Abstract
Background
Despite major advances in prevention and treatment, coronary artery disease (CAD) remains the leading cause of death worldwide. Whereas many sources of data are available on the epidemiology of acute coronary syndromes, fewer datasets reflect the contemporary management and outcomes of stable CAD patients.
Hypothesis
A worldwide contemporary registry would improve our knowledge about stable CAD. The main objectives are to describe the demographics, clinical profile, contemporary management and outcomes of outpatients with stable CAD; to identify gaps between evidence and treatment; and to investigate long‐term prognostic determinants.
Methods
CLARIFY (ProspeCtive observational LongitudinAl RegIstry oF patients with stable coronary arterY disease) is an ongoing international observational longitudinal registry. Stable CAD patients from 45 countries in Europe, Asia, America, Middle East, Australia and Africa were enrolled between November 2009 and June 2010. The inclusion criteria were previous myocardial infarction, evidence of coronary stenosis >50%, proven symptomatic myocardial ischemia or prior revascularization procedure. The main exclusion criteria were serious non‐cardiovascular disease, conditions interfering with life expectancy or severe other cardiovascular disease (including advanced heart failure). Follow‐up visits were planned annually for up to 5 years, interspersed with 6‐month telephone calls.
Results
Of the 32,703 patients enrolled, most (77.6%) were male, age (mean ± SD) was 64.2 ± 10.5 years, and 71.0% were receiving treatment for hypertension; mean ± SD resting heart rate was 68.2 ± 10.6 bpm. Patients were enrolled based on a history of myocardial infarction >3 months earlier (57.7%), having at least one stenosis >50% on coronary angiography (61.1%), proven symptomatic myocardial ischemia on non‐invasive testing (23.1%), or history of percutaneous coronary intervention or coronary artery bypass graft (69.8%). Baseline characteristics were similar across the four subgroups identified by the four inclusion criteria.
Conclusion
CLARIFY will provide a useful resource for understanding the current epidemiology of stable CAD.
Keywords: Stable Coronary Artery Disease, CLARIFY Registry, Baseline Characteristics
1. INTRODUCTION
Despite major advances in the prevention and treatment of atherothrombosis, coronary artery disease (CAD) is the primary cause of mortality worldwide, continues to be a major burden on public health,1, 2 and is expected to remain the world's leading cause of morbidity and mortality in 2020.3 The number of patients with CAD is likely to rise as life expectancy increases, as the prevalences of diabetes mellitus (DM) and obesity increase, and due to the improved survival of patients presenting with an acute coronary syndrome.4
The clinical characteristics, cardiovascular (CV) risk factors, treatment, and outcomes of patients with CAD have changed markedly over the years. Most of the existing data regarding the epidemiology of CAD are relatively old, often focus on 1 manifestation of disease (eg, stable angina)5 or pertain to acute coronary syndromes,6 and are often restricted to a single country or a specific geographic region, particularly North America or Western Europe.7, 8, 9 Thus, there is a need for robust contemporary data in stable CAD representing >1 region and addressing more than symptomatic angina. Moreover, despite the importance of heart rate (HR) in the prognosis of stable CAD,10, 11, 12, 13, 14 HR is not a routine component of CV risk assessment, nor a tool to decide whether treatment is indicated, and most datasets have not collected detailed information on HR in stable CAD.
Large datasets are available from randomized trials in stable CAD. However, although these are the gold standard to evaluate new therapies,15 they are generally performed in highly selected populations that often do not reflect patients encountered in daily practice in terms of their clinical characteristics, comorbidities, socioeconomic status, management, and outcomes.16 Large prospective registries often provide a more realistic description of the patients’ actual characteristics, management, and outcomes, provided their recruitment is unbiased and the sample size is sufficiently large.17, 18
The prospeCtive observational LongitudinAl RegIstry oF patients with stable coronary arterY disease (CLARIFY) was initiated to improve knowledge about the current management and outcomes of patients with stable CAD, to assess prognosis, and to subsequently design interventions to improve evaluation and treatment of these patients.
2. METHODS
2.1. Objectives
The first objective was to describe contemporary patients with stable CAD in terms of their demographic characteristics, clinical profile, management, and outcomes, with a global geographic reach, encompassing patients from high‐, middle‐, and low‐income regions. The second objective was to identify gaps between evidence‐based recommendations and current management. The third objective was to characterize the clinical determinants of long‐term prognosis in this population.
2.2. Study design
CLARIFY is an ongoing international, prospective, observational, longitudinal registry of outpatients with stable CAD, with yearly follow‐up for up to 5 years. This observational registry was designed to collect the current status of outpatients with stable CAD, including their demographic characteristics, clinical profiles, therapeutic strategies, and outcomes. Data were collected prospectively at annual visits every 12 ± 3 months. Owing to substantial geographic variations in the epidemiology of stable CAD, this registry is international to generate reliable data on several regions in the world. Patients were enrolled in 45 countries in Europe, Asia, North/Central/South America, the Middle East, Australia, and South Africa (Figure 1). Importantly, no patients were enrolled in the United States, due to a lack of sponsor support.
Figure 1.
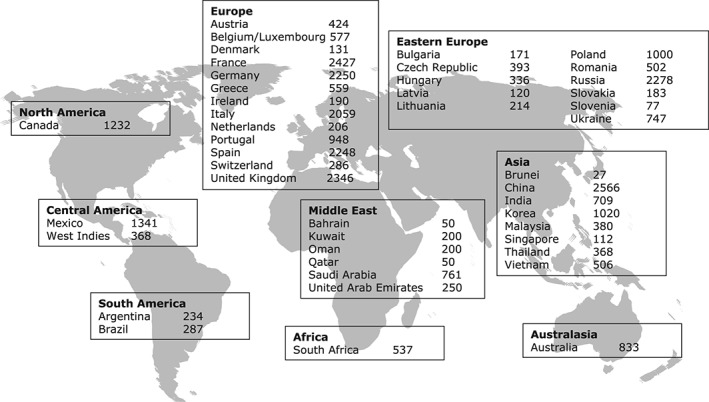
Worldwide distribution of study participants (n = 32703).
