Abstract
Objective
To compare clinical performance of a new resorbable non‐cross‐linked collagen membrane, creos xenoprotect (CXP), with a reference membrane (BG) for guided bone regeneration at dehisced implant sites.
Materials and methods
This randomized controlled clinical trial enrolled patients with expected dehiscence defects following implant placement to restore single teeth in the maxillary and mandibular esthetic zone and premolar area. Implants were placed using a two‐stage surgical protocol with delayed loading. Bone augmentation material placed at the implant surface was immobilized with CXP or BG membrane. Soft tissue health was followed during the healing period, and the defect size was measured at reentry and 6 months after implant placement.
Results
Of the 49 included patients, 24 were treated with CXP and 25 with BG. Patient characteristics did not differ between the two arms. In the CXP arm, the defect height at implant insertion was (mean ± SD) 5.1 ± 2.1 mm (n = 24) and reduced at reentry by 81% to 1.0 ± 1.3 mm (n = 23). In the BG arm, the defect height at implant insertion was 4.9 ± 1.9 mm (n = 25) and reduced at reentry by 62% to 1.7 ± 2.1 mm (n = 24). Assuming a margin of non‐inferiority of 1 mm, CXP was non‐inferior to BG. Membrane exposure rate was highest at week 3 in both arms, reaching 16.7% for BG and 8.7% for CXP.
Conclusions
The new resorbable non‐cross‐linked collagen membrane facilitates bone gain to support implant placement in expected dehiscence defects. The observed trend toward higher mean bone gain and lower exposure rate with CXP compared to BG should be further investigated.
Keywords: collagen membrane, dehisced implant sites, esthetic zone, Guided bone regeneration, randomized clinical trial, simultaneous implant placement
Over the last few decades, implant‐supported restorations have become an established treatment option for missing or hopeless teeth in several indications (Branemark et al. 1977, 1999; Buser et al. 1997; Lekholm et al. 1999; den Hartog et al. 2008; Pjetursson et al. 2012; Hof et al. 2013, 2015; Khzam et al. 2015). Historically, osseointegration was the sole factor defining success; however, this paradigm has shifted toward restoration‐driven implant placement and “backward planning,” in which the design of the prosthetic device determines the exact location of the implant (Garber & Belser 1995; Lindhe et al. 2005). Placing an implant precisely in the root socket of the extracted tooth may result in strong resorption of the alveolar ridge, primarily on the buccal side (Araújo & Lindhe 2005). Approximately 50% of the resorption in the buccolingual direction occurs in the first 12 months following tooth extraction (Schropp et al. 2003), and most of it occurs within the first 3 months (Araújo et al. 2006). To correct for bone resorption, which is particularly important when performing implant placement in the esthetic zone, bone augmentation is often the only technique that produces successful esthetic and functional results (Khzam et al. 2015; Nisand et al. 2015).
Guided bone regeneration (GBR) for lateral alveolar ridge augmentation has been described extensively. Numerous high‐quality studies have shown that this technique provides reproducible results and high long‐term implant survival rates (Aghaloo & Moy 2007; Sanz‐Sanchez et al. 2015). Similar survival rates are achieved when GBR is combined with either simultaneous or subsequent implant placement, indicating that the two approaches are comparable (Sanz‐Sanchez et al. 2015). Similar implant survival rates are also achieved when the implant is placed in augmented or pristine bone (Donos et al. 2008).
Bone augmentation of the alveolar ridge is based on the principles of tissue engineering and was first described for guided tissue regeneration in 1979 by Nyman & Karring (1979) and later investigated extensively (Gottlow et al. 1984; Dahlin et al. 1988; Buser et al. 1990; Lang et al. 1994; Simion et al. 1994). These early publications mainly deal with polytetrafluorethylene membranes as a barrier; however, the membrane exposure rate of this material ranges from 30% to 40% (Becker et al. 1994; Lang et al. 1994; Carpio et al. 2000). Additionally, these non‐resorbable membranes often require an extensive reentry procedure to remove the membrane (Becker et al. 1994; Lang et al. 1994; Carpio et al. 2000).
