Procedures. The ORF of MP98 contains 1,377 bp. Based on the MP98 gDNA sequence from the Stanford University C. neoformans genome project, there are four predicted introns. The N-terminal amino acid sequence obtained from the purified protein begins at amino acid 52, suggesting that the first 51 aa are not part of the mature protein. Fig. 6, which is published as supplemental data on the PNAS web site, www.pnas.org, shows the alignment of the sequences of MP98 cDNA and gDNA, as well as the predicted 458 aa of MP98.
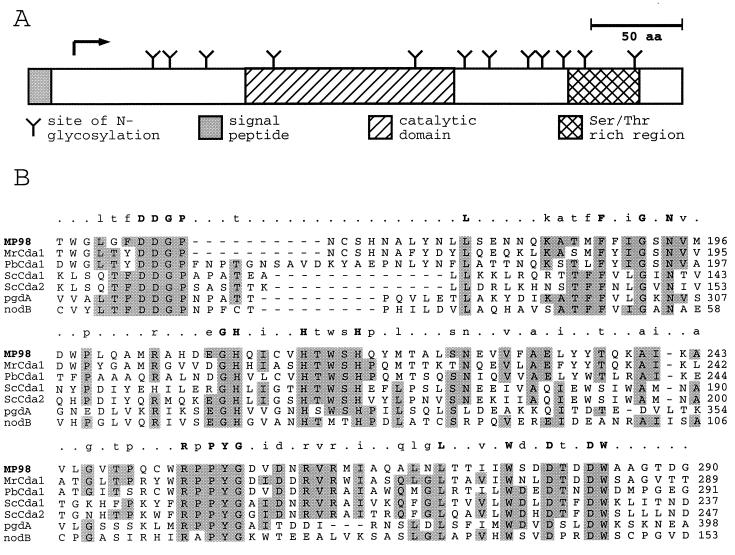
Analysis of the MP98 protein sequence revealed relevant features shown in Fig. 4A. A signal peptide was predicted (SIGNALP V2.0, Center for Biological Sequence Analysis, http://www.cbs.dtu.dk/) with a cleavage site between amino acids 20 and 21. There are also several potential glycosylation sites including a serine- and threonine-rich C-terminal region, which could serve as a site for extensive O-glycosylation. Moreover, of the 28 asparganine residues, 12 are part of the Asn-X-Ser/Thr glycosylation sequon and thus serve as potential N-glycosylation sites (25). The C-terminal portion of MP98 has a putative glycosylphosphatidylinositol anchor site. When subjected to a blastp search, Mp98 was found to have a significant degree of similarity with chitin deacetylases from the fungal species S. cerevisiae, Mucor rouxii, and Phycomyces blakesleeanus. MP98 was most similar to the M. rouxii Cda1 protein with 40% identity/55% similarity when the full-length sequences were aligned. A region of about 150 aa showed the greatest homology and is conserved in the other fungal chitin deacetylases, the chitooligosaccharide deacetylase nodB of Rhizobium species, and a peptidoglycan N-acetylglucosamine deacetylase of Streptococcus pneumoniae (Fig. 4B). This region probably represents the catalytic domain of each protein and suggests that MP98 can function as a chitin deacetylase.
Figure 4.
Features of the MP98 protein sequence (A) and its similarity to N-acetylglucosamine deacetylases (B). The signal sequence (shaded), catalytic domain (hatched), and Ser/Thr rich region (cross-hatch) of MP98 are boxed in A. Each putative site of N-glycosylation is indicated by a Y, and the position of the N terminus of the mature MP98 protein is indicated by an arrow. The putative catalytic domain of MP98 identified by blast searches was aligned by clustal with comparable regions of chitin deacetylases of M. rouxii (MrCDA1, A47713); P. blakesleeanus (PbCDA1, BAB03595); S. cerevisiae (ScCDA1, NP 013410 and ScCDA2, NP 013411); a peptidoglycan N-acetylglucosamine deacetylase of S. pneumonia (pgdA, CAB96552); and a chitooligosaccharide deacetylase of R. leguminosarum (nodB, P04676). Amino acids identical in four or more sequences are shaded. Above each section of the alignment amino acid identities in 6/6 sequences (uppercase letters), 4/6 or 5/6 sequences (lowercase letters), and nonidentity (periods) are summarized.
