Abstract
Introduction
Radial forearm free flap (RFFF) tube‐in‐tube phalloplasty is the most performed phalloplasty technique worldwide. The conspicuous donor‐site scar is a drawback for some transgender men. In search for techniques with less conspicuous donor‐sites, we performed a series of one‐stage pedicled anterolateral thigh flap (ALT) phalloplasties combined with RFFF urethral reconstruction. In this study, we aim to describe this technique and assess its surgical outcome in a series of transgender men.
Patients and Methods
Between January 2008 and December 2015, nineteen transgender men (median age 37, range 21–57) underwent pedicled ALT phalloplasty combined with RFFF urethral reconstruction in one stage. The surgical procedure was described. Patient demographics, surgical characteristics, intra‐ and postoperative complications, hospitalization length, and reoperations were recorded.
Results
The size of the ALT flaps ranged from 12 × 12 to 15 × 13 cm, the size of the RFFFs from 14 × 3 to 17 × 3 cm. Median clinical follow‐up was 35 months (range 3–95). Total RFFF failure occurred in two patients, total ALT flap failure in one patient, and partial necrosis of the ALT flap in one patient. Long‐term urinary complications occurred in 10 (53%) patients, of which 9 concerned urethral strictures.
Conclusions
In experienced hands, one‐stage pedicled ALT phalloplasty combined with RFFF urethral reconstruction is a feasible alternative surgical option in eligible transgender men, who desire a less conspicuous forearm scar. Possible drawbacks comprise flap‐related complications, difficult inner flap monitoring and urethral complications.
1. INTRODUCTION
Phalloplasty, the surgical creation of a penis, is indicated for transgender men and biological men with congenital or acquired (partial) absence of the penis. Transgender men report a good quality of life and experience satisfactory sexual function after phalloplasty (Wierckx et al., 2011). The radial forearm free flap (RFFF) is the most frequently performed phalloplasty technique in these patients (Fang, Lin, & Ma, 1994; Monstrey et al., 2005; Leriche et al., 2008). It is considered the gold‐standard technique for penile (re)construction, because of the thin pliable forearm skin, reliable vascularization pattern and easy resensibilisation (Monstrey et al., 2009). A tube‐in‐tube design of the flap facilitates voiding in a standing position (Gillies & Millard, 1957). However, disadvantages comprise donor‐site morbidity, a conspicuous large scar area on the forearm and penile color mismatch. Donor‐site morbidity is estimated to arise more frequently after RFFF phalloplasty than other RFFF‐based reconstructions because of the larger flap size, which is approximately as large as two thirds of the forearms’ circumference. A contraindication exists if the vascular arcades in the hand are incomplete and the ulnar artery therefore provides insufficient blood supply to the hand. The Allen test can be used to check collateral circulation of the hand. A surgical alternative is the pedicled anterolateral thigh flap (ALT) phalloplasty (Descamps, Hayes, & Hudson, 2009; Felici & Felici, 2006; Lumen, Monstrey, Ceulemans, van Laecke, & Hoebeke, 2008; Morrison et al., 2014). This also creates a large donor‐site scar, however this is easier to conceal for patients (eg underwear, swimwear) when compared to the RFFF scar. A tube‐in‐tube ALT phalloplasty is only possible in thin patients with minimal subcutaneous tissue. In patients who are not eligible for a tube‐in‐tube RFFF or ALT phalloplasty, but who do express the wish for voiding in the standing position, other surgical approaches can be performed. In search for phalloplasty techniques with less conspicuous donor‐sites we performed a series of one‐stage double flap phalloplastiy procedures with a pedicled ALT combined with RFFF urethral reconstruction. In this study, we aim to describe this technique and assess its surgical outcome in a series of transgender men.
2. PATIENTS AND METHODS
Between January 2008 and December 2015, 19 transgender men (median age 37, range 21–57) underwent a pedicled ALT phalloplasty combined with a RFFF urethral reconstruction in one stage with a median clinical follow‐up of 35 months (3–95). Patient demographics are presented in Table 1. All patients previously underwent laparoscopic hysterectomy with bilateral adnexectomy and colpectomy. Patient demographics, surgical characteristics, intra‐ and postoperative complications, hospitalization length and reoperations were recorded. Descriptive statistics were calculated for all variables. Continuous Gaussian variables were presented as means with standard deviations. Continuous non‐Gaussian variables were presented as medians and ranges. Categorical data were presented as frequencies and percentages. The cosmetic outcome of the neophallus was retrospectively assessed from our digital patient database and scored as excellent, good, fair or poor. The study was approved by our institutional medical ethical committee (reference number 2016.403) and written informed consent for the study was obtained from all patients. The study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. All portrayed patients consented for their photographs to be used in this publication.