The study was performed in accordance with the principles laid out in the Declaration of Helsinki; in the United Kingdom, it was approved by the national Research Ethics Service, Isle of Wight, Portsmouth, and Southeast Hampshire Research Ethics Committee. Local ethical approval was also obtained in all 45 countries before recruitment, according to national and local regulations at each site. All patients gave written informed consent.
The CLARIFY Registry is registered in the ISRCTN registry of clinical trials (ISRCTN43070564). For a complete list of CLARIFY Registry investigators, see Supporting Information, Appendix, in the online version of this article.
2.3. Study population
Patients were eligible for enrollment if they fulfilled ≥1 of the following (not mutually exclusive) criteria: documented myocardial infarction (MI) >3 months ago; coronary angiography showing ≥1 coronary stenosis of >50%; chest pain with myocardial ischemia proven by stress electrocardiography (ECG), stress echocardiography, or myocardial imaging; and coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) >3 months ago. The exclusion criteria were hospitalization for CV disease within the previous 3 months (including revascularization); planned revascularization; and conditions that could have affected participation or 5‐year follow‐up, such as limited cooperation, limited legal capacity, serious non‐CV disease, conditions interfering with life expectancy (eg, cancer, drug abuse), severe CV disease (eg, advanced heart failure [HF]), severe valve disease, and history of valve repair/replacement. The first patient was included on November 26, 2009, and recruitment was completed on June 30, 2010.
2.4. Site selection
To ensure that the enrolled population of outpatients with stable CAD was representative of the population of each country, sites were identified based on a predefined selection of physicians including cardiologists, general practitioners, internists, and hospital‐based physicians. In each country, selection of physicians was made by national coordinators using the best available epidemiological data reflecting the burden of CAD in that country, to provide a representative distribution of physicians across regions and location types (ie, urban, suburban, and rural areas). Epidemiologic and medical care data, published and endorsed by national or international societies, either local or regional, were used to identify the distribution of coronary patients in each country, to select physician types and locations, and subsequently patients. The executive committee validated the physician selection process for each country before starting enrollment. As an observational registry, physicians were instructed to manage their patients per usual practice, and no specific tests or therapies were prescribed as part of the registry, to ensure that patient care was not affected by participation in the study.
A total of 2898 physicians were selected. Each physician was requested to recruit 10 to 15 consecutive outpatients with stable CAD. In each country, the goal was to meet a predefined country target of approximately 25 patients per million inhabitants (range, 12.5–50) to ensure balanced representation of participating countries; one exception was China, where recruitment was expected to be representative of the fraction of the population having access to “Western‐type” medical care. Patients were enrolled over a brief period to minimize the risk of selection bias.
2.5. Data collection and evaluation
Data were collected anonymously using electronic standardized international case‐report forms (translated into the local language) at baseline and annually for up to 5 years, to ascertain clinical events, hospitalization, employment status, or sick leave. Between the baseline visit and each annual visit, to maximize follow‐up and retention rates, 6‐month telephone calls were made to collect vital status, confirm contact details, and ensure the next annual visit was planned.
At baseline, data collection included demographic data (sex, age, living status, employment status), risk factors and lifestyle, medical history, physical examination, and vital signs, including sitting arterial blood pressure (BP) and HR (determined by both pulse palpation and 12‐lead ECG performed within the previous 6 months), current symptoms and, if available, results of biological tests performed within the previous 12 months (fasting blood glucose; hemoglobin A1c; total cholesterol, high‐density lipoprotein cholesterol, and low‐density lipoprotein cholesterol; triglycerides; serum creatinine; and hemoglobin), measurement of left ventricular ejection fraction (LVEF), and results of coronary angiography and noninvasive stress tests. Finally, detailed current drug treatment data were collected by type of agent (without dosages, except for β‐blockers).
At annual follow‐up visits, data were collected on clinical outcomes since the last visit, demographic data, new physical examination and vital signs including HR, current symptoms, most recent available measurements, and medical treatments.
2.6. Outcomes
The main outcomes collected during the 5‐year follow‐up included mortality and CV morbidity data. Deaths were categorized into fatal MI, fatal stroke and other CV death (including sudden death), non‐CV death (death that was not definitely CV), and unknown cause. Events were collected as reported by investigators without central adjudication, but investigators were provided within the case‐report forms with a set of definitions for each outcome. Recognizing the difficulty in assigning definite causes in many cases of outpatient death, unknown‐cause deaths were grouped with other CV deaths for analysis. Nonfatal events collected were nonfatal MI, unstable angina, new‐onset or worsening HF requiring hospitalization, coronary revascularization (PCI or CABG), nonfatal stroke or transient ischemic attack, major bleeding, valve repair/replacement, pacemaker implantation, atrial fibrillation/flutter, peripheral artery disease surgery/amputation/interventions, carotid surgery/stenting, and abdominal aorta surgery/stenting.
To ensure data quality, every year, 1% of the centers were randomly selected to perform on‐site audits. In these selected centers, 100% of the data for all patients were checked for source documentation and accuracy. Data quality control was done at face‐to‐face quality‐control visits and involved review of source documents supporting the adequacy and accuracy of data collected on the case‐report forms.
2.7. Statistical analysis
CLARIFY is an observational registry, and the size of the population was not based on a planned treatment comparison. The number of patients to be included was computed based on the aim to build a robust risk model at the completion of follow‐up and depended on the CV event rate, number of subjects lost to follow‐up, and study duration. Based on data from the literature, the annual rates of CV death and of major adverse CV events were expected to be approximately 2% and 4.5%, respectively. CLARIFY had to screen ≥31 000 subjects and follow them up for 4 to 5 years (with approximately 5% per year loss to follow‐up). With these assumptions, it was expected that there would be approximately 2300 CV deaths at the end of follow‐up, providing ample power for risk modeling. Taking a conservative approach, based on the analysis of HR as a categorical variable (population split by quartiles of HR) comparing risk of CV death between the highest HR quartile to the other quartiles, there would be ≥80% power to identify a 20% increase in risk in the group with the highest HR. If HR was considered as a continuous variable, there would be 90% power at the 5% level of significance to detect an underlying hazard ratio of 1.06 per 10‐bpm increase in HR.