Stimulation of P1D6 by Recombinant MP98 Expressed in S. cerevisiae.
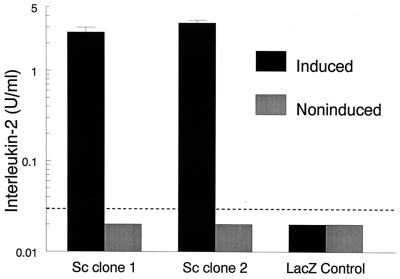
MP98 was inserted into the pYES2.1/V5-His-TOPO vector and then transformed into competent E. coli. Purified plasmid from two colonies with correct orientation and size were transformed into S. cerevisiae. Recombinant MP98 was induced or left noninduced by growth on SC minimal medium containing galactose or glucose, respectively. The transformed S. cerevisiae was disrupted with glass beads, and supernatants were used to stimulate hybridoma P1D6 in the presence of APCs. As a control, S. cerevisiae was transformed with the pYES2.1/V5-His/lacZ vector. Both strains of S. cerevisiae expressing MP98 stimulated P1D6 to produce IL-2 whereas the LacZ control strain did not (Fig. 5). Stimulation was seen only when recombinant protein was induced with galactose.
Figure 5.
Stimulation of P1D6 by recombinant MP98 expressed in S. cerevisiae. S. cerevisiae was transformed with the pYES2.1/V5-His-TOPO vector containing MP98 cDNA. Two clones were selected (Sc clone 1 and Sc clone 2) and yeast cells were grown under conditions where recombinant protein is induced or noninduced. Supernatants from disrupted S. cerevisiae then were tested for their capacity to stimulate hybridoma P1D6 to produce IL-2 in the presence of APCs. As a control, supernatants were used from disrupted S. cerevisiae transformed with pYES2.1/V5-His/lacZ (LacZ Control). The dotted line denotes the lower limit of the IL-2 bioassay, 0.03 units/ml. Values below this lower limit were arbitrarily assigned a value of 0.02 units/ml. Data are means ± SE of one representative duplicate experiment.
Discussion
MPs have been identified as the major antigens stimulating T cell responses to C. neoformans (10, 12, 22). However, the studies reported herein purify to homogeneity, clone, and express an immunoreactive cryptococcal MP. Two immunoreactive C. neoformans glycoproteins, with estimated molecular masses of 105 and 115 kDa, have been partially purified. The former is a MP involved in the lymphoproliferative response to Cryptococcus (26) whereas the latter is a glycoprotein recognized by immune patient sera (27). Sequences of these glycoproteins were not presented. MPs also have been identified as major targets of immune responses to other fungi (28, 29).
By immunoelectron microscopy, most cryptococcal MP is located in the vicinity of the inner cell wall (30). Purified C. neoformans cell walls lack mannose, suggesting that the MPs are not a structural element of the cell wall. Analysis of the predicted amino acid sequence of MP98 reveals multiple sites for N- and O-glycosylation. Of the 458 aa in the full-length protein, there are 28 asparganine residues, 53 serine residues, and 50 threonine residues. Twelve of the 28 asparganine residues have the Asn-X-Ser/Thr N-linked glycosylation sequon (25). Of particular note is a 53-aa stretch at the C-terminal end containing 34 (64%) serine and threonine residues. Crude preparations of cryptococcal MPs have been estimated to contain as much as 80% carbohydrate (12, 22).