Table 1.
Patient characteristics, surgical details and outcomes
| Case | Age | Somatic comorbidity | Psychiatric comorbidity | History of smoking | History of drug abuse | ALT flap size (cm) | No. ALT perforators | RFFF flap size (cm) | Surgery duration (min) | RFFF donor‐site closure | FU (months) | Early complications | Late complications | Cosmetic outcome | Functional outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | … | … | Yes | … | 13 × 10 | 2 | 15 x 3 | 582 | Primary | 95 | … |
Urethral stenosis Urethral fistula |
Excellent | Voiding while standing possible |
| 2 | 33 | … | … | … | … | 12 × 12 | 2 | 12 × 3 | 473 | <25% STSG | 75 | … | Urethral (hair) stenosis | Excellent | Voiding while standing possible |
| 3 | 55 | … | … | … | … | 14 × 14 | 2 | 15 × 3 | 583 | Primary | 83 | Complete RFFF loss | Urethral fistula | Good | Voiding while standing possible |
| 4 | 38 | Hypertension | … | … | … | 14 × 14 | 2 | 15 × 3 | 420 | FTG | 54 | … | … | Excellent | Voiding while standing possible |
| 5 | 40 | … | … | Yes | … | 13 × 13 | 2 | 14 × 3 | 693 | <25% STSG | 52 | … | … | Excellent |
Voiding while standing possible Genesis Malleable penile prosthesis successfully implanted |
| 6 | 30 | … | … | … | … | 14 × 14 | 2 | 15 × 3 | 518 | Primary | 52 | ‐ | Urethral stenosis | Good | Voiding while standing not possible |
| 7 | 35 | … | … | … | … | 14 × 14 | 2 | 14 × 3 | 572 | <25% STSG | 49 | ‐ | Urethral stenosis | Excellent | Voiding while standing possible |
| 8 | 38 | … | … | Yes | Poppers | 14 × 14 | 2 | 16 × 3.5 | 563 | <25% STSG | 43 | Complete RFFF loss | … | Poor | Voiding while standing not possible |
| 9 | 22 | … | … | … | … | 13 × 13 | 1 | 16 × 3 | 467 | <25% STSG | 38 | … | Urethral stenosis | Excellent | Voiding while standing possible |
| 10 | 32 | … | … | Yes | Cannabis | 13 × 13 | 3 | 15 × 3.5 | 408 | Primary | 35 | … | … | Excellent | Voiding while standing possible |
| 11 | 21 | Hypertension | … | … | … | 12 × 12 | 2 | 17 × 3 | 405 | <25% STSG | 30 | … | … | Not reported | Voiding while standing possible |
| 12 | 26 | … | Depression | … | … | 13 × 13 | 2 | 15 × 3.5 | 437 | Primary | 28 | … | Urethral stenosis | Good | Voiding while standing possible |
| 13 | 37 | … | ‐ | … | … | 15 × 13 | 1 | 14 × 3 | 530 | Primary | 24 | … | … | Excellent | Voiding while standing possible |
| 14 | 44 | … | Depression | … | … | 13 × 12 | 2 | 15 × 3 | 434 | Primary | 22 | … | Urethral stenosis | Excellent | Voiding while standing possible |
| 15 | 28 | … | … | … | Cocaine | 13 × 11 | 3 | 15 × 3 | 485 | Primary | 20 | … | ALT donor site infection | Excellent | Voiding while standing possible |
| 16 | 41 | Diabetes, hypercholesterolemia | … | … | … | 14 × 14 | 2 | 14 × 3 | 428 | Primary | 19 | Partial ALT loss | Urethral stenosis | Fair | Voiding while standing possible |
| 17 | 23 | … | … | Yes | … | 14 × 14 | 2 | 14 × 3,5 | 465 | Primary | 13 | Complete ALT loss | Urethral stenosis | Good | Voiding while standing possible |
| 18 | 51 | … | … | … | … | 13 × 13 | 2 | 15 × 3,5 | 376 | <25% STSG | 8 | … | … | Excellent | Voiding while standing possible |
| 19 | 57 | … | Social phobia | Yes | … | 13 × 10 | 2 | 15 × 3 | 363 | Primary | 3 | … | … | Excellent | Voiding while standing possible |
Abbreviation: ALT, anterolateral thigh flap; RFFF, radial forearm free flap; FU, follow‐up; CS, completely survived; CL, completely lost; PL. partially lost.