Data were recorded centrally and analyzed by an academic statistics center (Robertson Centre for Biostatistics, University of Glasgow, United Kingdom). Baseline results are presented for the overall population and for the 4 subgroups identified by their inclusion criteria: documented MI >3 months ago; coronary angiography showing ≥1 coronary stenosis of >50%; chest pain with myocardial ischemia proven by stress ECG, stress echocardiography, or myocardial imaging; and CABG or PCI performed >3 months ago. Baseline continuous variables are presented as mean ± SD or median and interquartile range, depending on the distribution of the data; categorical data are presented as counts and percentages. As the 4 patient groups largely overlap and there was no a priori hypothesis regarding differences between groups, no formal statistical comparison was made between these groups given the large number of variables available for comparison.
3. RESULTS
A total of 33 032 patients were enrolled in the CLARIFY Registry. Of these, 329 withdrew their consent or did not meet the inclusion criteria. The baseline study population was therefore 32 703 patients (Figure 2).
Figure 2.
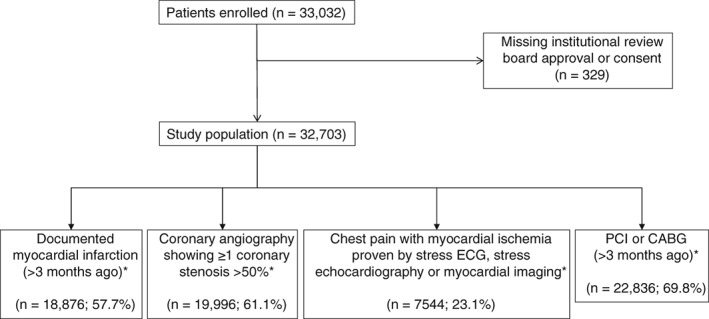
Study flow diagram. Abbreviations: CABG, coronary artery bypass graft; ECG, electrocardiogram; PCI, percutaneous coronary intervention. * Groups are not mutually exclusive.
Baseline demographics are detailed in Table 1. The mean age was 64.2 ± 10.5 years. Patients were predominantly male (77.6%) and Caucasian (64.6%). The median body mass index was 27.3, indicating that the majority of subjects were overweight or obese. Likewise, a majority of patients were either current or former smokers, dyslipidemic, and treated for hypertension. The majority of the patients did not work, and most reported only light physical activity.
Table 1.
Baseline clinical parameters
| All CLARIFY Patients, n = 32 703 | Prior MI >3 Months Ago,a n = 18 876 (57.7%) | Coronary Stenosis ≥50% on Angiography,a n = 19 996 (61.1%) | Chest Pain With Proven Myocardial Ischemia,a n = 7544 (23.1%) | History of PCI or CABG >3 Months Ago,a n = 22 836 (69.8%) | |
|---|---|---|---|---|---|
| Age, y | 64.2 ± 10.5 | 63.1 ± 10.7 | 64.1 ± 10.4 | 64.6 ± 10.2 | 64.2 ± 10.4 |
| Male sex | 25 365 (77.6) | 15 246 (80.8) | 15 877 (79.4) | 5571 (73.9) | 18 291 (80.1) |
| Ethnicity | |||||
| Caucasian | 21 112 (64.6) | 12 873 (68.2) | 12 124 (60.6) | 4772 (63.3) | 14 342 (62.8) |
| South Asian | 2444 (7.5) | 1336 (7.1) | 1549 (7.7) | 539 (7.1) | 1645 (7.2) |
| Chinese | 2753 (8.4) | 1242 (6.6) | 1938 (9.7) | 330 (4.4) | 2040 (8.9) |
| Japanese/Korean | 1035 (3.2) | 401 (2.1) | 685 (3.4) | 103 (1.4) | 853 (3.7) |
| Hispanic | 1624 (5.0) | 1008 (5.3) | 1058 (5.3) | 580 (7.7) | 1171 (5.1) |
| Black/African | 357 (1.1) | 247 (1.3) | 217 (1.1) | 94 (1.2) | 225 (1.0) |
| Unknown | 3378 (10.3) | 1769 (9.4) | 2425 (12.1) | 1126 (14.9) | 2560 (11.2) |
| BMI, kg/m2 | 27.3 (24.8–30.4) | 27.5 (25.0–30.5) | 27.2 (24.8–30.1) | 27.6 (25.0–30.5) | 27.2 (24.8–30.1) |
| Waist circumference, cm | 97 (89–105) | 97 (89–105) | 97 (89–105) | 97 (89–105) | 97 (89–105) |
| Family history of premature CAD | 9326 (28.5) | 5503 (29.2) | 5621 (28.1) | 2576 (34.2) | 6443 (28.2) |
| Treated hypertension | 23 210 (71.0) | 13 029 (69.1) | 14 231 (71.2) | 5619 (74.5) | 16 122 (70.6) |
| DM | 9502 (29.1) | 5388 (28.6) | 5956 (29.8) | 2277 (30.2) | 6799 (29.8) |
| Dyslipidemia | 24 504 (74.9) | 14 383 (76.2) | 15 318 (76.6) | 5991 (79.5) | 17 465 (76.5) |
| Smoking status | |||||
| Current | 4077 (12.5) | 2700 (14.3) | 2391 (12.0) | 870 (11.5) | 2706 (11.9) |
| Former | 15 109 (46.2) | 9263 (49.1) | 9570 (47.9) | 3281 (43.5) | 11 038 (48.3) |
| Never | 13 513 (41.3) | 6911 (36.6) | 8032 (40.2) | 3391 (45.0) | 9088 (39.8) |