Two decades of research into native resorbable collagen membranes for lateral ridge augmentation with GBR have shown this material to be comparable to non‐resorbable membranes with respect to the amount of bone gain (Zitzmann et al. 1997; Carpio et al. 2000; Sanz‐Sanchez et al. 2015). While the primary goal of using resorbable membranes was to avoid extensive membrane removal procedures, these materials also had a much lower membrane exposure rate, ranging from 8.7% to 31.8% (Carpio et al. 2000; Nemcovsky & Artzi 2002; Moses et al. 2005; Jung et al. 2009). Chemically cross‐linked collagen membranes have longer degradation times but also have significantly higher membrane exposure rates, up to 70.5% (Moses et al. 2005; Tal et al. 2008; Friedmann et al. 2011). Wound dehiscence with membrane exposure has a substantial negative effect on GBR around dental implants and often results in reduced bone regeneration (Machtei 2001).
It has been hypothesized that a longer degradation time can lead to higher regenerated bone quality or quantity; however, the evidence supporting or refuting this hypothesis is lacking. Recently, a native collagen membrane with a significantly longer degradation time and increased mechanical properties, such as higher tensile and suture pull‐out strength, has been developed (Bozkurt et al. 2014). This membrane had a low membrane exposure rate (12%) in a retrospective analysis of 36 patients with simultaneous or subsequent implant placement (Wessing et al. 2016).
The aim of this study was to evaluate bone formation and soft tissue healing 6 months after single‐tooth implant placement in patients with dehiscence defects treated using either creos xenoprotect (CXP; Nobel Biocare, Göteborg, Sweden) or Bio‐Gide (BG; Geistlich, Wolhusen, Switzerland) collagen membranes as part of a GBR procedure. This report presents the interim bone augmentation outcomes of a 5‐year prospective randomized controlled study.
Material and Methods
Ethical considerations
This study was conducted following the ethical principles founded in the Declaration of Helsinki. Written informed consent was obtained from all eligible patients prior to enrollment. Patients had the option to terminate their participation in the study at any time. Ethical approval was obtained from an ethical committee from each involved center, and the study was registered on ClinicalTrials.gov (NCT02373787). The Consolidated Standards of Reporting Trials (CONSORT) guidelines were used as the framework for this study and report (Schulz et al. 2010).
Study design and participants
This multicenter clinical investigation was performed at seven university clinics and private practices in Europe, including Austria, Germany, Hungary, Italy, and Spain. One study site withdrew before patient inclusion. The primary objective of this prospective study was to assess the efficacy, that is, non‐inferiority of CXP in comparison with a reference membrane (BG) in a randomized controlled clinical study. All patients were in need of a single‐tooth implant‐supported restoration(s) with expected dehiscence defects in the anterior and premolar areas of the maxilla or the mandible. Patients were randomized on the day of surgery based on a randomization list supplied by the electronic data capturing system (Viedoc©; Pharma Consulting Group, Uppsala, Sweden) used to collect all study data. The patients and the evaluators were blinded to treatment. If patients had two or more sites needing single‐unit implant restorations with bone augmentation, only one site was randomly included in the study. The other site was treated with standard care.
Outcome measures
The primary outcome measure was defined as the defect height (DH) measured from the top of the implant shoulder to the first bone‐to‐implant contact perpendicular to the long implant axis at 6 months following the augmentation procedure. The secondary outcome measures included bone formation with respect to the following:
Defect width (DW) defined as the distance between the mesial and distal bone crest measured at the level of the implant shoulder.
Defect depth (DD) defined as the distance from the bone crest to the implant surface measured perpendicular to the long axis of the implant.
Infrabony defect (ID) defined as the distance from the bone crest to the first bone‐to‐implant contact parallel to the implant axis.
The osseous defect measurements are shown in Fig. 1 and were performed according to Jung et al. (2009).
Figure 1.