Preoperative counseling was performed by a specialized psychologist with experience in the gender field to assess psychological eligibility. This also included sexual (history) anamnesis to assert the postoperative sexual expectations and desires. Surgical eligibility was subsequently performed by the genital surgeon. An Allen test was used to determine vascular competence of the palmar arch. The donor‐site forearm was depilated to prevent neourethral hair stenosis. Intravenous prophylactic antibiotics, 1,500 mg cefuroxime and 500 mg metronidazole, were administered 30 min before surgery. Since 2013, a standard preoperative urological evaluation, which consists of a 24‐hr voiding diary the International Prostate Symptom Score and a uroflowmetry, was performed.
2.1. Surgical technique
Under general anesthesia, the patient was placed on a short grain mattress in extended lithotomy Trendelenburg position with the legs placed in stirrups. The arm not operated was positioned alongside the body, sufficiently padded to prevent iatrogenic neuropathy and other pressure‐related problems. Perforators of the ALT were preoperatively located with a handheld Doppler device and marked on the skin. Preoperative evaluation of ALT perforators and the subcutaneous fat tissue layer using CT angiography, may facilitate adequate patient and perforator selection, and determine a feasible flap size. (Sinove et al., 2013) Flap dimensions were marked preoperatively. To minimize surgery time, this procedure is ideally performed in three teams simultaneously, in which one team harvests the RFFF, one team the pedicled ALT and one team performs a scrotoplasty and pars fixa urethral lengthening. In our institution, this procedure is generally performed by two plastic surgeons with assistance of plastic surgery residents or scrub nurses. The RFFF is generally harvested from the nondominant forearm. The ALT is harvested from the contralateral side.
The design of the ALT, an almost square design of approximately 13 × 13 cm, depending on the length of the patient, was preoperatively marked on the skin. The incision was lengthened proximally in a lazy‐S fashion, to be able to dissect the lateral cutaneous femoral nerve and include it in the flap. The lateral femoral cutaneous nerve was identified, and cut proximally, when sufficient length for neurorrhaphy was reached. The motor branch nerve of the vastus lateralis was spared. Subsequently, the ALT was dissected subfascially. Its perforator, originating from the descending branch of lateral femoral circumflex artery, was identified and carefully separated from the femoral nerve motor branches, running parallel to the pedicle. The pedicled ALT was relocated to the genital area through a tunnel created underneath the rectus femoris and adductor muscles without tension or torsion on its vascular pedicle. Although in this series pedicle length was always large enough to be able to transpose and tunnel the ALT flap as a pedicled flap, one should decide to convert to a free flap if there is too much tension on the pedicle. One big enough perforator is usually sufficient for the vascularization of the ALT flap, but we are accustomed to include close‐by perforators to the dominant perforator, if they can easily be followed back to the same pedicle without sacrificing muscle tissue. Subsequently, the donor‐site was closed with a split‐thickness skin graft taken from the inner site of the upper leg.
The design of the RFFF, a rectangular design of approximately 3 × 16 cm, was preoperatively marked on the skin, directly above the radial artery. Simultaneously to the ALT, the RFFF was harvested from the nondominant forearm. The vascular pedicle was identified and carefully dissected form distal to proximal, to its origin at the brachial artery. The RFFF was tabularized in two layers around a 18 Ch foley catheter with the skin facing the catheter. Donor‐sites were closed primarily or, when this was not (entirely) possible, covered with a split‐thickness skin graft.
The scrotoplasy with creation of the fixed part of the urethra was constructed according to the technique described by Selvaggi et al. (2009) The pars fixa urethral lengthening was achieved by tubularization of the infundibular tissue in between the labia minora over a 18 French (FR) foley catheter (Djordjevic et al., 2009). The clitoris was denuded and one dorsal clitoral nerve was dissected. A suprapubic bladder catheter was placed. Contralateral to the ALT harvesting site, the groin was dissected to isolate the common femoral artery and greater saphenous vein. Subsequently, the legs were taken out of the stirrups and placed in neutral position.