| Alcohol intake (drinks per week)b | |||||
| 0 | 15 613 (47.8) | 8813 (46.7) | 9562 (47.8) | 3451 (45.8) | 10 797 (47.3) |
| 1–19 | 15 898 (48.6) | 9305 (49.3) | 9743 (48.7) | 3778 (50.1) | 11 269 (49.4) |
| 20–40 | 1068 (3.3) | 688 (3.6) | 617 (3.1) | 279 (3.7) | 684 (3.0) |
| >40 | 113 (0.3) | 67 (0.4) | 65 (0.3) | 29 (0.4) | 75 (0.3) |
| Stimulant drinks consumed | |||||
| Coffee | 15 500 (47.4) | 8842 (46.9) | 9642 (48.3) | 3502 (46.6) | 11 438 (50.1) |
| Tea | 10 040 (30.7) | 6247 (33.1) | 5594 (28.0) | 2254 (30.0) | 6195 (27.2) |
| Neither | 7129 (21.8) | 3774 (20.0) | 4733 (23.7) | 1763 (23.4) | 5178 (22.7) |
| Intake of stimulant drinks, cups/d | 2 (2–4) | 2 (2–4) | 2 (2–4) | 2 (2–4) | 2 (2–4) |
| Employment status | |||||
| Employed full‐time | 7980 (24.4) | 4955 (26.3) | 5038 (25.2) | 1814 (24.1) | 5659 (24.8) |
| Employed part‐time | 2266 (6.9) | 1435 (7.6) | 1284 (6.4) | 520 (6.9) | 1471 (6.4) |
| Unable to work | 1284 (3.9) | 902 (4.8) | 802 (4.0) | 337 (4.5) | 846 (3.7) |
| Unemployed | 1852 (5.7) | 1079 (5.7) | 1062 (5.3) | 400 (5.3) | 1234 (5.4) |
| Retired | 18 081 (55.3) | 9860 (52.2) | 10 979 (54.9) | 4173 (55.4) | 12 721 (55.7) |
| Other | 1232 (3.8) | 640 (3.4) | 824 (4.1) | 294 (3.9) | 897 (3.9) |
| Weekly physical activity | |||||
| None | 5287 (16.2) | 2899 (15.4) | 3094 (15.5) | 1323 (17.6) | 3640 (15.9) |
| Light activity most weeks | 16 810 (51.4) | 9970 (52.8) | 10 071 (50.4) | 3821 (50.7) | 11 264 (49.4) |
| ≥20 minutes vigorous activity 1–2 times per week | 5470 (16.7) | 3130 (16.6) | 3432 (17.2) | 1277 (16.9) | 3968 (17.4) |
| ≥20 minutes vigorous activity ≥3 times per week | 5121 (15.7) | 2870 (15.2) | 3387 (16.9) | 1115 (14.8) | 3950 (17.3) |
| Education level | |||||
| Primary school (or less) | 8648 (26.5) | 4836 (25.6) | 5495 (27.5) | 1972 (26.2) | 6245 (27.4) |
| Secondary school | 15 204 (46.5) | 8797 (46.6) | 9119 (45.6) | 3499 (46.4) | 10 535 (46.2) |
| College/university | 8841 (27.0) | 5238 (27.8) | 5373 (26.9) | 2065 (27.4) | 6046 (26.5) |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CLARIFY, Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease; DM, diabetes mellitus; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Data are presented as n (%), mean ± SD, or median (IQR).
Inclusion criteria are not mutually exclusive; some patients may be included in >1 group.
1 drink = 1 standard measure of spirits, 1 glass of wine, 1 bottle of beer.
Overall, based on the 4 main (not mutually exclusive) inclusion criteria, 57.7% of patients were enrolled on the basis of a medical history of MI >3 months ago, 61.1% on the basis of having had coronary angiography showing ≥1 coronary stenosis of >50%, 23.1% on the basis of having experienced chest pain with evidence of myocardial ischemia on noninvasive testing, and 69.8% due to a history of myocardial revascularization by PCI or CABG.
At the time of enrollment, most patients were asymptomatic, without symptoms of angina or HF. Mean BP readings were within the normal range, as were the values for creatinine and fasting blood glucose (Table 2). Mean resting HR was 68.2 ± 10.6 bpm when measured by pulse (available in 32 673 patients) and 67.1 ± 11.4 bpm when measured by ECG (available in 24 438 patients), and most patients were in sinus rhythm. Among the 22 519 patients in whom a measurement was available, mean LVEF was 56.1% ± 11.1%. Among patients with results of coronary angiography within the past 12 months, almost all patients had ≥1 significant coronary stenosis: 58.3% had a significant stenosis localized in the left anterior descending artery, 36.1% in the left circumflex artery, 43.5% in the right coronary artery, and 8.7% in the left main stem. Of note, 3.2% of patients with angiographic data had no stenosis >50%.
Table 2.