Amino acids 160–290 of MP98 contain the polysaccharide deacteylase domain. This domain is found in fungal chitin deacetylases (31), nodulation (chitooligosaccharide deacetylase) proteins (32), peptidoglycan N-acetylglucosamine deacetylase of S. pneumoniae (33), and endoxylanases (34). MP98 has the strongest homology with fungal chitin deacetylases. Although definitive proof will require biochemical confirmation, MP98 is likely a chitin deacetylase. Chitin deacetylases catalyze the hydrolysis of N-acetamido bonds of chitin (a homopolymer comprising β1,4-linked N-acetyl-d-glucosamine residues), converting it to chitosan (35). The major biological role of chitin deacetylases appears to be in cell wall formation, although a role in plant pathogenesis has been suggested (35, 36). Our data demonstrate the immunogenicity of chitin deacetylases. S. cerevisiae has two functionally redundant chitin deacetylase genes, CDA1 and CDA2 (31, 37). Disruption of both genes results in viable cells that lack dityrosine on the outermost ascospore wall and are hypersensitive to lytic enzymes (31, 37). Interestingly, the CDA1 and CDA2 genes are expressed only during a distinct time period during sporulation. Vegetative cells of S. cerevisiae lack chitin deacetylase activity as well as chitosan in the cell wall (31). In contrast, both MP98 protein and MP98 mRNA were isolated from C. neoformans grown in the vegetative phase. A blast search of the C. neoformans genome project database reveals a second gene with homology to chitin deacetylases.
The antigen, MP98, which stimulates hybridoma P1D6, is likely to be one of many that elicit T cell responses, and ongoing studies have been directed at identifying additional antigens. A cryptococcal gene, designated DHA1, was recently cloned that encodes for a 20-kDa immunoreactive protein (38). Immunization of mice with a crude MP preparation containing MP98 is partially protective (M.K.M. and S.M.L., unpublished data). Efforts are directed at expressing recombinant MP98 in sufficient qualities to test its protective efficacy. Other approaches to cryptococcal vaccine development involve antibody-mediated protection (39, 40). Ultimately, for a vaccine against cryptococcosis to be successful, a combination vaccine that elicits both humoral and cell-mediated immunity responses may confer the greatest protection.
Supplementary Material
Acknowledgments
We thank Eleanor North and Xiulin Liu for technical assistance, George Deepe for stimulating discussions, and Herbert Kagan for help with protein purification. This work was supported by National Institutes of Health Grants AI37532, AI25780, GM-54380, and GM-31318. S.M.L. is the recipient of a Burroughs Wellcome Fund Scholar Award in Pathogenic Mycology.
Abbreviations
- APC
antigen-presenting cell
- MP
mannoprotein
- TCR
T cell receptor
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF361369).
References
- 1.Levitz S M. Rev Infect Dis. 1991;13:1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, DC: Am. Soc. Microbiol.; 1998. [Google Scholar]
- 3.Currie B P, Casadevall A. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 4.Saag M S, Graybill R J, Larsen R A, Pappas P G, Perfect J R, Powderly W G, Sobel J D, Dismukes W E. Clin Infect Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 5.Diamond R D. Infect Immun. 1977;17:187–194. doi: 10.1128/iai.17.1.187-194.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mody C H, Lipscomb M F, Street N E, Toews G B. J Immunol. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 7.Hill J O. J Exp Med. 1992;175:1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon D M, Casadevall A, Klein B, Mendoza L, Travassos L, Deepe G S., Jr Med Mycol. 1998;36:57–67. [PubMed] [Google Scholar]
- 9.Murphy J W. Rev Infect Dis. 1988;10:S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- 10.Murphy J W, Mosley R L, Cherniak R, Reyes G H, Kozel T R, Reiss E. Infect Immun. 1988;56:424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoy J F, Murphy J W, Miller G G. J Infect Dis. 1989;159:116–119. doi: 10.1093/infdis/159.1.116. [DOI] [PubMed] [Google Scholar]