After elevation of the RFFF, and pedicle dissection, the RFFF was transferred to the genital region. First, the urethral anastomosis was made in a spatulated two layer fashion with interrupted resorbable monofilament sutures. Subsequently, the vascular pedicle was tunneled to the groin and the radial artery was microscopically anastomosed side‐to‐end to the common femoral artery and the cephalic vein end‐to‐end to the great saphenous vein. Coapting the medial antebrachial cutaneous nerve to the ilioinguinal nerve was performed in the first few cases and subsequently abandoned, because having a sensate neourethra was reported to be very unpleasant during urinary catheterization. Subsequently, the lateral femoral cutaneous nerve was coapted microscopically to one of the dorsal clitoral nerves and the ALT was wrapped around the RFFF. Care was taken not to close the ALT too tight around the RFFF, because this may compromise its vascular supply due to postoperative swelling. The ALT was closed and attached to the neoscrotum in layers with resorbable stitches. Scars were placed innocuously at the ventral site of the neophallus, resembling a natural raphe.
3. RESULTS
The size of the ALT flaps ranged from 12 × 12 cm to 15 × 13 cm (Table 1). The size of the neourethral RFFFs ranged from 14 × 3 cm to 17 × 3 cm. The median number of perforators included in the ALT flap was 2 (range 1–3). Most perforators were intramuscular. There was no intra‐ or postoperative mortality. Primary closure of the RFFF donor‐site was possible in 11 (58%) patients. In the other 8 (42%) patients, a small split skin graft of <25% of the initial donor‐site defect was used to cover the remaining defect, thus still resulting in a less conspicuous scar. Urethral RFFF failure occurred in two patients due to venous thrombosis in one patient and lack of arterial inflow problem in the other. One was salvaged with a contralateral RFFF on the tenth postoperative day. The other underwent scrotal urethrostomy and is currently on the waiting list for urethral reconstruction with a contralateral RFFF. Total failure of the pedicled ALT due to venous thrombosis occurred in one patient. This was salvaged with the contralateral ALT on the fifth postoperative day. Partial necrosis of the ALT flap occurred in one patient, which was salvaged with split‐thickness skin grafting of an area of 2 × 1 cm. ALT donor site infection occurred in one patient, which was adequately treated with oral antibiotics. The mean hospitalization length was 9.2 ± 3.3 days.
Long‐term urinary complications occurred in 10 (53%) patients. Nine of these patients had urethral stenosis after a median postoperative time of 9 months (range 7–56). The stenosis developed at the anastomosis between the pars pendulans and the pars fixa of the urethra in eight cases and in the phallic part of the urethra due to neourethral hair growth in one case. The urethral stenosis was treated with end‐to‐end urethroplasty in five, with transurethral urethrotomy in two, and with scrotal urethrostomy in two patients. Urethral fistulas occurred in two (11%) patients. Reoperations for cosmetic reasons were performed in four patients, and comprised neophallic thinning by liposuction (n = 2), donor site liposuction (n = 1) and glans penis coronaplasty (n = 1). One patient underwent erectile prosthesis implantation 1.6 years after the phalloplasty procedure. A Genesis Malleable penile prosthesis (9.5 mm) was successfully implanted without intra‐ or postoperative complications.
3.1. Case report
A 38‐year old transgender man consulted our outpatient clinic with the request for genital reassignment surgery. He previously underwent a colpectomy and bilateral mastectomy. His BMI was 27.5. He preferred an inconspicuous donor‐site scar and his thigh contained too much subcutaneous fat to perform an ALT phalloplasty. After discussing the advantages and disadvantages of the surgical possibilities, we agreed to perform a pedicled ALT phalloplasty combined with RFFF urethral reconstruction (Figure 1). Flap dimensions and perforators were preoperatively marked as described previously. After dissection, the RFFF was wrapped around an 18 FR foley catheter with the skin facing the catheter. The ALT was carefully dissected leaving the descending branch of lateral femoral circumflex artery intact. The radial artery was microscopically anastomosed to the common femoral artery and the cephalic vein to the great saphenous vein. Subsequently, the ALT was relocated to the genital area through a tunnel underneath the rectus femoris and adductor muscles. The lateral femoral cutaneous nerve was coapted to a dorsal clitoral nerve. The operative procedure was uneventful and took 467 min. The RFFF donor‐site was closed primarily. Primary closure of the ALT donor‐site was not entirely possible, and the remainder was covered with a split‐thickness skin graft. The long‐term postoperative cosmetic outcome of the donor‐site scar was good, as perceived by both patient and physician (Figure 2).