Baseline medical history, symptoms, and paraclinical parameters
| All CLARIFY Patients, n = 32 703 | Prior MI >3 Months Ago,a n = 18 876 (57.7%) | Coronary Stenosis ≥50% on Angiography,a n = 19 996 (61.1%) | Chest Pain With Proven Myocardial Ischemia,a n = 7544 (23.1%) | History of PCI or CABG >3 Months Ago,a n = 22 836 (69.8%) | |
|---|---|---|---|---|---|
| MI | 19 595 (59.9) | NA | 11 521 (57.6) | 3219 (42.7) | 13 350 (58.5) |
| PCI | 19 162 (58.6) | 11 236 (59.5) | 13 797 (69.0) | 3746 (49.7) | NA |
| CABG | 7703 (23.6) | 3966 (21.0) | 5093 (25.5) | 1635 (21.7) | NA |
| AAA | 504 (1.5) | 300 (1.6) | 314 (1.6) | 130 (1.7) | 384 (1.7) |
| Carotid disease | 2474 (7.6) | 1279 (6.8) | 1617 (8.1) | 725 (9.6) | 1806 (7.9) |
| Internal cardiac defibrillator | 418 (1.3) | 334 (1.8) | 278 (1.4) | 74 (1.0) | 316 (1.4) |
| Pacemaker | 788 (2.4) | 408 (2.2) | 503 (2.5) | 197 (2.6) | 565 (2.5) |
| TIA | 1001 (3.1) | 522 (2.8) | 580 (2.9) | 327 (4.3) | 624 (2.7) |
| Hospitalization for CHF | 1531 (4.7) | 1051 (5.6) | 914 (4.6) | 391 (5.2) | 941 (4.1) |
| Current or previous clinical trial participation | 1135 (3.5) | 778 (4.1) | 663 (3.3) | 270 (3.6) | 760 (3.3) |
| Stroke | 1314 (4.0) | 777 (4.1) | 758 (3.8) | 300 (4.0) | 848 (3.7) |
| AF/flutter | 2313 (7.1) | 1190 (6.3) | 1369 (6.8) | 562 (7.5) | 1565 (6.9) |
| Asthma/COPD | 2419 (7.4) | 1393 (7.4) | 1441 (7.2) | 718 (9.5) | 1553 (6.8) |
| PAD | 3239 (9.9) | 1862 (9.9) | 1983 (9.9) | 914 (12.1) | 2221 (9.7) |
| Any angina | 7212 (22.1) | 4423 (23.4) | 3675 (18.4) | 2541 (33.7) | 3553 (15.6) |
| Angina and CCS class | |||||
| No angina | 25 479 (77.9) | 14 446 (76.6) | 16 312 (81.6) | 4996 (66.3) | 19 273 (84.4) |
| Angina class I | 2063 (6.3) | 1160 (6.1) | 1141 (5.7) | 706 (9.4) | 1136 (5.0) |
| Angina class II | 3834 (11.7) | 2355 (12.5) | 1966 (9.8) | 1378 (18.3) | 1898 (8.3) |
| Angina class III | 1236 (3.8) | 863 (4.6) | 527 (2.6) | 435 (5.8) | 477 (2.1) |
| Angina class IV | 78 (0.2) | 45 (0.2) | 40 (0.2) | 21 (0.3) | 41 (0.2) |
| CHF symptoms including NYHA class | |||||
| No CHF | 27 766 (84.9) | 15 328 (81.3) | 17 483 (87.5) | 6358 (84.4) | 20 233 (88.6) |
| CHF NYHA class II | 4113 (12.6) | 2953 (15.7) | 2131 (10.7) | 968 (12.8) | 2203 (9.7) |
| CHF NYHA class III | 808 (2.5) | 584 (3.1) | 369 (1.8) | 209 (2.8) | 389 (1.7) |
| HbA1c, % | 6.8 ± 1.8 | 6.9 ± 2.1 | 6.8 ± 1.9 | 6.8 ± 1.3 | 6.8 ± 1.4 |
| Cr, mmol/L | 0.088 (0.076–0.102) | 0.088 (0.077–0.103) | 0.088 (0.076–0.102) | 0.088 (0.076–0.102) | 0.088 (0.076–0.102) |
| Hgb, g/dL | 14.0 (13.0–15.0) | 14.1 (13.1–15.1) | 14.1 (13.0–15.0) | 14.0 (13.0–15.0) | 14.1 (13.0–15.0) |
| Fasting blood glucose, mmol/L | 5.7 (5.1–6.6) | 5.7 (5.1–6.6) | 5.7 (5.1–6.7) | 5.7 (5.1–6.6) | 5.7 (5.2–6.7) |
| TC, mmol/L | 4.3 (3.7–5.0) | 4.3 (3.7–5.1) | 4.2 (3.6–4.9) | 4.4 (3.7–5.2) | 4.2 (3.6–4.9) |
| HDL‐C, mmol/L | 1.1 (1.0–1.4) | 1.1 (1.0–1.3) | 1.1 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (1.0–1.4) |
| LDL‐C, mmol/L | 2.4 (1.9–2.9) | 2.4 (1.9–3.0) | 2.3 (1.9–2.9) | 2.4 (1.9–3.0) | 2.3 (1.9–2.9) |
| Fasting TG, mmol/L | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | 1.4 (1.0–2.0) | 1.4 (1.0–1.9) |
| HR (palpation), bpm | 68.2 ± 10.6 | 68.3 ± 10.6 | 67.7 ± 10.4 | 68.4 ± 10.9 | 67.7 ± 10.3 |
| ECG heart rate, bpm | 67.1 ± 11.4 | 67.2 ± 11.3 | 66.6 ± 11.1 | 67.6 ± 11.8 | 66.5 ± 11.0 |
| SBP, mm Hg | 131.0 ± 16.7 | 130.1 ± 16.6 | 130.4 ± 16.4 | 131.8 ± 16.1 | 130.5 ± 16.4 |
| DBP, mm Hg | 77.3 ± 10.0 | 77.3 ± 10.1 | 77.0 ± 9.7 | 77.5 ± 10.0 | 76.9 ± 9.7 |
| LVEF, % | 56.1 ± 11.1 | 53.7 ± 11.2 | 56.5 ± 11.0 | 57.3 ± 10.8 | 56.4 ± 11.0 |
| Coronary artery territories with stenosis >50% | |||||
| LM stem | 2848 (8.7) | 1485 (7.9) | 2014 (10.1) | 713 (9.5) | 2465 (10.8) |
| LAD | 19 062 (58.3) | 10 420 (55.2) | 14 022 (70.2) | 3965 (52.6) | 15 970 (70.0) |
| LCX | 11 793 (36.1) | 6547 (34.7) | 8920 (44.6) | 2595 (34.4) | 10 028 (43.9) |
| RCA | 14 233 (43.5) | 8320 (44.1) | 10617 (53.1) | 2982 (39.5) | 11 951 (52.4) |
| Bypass graft | 2630 (8.0) | 1427 (7.6) | 1703 (8.5) | 651 (8.6) | 2482 (10.9) |
| No significant stenosis | 1057 (3.2) | 609 (3.2) | 205 (1.0) | 417 (5.5) | 221 (1.0) |
| Coronary angiography not done in the past 12 months | 4763 (14.6) | 3247 (17.2) | 366 (1.8) | 1750 (23.2) | 520 (2.3) |
| ECG rhythm | |||||
| Sinus rhythm | 23 179 (94.9) | 13 622 (95.4) | 14 694 (95.1) | 5375 (94.6) | 16 482 (95.2) |
| AF/flutter | 836 (3.4) | 427 (3.0) | 503 (3.3) | 200 (3.5) | 537 (3.1) |
| Paced rhythm | 402 (1.6) | 227 (1.6) | 251 (1.6) | 105 (1.8) | 288 (1.7) |
| LBBB | 1201 (4.9) | 739 (5.2) | 724 (4.7) | 292 (5.1) | 767 (4.4) |
| Vessel disease | |||||
| 0 | 1007 (3.6) | 576 (3.7) | 199 (1.0) | 394 (6.8) | 213 (1.0) |
| 1 | 11 458 (41.1) | 6498 (41.7) | 8059 (41.1) | 2001 (34.6) | 8783 (39.4) |
| ≥2 | 15 413 (55.3) | 8519 (54.6) | 11 341 (57.9) | 3383 (58.5) | 13 275 (59.6) |
Abbreviations: AAA, abdominal aortic aneurysm; AF, atrial fibrillation; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; CHF, chronic heart failure; CLARIFY, Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DBP, diastolic blood pressure; ECG, electrocardiogram; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; Hgb, hemoglobin; HR, heart rate; IQR, interquartile range; LAD, left anterior descending artery; LBBB, left bundle branch block; LCX, left circumflex artery; LDL‐C, low‐density lipoprotein cholesterol; LM, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack.