- 12.Levitz S M, North E A. J Med Vet Mycol. 1997;35:229–236. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 13.Bentley G A, Mariuzza R A. Annu Rev Immunol. 1996;14:563–590. doi: 10.1146/annurev.immunol.14.1.563. [DOI] [PubMed] [Google Scholar]
- 14.Kruisbeek A M. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Vol. 1. New York: Green/Wiley-Interscience; 1992. pp. 3.14.1–3.14.11. [Google Scholar]
- 15.Jacobson E S, Ayers D J, Harrell A C, Nicholas C C. J Bacteriol. 1982;150:1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan L H, Klein B S, Levitz S M. Clin Microbiol Rev. 1996;9:469–488. doi: 10.1128/cmr.9.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung K J, Edman J C, Wickes B L. Infect Immun. 1992;60:602–605. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepe G S, Jr, Brunner G D. Infect Immun. 1990;58:1538–1544. doi: 10.1128/iai.58.6.1538-1544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S Y, Ardouin L, Gillet A, Malissen M, Malissen B. J Exp Med. 1997;185:707–715. doi: 10.1084/jem.185.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treseler C B, Maziarz R T, Levitz S M. Infect Immun. 1992;60:183–188. doi: 10.1128/iai.60.1.183-188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mambula S S, Simons E R, Hastey R, Selsted M E, Levitz S M. Infect Immun. 2000;68:6257–6264. doi: 10.1128/iai.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orendi J M, Verheul A F M, DeVos N M, Visser M R, Snippe H, Cherniak R, Vaishnav V V, Rijkers G T, Verhoef J. Clin Exp Immunol. 1997;107:293–299. doi: 10.1111/j.1365-2249.1997.283-ce1169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou-Zeid C, Filley E, Steele J, Rook G A. J Immunol Methods. 1987;98:5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- 24.Cherniak R, Sundstrom J B. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helenius A, Aebi M. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 26.Pitzurra L, Vecchiarelli A, Peducci R, Cardinali A, Bistoni F. J Med Vet Mycol. 1997;35:299–303. [PubMed] [Google Scholar]
- 27.Hamilton A J, Goodley J. J Clin Microbiol. 1993;31:335–339. doi: 10.1128/jcm.31.2.335-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Valle R, Sandini S, Gomez M J, Mondello F, Romagnoli G, Nisini R, Cassone A. Infect Immun. 2000;68:6777–6784. doi: 10.1128/iai.68.12.6777-6784.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L, Chan C M, Lee C, Wong S S, Yuen K Y. Infect Immun. 1998;66:966–973. doi: 10.1128/iai.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vartivarian S E, Reyes G H, Jacobson E S, James P G, Cherniak R, Mumaw V R, Tingler M J. J Bacteriol. 1989;171:6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra C, Semino C E, McCreath K J, de la Vega H, Jones B J, Specht C A, Robbins P W. Yeast. 1997;13:327–336. doi: 10.1002/(SICI)1097-0061(19970330)13:4<327::AID-YEA96>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Nature (London) 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 33.Vollmer W, Tomasz A. J Biol Chem. 2000;275:20496–20501. doi: 10.1074/jbc.M910189199. [DOI] [PubMed] [Google Scholar]
- 34.Millward-Sadler S J, Poole D M, Henrissat B, Hazlewood G P, Clarke J H, Gilbert H J. Mol Microbiol. 1994;11:375–382. doi: 10.1111/j.1365-2958.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsigos I, Martinou A, Kafetzopoulos D, Bouriotis V. Trends Biotechnol. 2000;18:305–312. doi: 10.1016/s0167-7799(00)01462-1. [DOI] [PubMed] [Google Scholar]
- 36.Vander P, Vårum K M, Domard A, Eddine El Gueddari N, Moerschbacher B M. Plant Physiol. 1998;118:1353–1359. doi: 10.1104/pp.118.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christodoulidou A, Bouriotis V, Thireos G. J Biol Chem. 1996;271:31420–31425. doi: 10.1074/jbc.271.49.31420. [DOI] [PubMed] [Google Scholar]
- 38.Mandel M A, Grace G G, Orsborn K I, Schafer F, Murphy J W, Orbach M J, Galgiani J N. Infect Immun. 2000;68:6196–6201. doi: 10.1128/iai.68.11.6196-6201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devi S J N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zebedee S L, Koduri R K, Mukherjee J, Mukherjee S, Lee S, Sauer D F, Scharff M D, Casadevall A. Antimicrob Agents Chemother. 1994;38:1507–1514. doi: 10.1128/aac.38.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.