Figure 1.
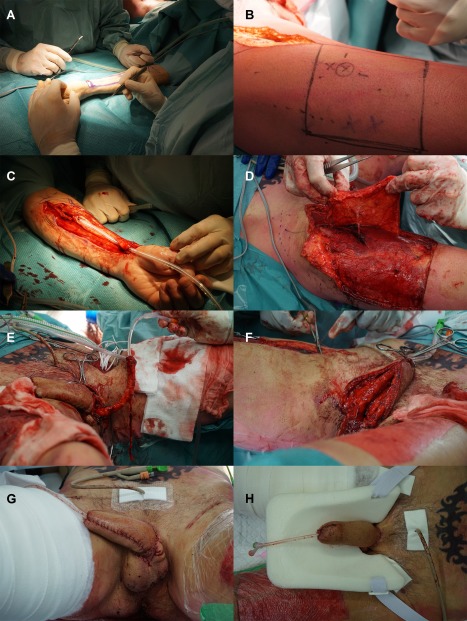
One‐stage double flap phalloplasty (pedicled ALT phalloplasty combined with RFFF urethral reconstruction) in a transgender man. (A) Preoperative marking of the RFFF. (B) Preoperative marking of the ALT and its perforator. (C). After dissection, the RFFF was wrapped around an 18 FR foley catheter with the skin facing the catheter. (D) The ALT was carefully dissected leaving the descending branch of lateral femoral circumflex artery intact. (E) The radial artery was microscopically anastomosed to the common femoral artery and the cephalic vein to the great saphenous vein. (F) The ALT was relocated to the genital area through a tunnel underneath the rectus femoris and adductor muscles. The lateral femoral cutaneous nerve was coapted to a dorsal clitoral nerve. (G,H) Postoperative end result
Figure 2.
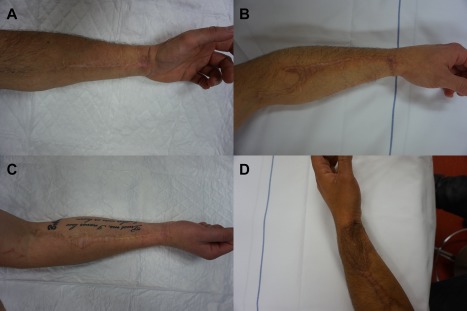
Inconspicuous donor site scars after RFFF urethral reconstruction. (A) RFFF donor‐site after primary closure. The photograph was taken 21 months after surgery. (B) RFFF donor‐site after primary closure. The photograph was taken 50 months after surgery. (C) RFFF donor‐site after primary closure. The photograph was taken 48 months after surgery. (D) RFFF donor‐site off which a small portion was closed with a STSG. The photograph was taken 20 months after surgery
4. DISCUSSION
In this study, we described the surgical technique of double flap phalloplasty consisting of a pedicled ALT combined with RFFF urethral reconstruction in one stage and our initial experience with this procedure performed in nineteen transgender men. Short‐term complications comprised total RFFF failure in two patients and ALT failure in one. Partial necrosis of the ALT occurred in one patient, which was salvaged with split‐thickness skin grafting. Neourethral complications, especially strictures, occurred frequently, but were successfully managed by minor operations.