Data are presented as n (%), mean ± SD, or median (IQR).
Inclusion criteria are not mutually exclusive; some patients may be included in >1 group.
At baseline, the CLARIFY population had high rates of use of evidence‐based drugs for prevention in CAD (Table 3). Most patients were receiving aspirin (ASA; 87.8%) and lipid‐lowering drugs (92.3%). Rates of β‐blocker, angiotensin‐converting enzyme inhibitor (ACEI), and angiotensin II receptor blocker therapies were 75.3%, 51.7%, and 26.5%, respectively. Three‐quarters of patients received either full (39.2%) or partial (37.7%) reimbursement for their CV agents.
Table 3.
Baseline medications
| All CLARIFY Patients, n = 32 703 | Prior MI >3 Months Ago,a n = 18 876 (57.7%) | Coronary Stenosis ≥50% on Angiography,a n = 19 996 (61.1%) | Chest Pain With Proven Myocardial Ischemia,a n = 7544 (23.1%) | History of PCI or CABG >3 Months Ago,a n = 22 836 (69.8%) | |
|---|---|---|---|---|---|
| ASA | 28 687 (87.8) | 16 806 (89.1) | 17 652 (88.3) | 6548 (86.8) | 20 298 (88.9) |
| Thienopyridine | 8881 (27.2) | 5179 (27.5) | 6244 (31.3) | 1913 (25.4) | 7217 (31.6) |
| Other antiplatelets | 3023 (9.3) | 1659 (8.8) | 1984 (9.9) | 713 (9.5) | 2213 (9.7) |
| Oral anticoagulants | 2670 (8.2) | 1501 (8.0) | 1614 (8.1) | 610 (8.1) | 1795 (7.9) |
| β‐Blockers | 24 611 (75.3) | 14 887 (78.9) | 15 317 (76.6) | 5435 (72.1) | 17 391 (76.2) |
| Symptoms indicative of intolerance or contraindication to β‐blockers | 4718 (14.4) | 2822 (15.0) | 2816 (14.1) | 1278 (17.0) | 3078 (13.5) |
| Ivabradine | 3218 (9.8) | 1990 (10.5) | 1790 (9.0) | 1167 (15.5) | 1810 (7.9) |
| Calcium antagonists | 8909 (27.3) | 4363 (23.1) | 5592 (28.0) | 2359 (31.3) | 6090 (26.7) |
| Verapamil or diltiazem | 1896 (5.8) | 898 (4.8) | 1135 (5.7) | 579 (7.7) | 1247 (5.5) |
| ACEIs | 16 895 (51.7) | 10 963 (58.1) | 10 092 (50.5) | 3620 (48.0) | 11 548 (50.6) |
| ARBs | 8674 (26.5) | 4444 (23.6) | 5435 (27.2) | 2175 (28.9) | 6232 (27.3) |
| Lipid‐lowering drugs | 30 191 (92.3) | 17 657 (93.6) | 18 718 (93.6) | 6915 (91.7) | 21 415 (93.8) |
| Long‐acting nitrates | 7152 (21.9) | 4196 (22.2) | 4262 (21.3) | 2002 (26.6) | 4370 (19.1) |
| Other antianginal agents | 4541 (13.9) | 2687 (14.2) | 2653 (13.3) | 1185 (15.7) | 2602 (11.4) |
| Diuretics | 9585 (29.3) | 5761 (30.5) | 5668 (28.4) | 2312 (30.7) | 6471 (28.4) |
| Other antihypertensive agents | 2251 (6.9) | 1209 (6.4) | 1341 (6.7) | 575 (7.6) | 1511 (6.6) |
| Digoxin and derivatives | 828 (2.5) | 523 (2.8) | 464 (2.3) | 204 (2.7) | 511 (2.2) |
| Amiodarone/dronedarone | 962 (2.9) | 594 (3.1) | 614 (3.1) | 247 (3.3) | 667 (2.9) |
| Other antiarrhythmics | 306 (0.9) | 151 (0.8) | 185 (0.9) | 88 (1.2) | 194 (0.9) |
| NSAIDs | 1614 (4.9) | 902 (4.8) | 897 (4.5) | 472 (6.3) | 1049 (4.6) |
| Anti‐DM agents | 8016 (24.5) | 4502 (23.9) | 5075 (25.4) | 1963 (26.0) | 5761 (25.2) |
| PPIs | 8106 (24.8) | 4770 (25.3) | 5106 (25.5) | 2178 (28.9) | 5948 (26.1) |
| Thyroid HRT | 1420 (4.3) | 738 (3.9) | 804 (4.0) | 355 (4.7) | 957 (4.2) |
| HRT in postmenopausal women | 99 (0.3) | 44 (0.2) | 52 (0.3) | 37 (0.5) | 59 (0.3) |
| ED | 529 (1.6) | 329 (1.7) | 340 (1.7) | 152 (2.0) | 367 (1.6) |
| Reimbursement of CV agents | |||||
| Full | 12 792 (39.2) | 7324 (38.9) | 7412 (37.2) | 3113 (41.4) | 9134 (40.1) |
| Partial | 12 318 (37.7) | 7114 (37.8) | 8061 (40.4) | 2678 (35.6) | 8992 (39.5) |
| None | 7521 (23.0) | 4392 (23.3) | 4478 (22.4) | 1721 (22.9) | 4654 (20.4) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin II receptor blockers; ASA, acetylsalicylic acid (aspirin); CABG, coronary artery bypass grafting; CLARIFY, Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease; CV, cardiovascular; DM, diabetes mellitus; ED, erectile dysfunction; HRT, hormone replacement therapy; MI, myocardial infarction; NSAIDs, nonsteroidal anti‐inflammatory drugs; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor.
Data are presented as n (%).
Inclusion criteria are not mutually exclusive; some patients may be included in >1 group.
Overall, the clinical characteristics of the 4 groups were similar, although patients in the group with symptomatic angina were slightly older, with a higher prevalence of DM, treated hypertension, and dyslipidemia, and less physical activity (Table 1). Patients with angina also more frequently had a history of atrial fibrillation/flutter, peripheral artery disease, and asthma/chronic obstructive pulmonary disease (Table 2). Given the size of the cohort, these modest differences were significant. There were, however, notable differences in management between groups: patients with a history of MI were more likely to receive ASA, β‐blockers, and ACEIs and less likely to receive calcium antagonists than the other groups (Table 3). Also, the use of some non‐CV drug classes was substantial (eg, proton pump inhibitors [24.8%] and anti‐DM agents [24.5%]).
4. DISCUSSION
The CLARIFY Registry enrolled a large, worldwide population representative of contemporary established outpatients with CAD. This population was composed of a relatively young and mostly hypertensive male population, mainly retired, with few current smokers or patients with DM, with preserved LVEF, and with high rates of use of evidence‐based drugs for secondary prevention. This probably reflects the exclusion of patients with severe noncardiac conditions or advanced other cardiac conditions, such as HF or advanced valvular disease.
Compared with the Euro Heart Survey in 20055 or the REACH Registry in 200717 and 2010,18 the rates of use of evidence‐based medications for secondary prevention appear to be higher in the CLARIFY stable‐CAD population, reflecting increasing adherence to international guidelines in routine clinical practice.19, 20, 21, 22, 23 Despite this improvement, prevalence and control of major CV risk factors vary markedly worldwide, with many outpatients with stable CAD being treated suboptimally.24
With a very detailed 5‐year follow‐up—including medical events; clinical, biological, and paraclinical variables; and medication—the CLARIFY Registry will provide the opportunity to describe the prognostic determinants of stable CAD. Some preliminary findings from CLARIFY already have been reported. In patients with hypertension and stable CAD, systolic BP <120 mm Hg and diastolic BP <70 mm Hg were each associated with CV events, including mortality, supporting the existence of a J‐curve phenomenon and suggesting that caution should be taken with the use of BP‐lowering treatment in patients with hypertension and CAD.25
Despite high rates of use of β‐blockers, patients with stable CAD often have resting HR ≥70 bpm, which has been associated with an overall worse health status, more frequent angina, and ischemia.26 Sex‐ and age‐related differences have also been identified. Women were more likely to have angina but less likely to have undergone revascularization procedures; and patients ≥75 years' old were less often treated with β‐blockers, ASA, and ACEIs than were patients ≤65 years' old.27 However, after 1‐year follow‐up, there was no clear difference in age‐adjusted outcomes between men and women with stable CAD.28 Compared with normal renal function, chronic renal insufficiency was associated with a lower use of evidence‐based medications for secondary prevention, including antiplatelet drugs, statins, β‐blockers, and ACEIs.29
In patients with atrial fibrillation within CLARIFY, anticoagulants were markedly underused, whereas antiplatelet therapy was still widely used, both of which are at odds with contemporary international guidelines.30, 31, 32
Finally, in patients who underwent noninvasive testing, the presence of anginal symptoms (with or without ischemia) appeared to be associated with a higher risk of adverse CV outcomes than ischemia per se.33 An additional finding of that analysis was that approximately 70% of events occurred in patients with no evidence of myocardial ischemia on noninvasive testing, indicating that focusing the management of stable CAD solely on the prevention or treatment of ischemia does not address the risks that these patients face.33 A particular focus will be given to these populations (ie, with or without anginal symptoms and with or without proven ischemia on noninvasive testing) to try to explain the differences in CV outcomes.
Despite the size and scope of CLARIFY, the registry is not without important limitations. First, as with any observational database, it is difficult to rule out selection biases and confounding. We attempted to improve the representativeness of the cohort: minimizing the risk of selection bias by drastically limiting the enrollment period and attempting to balance representation of each country by targeting a fixed proportion of patients in relation to each country's population. Second, although patients were enrolled in North and Central America, there was no enrollment in the United States. Third, there was not 100% source data monitoring, but audits were performed in randomly selected sites and data were reviewed and queried remotely. Finally, events were collected as reported by investigators and there was no central adjudication, although a set of short definitions was included in the case‐report forms to assist investigators in defining and identifying clinical characteristics and outcomes.
5. CONCLUSION
The CLARIFY Registry will provide a large database of contemporary international data regarding the characteristics, management, and outcomes of patients with stable CAD.
Supporting information
On‐line only appendix
ACKNOWLEDGMENTS
Editorial support was provided by Jenny Lloyd (MedLink Healthcare Communications Limited) and was compensated by the sponsor.