Free or pedicled flap phalloplasty is a complex surgical reconstructive procedure, performed by a limited number of surgeons. Ideally, the phalloplasty is performed in one‐stage and provides a neourethra which facilitates voiding while standing, erogenous sensibility, enough bulge to insert a stiffener, an acceptable aesthetic result, and minimal donor‐site scarring, morbidity and functional loss (Hage & De Graaf, 1993). In transgender men, RFFF phalloplasty is considered the surgical gold standard by most reconstructive surgeons. As shown in larger series, total RFFF failure in RFFF phalloplasty procedures occurs in approximately 1%–5% of cases and partial flap failure in 2%–11% (Baumeister, Sohn, Domke, & Exner, 2011; Doornaert et al., 2011; Leriche et al., 2008; Monstrey et al., 2005). The pedicled ALT is often mentioned a feasible alternative, mostly because of its vascular reliability. However, variability in vascular anatomy has been reported (Valdatta et al., 2002). Using the ALT as penile shaft has a cosmetic advantage, since its color resembles the perineal skin color and the donor scar is concealed when dressed. A tube‐in‐tube design facilitates one‐stage reconstruction of urethra and penis (Hasegawa, Namba, & Kimata, 2013; Lumen et al., 2008). However, this is only possible in thin patients with minimal subcutaneous tissue. A suprafascial dissection of the ALT flap may be performed in these patients, which allows the harvest of a thinner flap. However, in some patients with overweight, the flap will still be too bulky. Although in this series pedicle length was always large enough to be able to transpose and tunnel the ALT flap as a pedicled flap, one should decide to convert to a free flap if there is too much tension on the pedicle. In patients wishing to be able to void in standing position and unwilling to have a conspicuous forearm scar, the described double flap phalloplasty in one stage, a novel combination of techniques, can be performed. The RFFF has a thin skin and a long vascular pedicle, which makes is suitable for urethral reconstruction and vascular anastomosis in the groin. Using a small RFFF for urethral reconstruction creates a smaller, less conspicuous donor site scar on the forearm which often can be closed primarily when compared to RFFF phalloplasty. The reported RFFF failure rate in this series can be explained by several factors. Outer flap problems such as swelling or hematoma, may compromise vascularization of the inner flap used for urethral reconstruction. One of the disadvantages of free flap urethral reconstruction in general is the difficulty of flap monitoring. Another disadvantage of the described technique comprises the long operative time. A three‐team approach is frequently chosen for this procedure at our institution to minimize the operative time. This however does require significant resources.
Free flap urethral reconstruction has been described predominantly in oncological cases, but also as salvage option after partial necrosis of the neourethral part of a tube‐in‐tube phalloplasty (Camp, Cartwright, & Siddiqi, 2011; Dabernig, Shelley, Cuccia, & Schaff, 2007; Lee, Hur, Song, & Tark, 2001; Tchang et al., 2014). Single‐stage double flap phalloplasty with free or pedicled flap urethral reconstruction has been described only in case reports and small case series (Harashina et al., 2002; Koshima et al., 2006; Yoo, Shin, and Lee, 2012). For urethral reconstruction, use of the RFFF, gracilis flap, ulnar flap and pedicled superficial circumflex iliac artery perforator flap (SCIPF) were described. The use of the SCIPF has both surgical and aesthetic advantages, as primary closure is frequently possible and its surgical site is close to the genital region, which facilitates easy surgical preparation and leaves concealable scars. Disadvantages comprise that it might be hair bearing and may be bulky when combined with an ALT.
In our study, long‐term urinary complications occurred in 10 (53%) patients, and comprised predominantly urethral strictures. Urethral complications, especially urethral strictures and fistulas, are common after phalloplasty procedures combined with urethroplasty. In larger series, urethral fistulas are reported to occur in 21%–68% and urethral strictures in 15%–32% after RFFF phalloplasty with urethroplasty (Baumeister et al., 2011; Doornaert et al., 2011; Fang et al., 1994; Leriche et al., 2008; Monstrey et al., 2005). Fistula rates are reported to be lower if local flaps, for example, a vascularized labia minora skin flap, are used in conjunction with the RFFF for neourethroplasty (Kim, Moon, Heo, Kwon, & Lee, 2010). Strictures most frequently occur at the anastomosis between the phallic and the fixed part of the urethra (Lumen, Monstrey, Goessaert, Oosterlinck, & Hoebeke, 2011). This is probably due to suboptimal vascularization at the borders of the local and free flap where they are anastomosed.
5. CONCLUSION
Double flap phalloplasty with pedicled ALT and RFFF urethral reconstruction is a feasible surgical technique in transgender men (1) wishing to be able to void in standing position and (2) who desire a less conspicuous forearm scar. Using the ALT as penile shaft has the cosmetic advantage of perineal skin color resemblance and a hidden donor scar. Using a small RFFF solely for urethral reconstruction creates a small donor site scar on the forearm. As in any pedicled or free flap phalloplasty, possible drawbacks comprise flap‐related complications and urethral complications.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest with the contents of this article.