Conflicts of interest
R.F. has served on speaker's bureau for Bayer, Merck Serono, Novartis, Amgen, Servier International, and Pfizer; discloses research grants/contracts from Boehringer Ingelheim, Novartis, Irbetch, and Servier International; has served on advisory board for Boehringer Ingelheim, Novartis, and Servier International; has received an honorarium from Servier for steering committee membership consulting and speaking plus support for travel to study meetings; has received personal fees from Boehringer‐Ingelheim, Novartis, Merck Serono, and Irbtech; and has been a stockholder in Medical Trials Analysis. I.F. discloses honoraria and research grants from Servier and Amgen. K.F. discloses honoraria and/or consultation fees and/or travel expenses from Servier, AstraZeneca, TaurX, Armgo, Broadview Ventures, and CellAegis; he is on the scientific advisory board of Celixer and is a director of Vesalius Trials Ltd. J.‐C.T. discloses research grants from Amarin, AstraZeneca, DalCor, Esperion, Ionis, Merck, Pfizer, Sanofi, and Servier, and honoraria from DalCor, Pfizer, Sanofi, and Servier. M.T. discloses honoraria and consultation fees from Servier, Bayer, Janssen‐Cilag, Celyad, and Kowa. P.G.S. discloses research grants from Merck, Sanofi, and Servier, and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, CSL Behring, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and The Medicines Company. E.S. and N.G. have no conflicts to disclose.
Sorbets E, Greenlaw N, Ferrari R, et al. Rationale, design, and baseline characteristics of the CLARIFY registry of outpatients with stable coronary artery disease. Clin Cardiol. 2017;40:797–806. 10.1002/clc.22730
Funding information The CLARIFY Registry is supported by Servier. The sponsor had no role in the study design or data analysis and interpretation, nor in the decision to submit the manuscript for publication. The sponsor assisted with the setup, data collection, and management of the study in each country. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1. Leal J, Luengo‐Fernández R, Gray A, et al. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J. 2006;27:1610–1619. [DOI] [PubMed] [Google Scholar]
- 2. Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. [DOI] [PubMed] [Google Scholar]
- 3. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. [DOI] [PubMed] [Google Scholar]
- 4. Tunstall‐Pedoe H, Kuulasmaa K, Mähönen M, et al. Contribution of trends in survival and coronary‐event rates to changes in coronary heart disease mortality: 10‐year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557. [DOI] [PubMed] [Google Scholar]
- 5. Daly CA, Clemens F, López‐Sendón J, et al; Euro Heart Survey Investigators . The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: from the Euro Heart Survey of Stable Angina. Eur Heart J. 2005;26:996–1010. [DOI] [PubMed] [Google Scholar]
- 6. GRACE Investigators . Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141:190–199. [DOI] [PubMed] [Google Scholar]
- 7. Puymirat E, Simon T, Steg PG, et al; USIK USIC 2000 Investigators; FAST‐MI Investigators . Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308:998–1006. [DOI] [PubMed] [Google Scholar]
- 8. Carrero JJ, Evans M, Szummer K, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–928. [DOI] [PubMed] [Google Scholar]
- 9. Zarifis J, Kallistratos M, Katsivas A, et al. Antianginal efficacy of ivabradine/metoprolol combination in patients with stable angina. Clin Cardiol. 2016;39:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz A, Bourassa MG, Guertin MC, et al. Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. [DOI] [PubMed] [Google Scholar]
- 11. Fox K, Ford I, Steg PG, et al; BEAUTIFUL Investigators . Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet . 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 12. Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. [DOI] [PubMed] [Google Scholar]
- 13. Hsia J, Larson JC, Ockene JK, et al. Resting heart rate as a low tech predictor of coronary events in women: prospective cohort study. BMJ. 2009;338:b219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the International Verapamil‐SR/trandolapril Study (INVEST). Eur Heart J. 2008;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mauri L. Why we still need randomized trials to compare effectiveness. N Engl J Med. 2012;366:1538–1540. [DOI] [PubMed] [Google Scholar]
- 16. Steg PG, López‐Sendón J, Lopez de Sa E, et al. External validity of clinical trials in acute myocardial infarction. Arch Intern Med . 2007;167:68–73. [DOI] [PubMed] [Google Scholar]
- 17. Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators . One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA . 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 18. Bhatt DL, Eagle KA, Ohman EM, et al; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 19. Steg PG, James SK, Atar D, et al; Task Force on the Management of ST‐segment Elevation Acute Myocardial Infarction of the European Society of Cardiology . ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J . 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 20. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 21. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol . 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 22. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST‐Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. [DOI] [PubMed] [Google Scholar]
- 23. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2013;62:1039]. J Am Coll Cardiol . 2013;61:485–510. [DOI] [PubMed] [Google Scholar]
- 24. Ferrari R, Ford I, Greenlaw N, et al; CLARIFY Registry Investigators . Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: data from the contemporary CLARIFY registry. Eur J Prev Cardiol . 2015;22:1056–1065. [DOI] [PubMed] [Google Scholar]
- 25. Vidal‐Petiot E, Ford I, Greenlaw N, et al; CLARIFY Investigators . Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet . 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 26. Steg PG, Ferrari R, Ford I, et al; CLARIFY Investigators . Heart rate and use of β‐blockers in stable outpatients with coronary artery disease. PLoS One . 2012;7:e36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrari R, Abergel H, Ford I, et al; CLARIFY Investigators . Gender‐ and age‐related differences in clinical presentation and management of outpatients with stable coronary artery disease. Int J Cardiol . 2013;167:2938–2943. [DOI] [PubMed] [Google Scholar]
- 28. Steg PG, Greenlaw N, Tardif JC, et al; CLARIFY Registry Investigators . Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J . 2012;33:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalra PR, García‐Moll X, Zamorano J, et al. Impact of chronic kidney disease on use of evidence‐based therapy in stable coronary artery disease: a prospective analysis of 22 272 patients. PLoS One. 2014;9:e102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fauchier L, Greenlaw N, Ferrari R, et al; CLARIFY Investigators . Use of anticoagulants and antiplatelet agents in stable outpatients with coronary artery disease and atrial fibrillation. International CLARIFY Registry. PLoS One. 2015;10:e0125164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 32. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 33. Steg PG, Greenlaw N, Tendera M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174:1651–1659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
On‐line only appendix