van der Sluis WB, Smit JM, Pigot GLS, et al. Double flap phalloplasty in transgender men: Surgical technique and outcome of pedicled anterolateral thigh flap phalloplasty combined with radial forearm free flap urethral reconstruction. Microsurgery. 2017;37:917–923. https://doi.org/10.1002/micr.30190
REFERENCES
- Baumeister, S. , Sohn, M. , Domke, C. , & Exner, K. (2011). Phalloplasty in female‐to‐male transsexuals: Experience from 259 cases. Handchirurgie, Mikrochirurgie, Plastische Chirurgie, 43, 215–221. [DOI] [PubMed] [Google Scholar]
- Camp, S. , Cartwright, P. , & Siddiqi, F. (2011). The prefabricated gracilis muscle flap with full‐thickness skin graft and delay for urethral channel reconstruction. Annals of Plastic Surgery, 67, 59–61. [DOI] [PubMed] [Google Scholar]
- Dabernig, J. , Shelley, O. P. , Cuccia, G. , & Schaff, J. (2007). Urethral reconstruction using the radial forearm free flap: Experience in oncologic cases and gender reassignment. European Urology, 52, 547–553. [DOI] [PubMed] [Google Scholar]
- Descamps, M. J. , Hayes, P.M. , & Hudson, D. A. (2009). Phalloplasty in complete aphallia: Pedicled anterolateral thigh flap. Journal of Plastic, Reconstructive & Aesthetic Surgery, 62, e51–e54. [DOI] [PubMed] [Google Scholar]
- Djordjevic, M. L. , Bizic, M. , Stanojevic, D. , Bumbasirevic, M. , Kojovic, V. , Majstorovic, M. , … Perovic, S. V. (2009). Urethral Lengthening in metoidioplasty (female‐to‐male sex reassignment surgery) by combined buccal mucosa graft and labia minora flap. Urology, 74, 349–353. [DOI] [PubMed] [Google Scholar]
- Doornaert, M. , Hoebeke, P. , Ceulemans, P. , T'Sjoen, G. , Heylens, G. , & Monstrey, S. (2011). Penile reconstruction with the radial forearm flap: An update. Handchirurgie, Mikrochirurgie, Plastische Chirurgie, 43, 208–214. [DOI] [PubMed] [Google Scholar]
- Fang, R. H. , Lin, J. T. , & Ma, S. (1994). Phalloplasty for female transsexuals with sensate free forearm flap. Microsurgery, 15, 349–352. [DOI] [PubMed] [Google Scholar]
- Felici, N. , & Felici, A. (2006). A new phalloplasty technique: The free anterolateral thigh flap phalloplasty. Journal of Plastic, Reconstructive & Aesthetic Surgery, 59,153–7. [DOI] [PubMed] [Google Scholar]
- Gillies, H. , & Millard, R. (1957). The principles and art of plastic surgery (Vol. II). Boston, MA: Little, Brown & Company. [Google Scholar]
- Hage, J. J. , & De Graaf, F. H. (1993). Addressing the ideal requirements by free flap phalloplasty: Some reflections on refinements of technique. Microsurgery, 14, 592–598. [DOI] [PubMed] [Google Scholar]
- Harashina, T. , Inoue, Y. , Takamatsu, A. , Wakamatsu, K. , Takeshita, A. , & Miura, H. (2002). Construction of penis with two free flaps. Annals of Plastic Surgery, 49, 302–306. [DOI] [PubMed] [Google Scholar]
- Hasegawa, K. , Namba, Y. , & Kimata, Y. (2013). Phalloplasty with an innervated island pedicled anterolateral thigh flap in a female‐to‐male transsexual. Acta Medica Okayama, 67, 325–331. [DOI] [PubMed] [Google Scholar]
- Kim, S. K. , Moon, J. B. , Heo, J. , Kwon, Y. S. , & Lee, K. C. (2010). A new method of urethroplasty for prevention of fistula in female‐to‐male gender reassignment surgery. Annals of Plastic Surgery, 64, 759–764. [DOI] [PubMed] [Google Scholar]
- Koshima, I. , Nanba, Y. , Nagai, A. , Nakatsuka, M. , Sato, T. , & Kuroda, S. (2006). Penile reconstruction with bilateral superficial circumflex iliac artery perforator (SCIP) flaps. Journal of Reconstructive Microsurgery, 22, 137–142. Apr [DOI] [PubMed] [Google Scholar]
- Lee, H. B. , Hur, J. Y. , Song, J. M. , & Tark, K. C. (2008). Long anterior urethral reconstruction using a sensate ulnar forearm free flap. Plastic and Reconstructive Surgery, 108, 2053–2056. [DOI] [PubMed] [Google Scholar]
- Leriche, A. , Timsit, M. O. , Morel‐Journel, N. , Bouillot, A. , Dembele, D. , & Ruffion, A. (2008). Long‐term outcome of forearm flee‐flap phalloplasty in the treatment of transsexualism. BJU International, 101, 1297–1300. [DOI] [PubMed] [Google Scholar]
- Lumen, N. , Monstrey, S. , Ceulemans, P. , van Laecke, E. , & Hoebeke, P. (2008). Reconstructive surgery for severe penile inadequacy: Phalloplasty with a free radial forearm flap or a pedicled anterolateral thigh flap. Advances in Urology, 2008, 704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumen, N. , Monstrey, S. , Goessaert, A. S. , Oosterlinck, W. , & Hoebeke, P. (2011). Urethroplasty for strictures after phallic reconstruction: A single‐institution experience. European Urology, 60, 150–158. [DOI] [PubMed] [Google Scholar]
- Monstrey, S. , Hoebeke, P. , Dhont, M. , Selvaggi, G. , Hamdi, M. , Van Landuyt, K. , & Blondeel, P. (2005). Radial forearm phalloplasty: A review of 81 cases. European Journal of Plastic Surgery, 28, 206–212. [Google Scholar]
- Monstrey, S. , Hoebeke, P. , Selvaggi, G. , Ceulemans, P. , Van Landuyt, K. , Blondeel, P. , … De Cuypere, G. (2009). Penile reconstruction: Is the radial forearm flap really the standard technique?. Plastic and Reconstructive Surgery, 124, 510–518. [DOI] [PubMed] [Google Scholar]
- Morrison, S. D. , Son, J. , Song, J. , Berger, A. , Kirby, J. , Ahdoot, M. , & Lee, G. K. (2014). Modification of the tube‐in‐tube pedicled anterolateral thigh flap for total phalloplasty: The mushroom flap. Annals of Plastic Surgery, 72, S22–S26. [DOI] [PubMed] [Google Scholar]
- Selvaggi, G. , Hoebeke, P. , Ceulemans, P. , Hamdi, M. , Van Landuyt, K. , Blondeel, P. , … Monstrey S. (2009). Scrotal reconstruction in female‐to‐male transsexuals: A novel scrotoplasty. Plastic and Reconstructive Surgery, 123, 1710–1718. [DOI] [PubMed] [Google Scholar]
- Sinove, Y. , Kyriopoulos, E. , Ceulemans, P. , Houtmeyers, P. , Hoebeke, P. , & Monstrey, S. (2013). Preoperative planning of a pedicled anterolateral thigh (ALT) flap for penile reconstruction with the multidetector CT scan. Handchirurgie, Mikrochirurgie, Plastische Chirurgie, 45, 217–222. [DOI] [PubMed] [Google Scholar]
- Tchang, L. A. , Largo, R. D. , Babst, D. , Wettstein, R. , Haug, M. D. , Kalbermatten, D.F. , & Schaefer, D. J. (2014). Second free radial forearm flap for urethral reconstruction after partial flap necrosis of tube‐in‐tube phalloplasty with radial forearm flap: A report of two cases. Microsurgery, 34, 58–63. [DOI] [PubMed] [Google Scholar]
- Valdatta, L. , Tuinder, S. , Buoro, M. , Thione, A. , Faga, A. , & Putz, R. (2002). Lateral circumflex femoral arterial system and perforators of the anterolateral thigh flap: An anatomic study. Annals of Plastic Surgery, 49, 145–150. [DOI] [PubMed] [Google Scholar]
- Wierckx, K. , Van Caenegem, E. , Elaut, E. , Dedecker, D. , Van de Peer, F. , Toye, K. , … T'Sjoen, G. (2011). Quality of life and sexual health after sex reassignment surgery in transsexual men. The Journal of Sexual Medicine, 8, 3379–3388. [DOI] [PubMed] [Google Scholar]
- Yoo, K. W. , Shin, H. W. , & Lee, H. K. (2012) A case of urethral reconstruction using a superficial circumflex iliac artery. Archives of Plastic Surgery, 39, 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