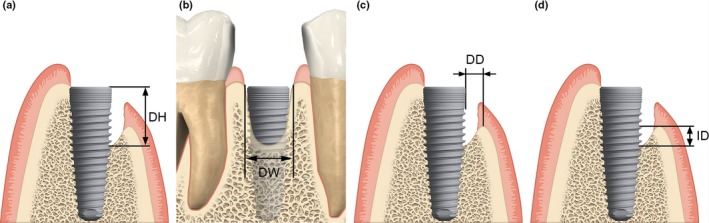
Clinical measurements of the peri‐implant bone defect performed at implant placement. (a) Defect height (DH), (b) defect width (DW), (c) defect depth (DD), (d) infrabony defect (ID).
Other secondary outcome measures included soft tissue healing assessed using clinical measurements of wound dehiscence, membrane exposure, redness and swelling, and implant survival rates. Implant stability was evaluated by hand testing. Patient's subjective pain was assessed using a visual analog scale with ratings from 0 to 10 where 0 = no pain to 10 = very intense pain. The oral health impact profile (OHIP‐14) was assessed with the OHIP‐14 questionnaire in respective local languages using validated translations. Patients rated the prevalence of their functional limitations, physical pain, psychological discomfort, physical, psychological and social disability, and handicap on a 0–4 scale, where 0 = never, 1 = hardly ever, 2 = occasionally, 3 = fairly often, and 4 = very often (Slade 1997). The follow‐up schedule for each parameter is listed in Table 1.
Table 1.
Time schedule for parameter assessment
| Pretreatment examination | Implant insertion and GBR procedure | 1‐, 3‐, 6‐week and 3‐month follow‐up | Reentry surgery at 6 months | |
|---|---|---|---|---|
| Efficacy parameters | ||||
| Defect size measurements | X | X | ||
| Soft tissue healing parameters | X | X | ||
| Clinical photographs | X | X | X | X |
| Radiographic examinations | X | |||
| Implant survival | X | X | ||
| OHIP‐14 | X | X | X | X |
| Pain | X | X | ||
| Safety parameters | ||||
| Adverse event reporting | X | X | X | |
| Other parameters | ||||
| Patient information and informed consent procedure | X | |||
| Inclusion/exclusion criteria | X | X | ||
| Demographic data/medical history | X | |||
| Medical history update | X | X | X | |
| Implant parameters | X | |||
| Prosthesis parameters | X | |||
Inclusion criteria
The primary inclusion criteria were as follows. Patients were included if they (i) provided written informed consent; (ii) were ≥18 years of age and ceased growth; (iii) were physically and mentally capable of participating through the 5‐year follow‐up period; (iv) were willing and able to comply with all study‐related procedures; (v) required a single‐unit implant restoration in the anterior and premolar areas of maxilla or mandible with GBR of bony defects; (vi) had an American Society of Anesthesiologists (ASA) score of I or II; (vii) had an implant site free of infection and extraction remnants; (viii) had a full‐mouth gingival index lower than 25%, a full‐mouth bleeding score lower than 25%, and a full‐mouth plaque score lower or equal to 25%; (ix) had a favorable and stable occlusal relationship and natural roots adjacent to the implant site; and (x) could undergo a two‐stage surgical procedure.
Subjects were excluded if they (i) had previous bone augmentation at the implant site; (ii) had the tooth extraction at the implant placement site performed within 3 months of implant placement surgery; (iii) had acute, untreated periodontitis; (iv) had a health condition that does not permit surgical treatment; (v) had any disorders in the planned implant area, such as previous tumors, chronic bone disease, or previous irradiation; (vi) had an infection in the planned implant placement site or adjacent tissue; (vii) were undergoing treatment with an interfering medication, such as steroid therapy or bisphosphonates; (viii) had a history of past or ongoing alcohol or substance abuse; (ix) were a heavy smoker (>10 cigarettes/day); (x) had uncontrolled diabetes; (xi) have severe bruxism or other destructive habits; and (xii) were pregnant or lactating at the time of collagen membrane insertion.
Secondary inclusion criteria were determined at the time of surgery. Subjects were included if they (i) had sufficient bone volume at the implant site to place a tapered implant that was 10 mm in length, (ii) had initial implant stability as assessed by hand testing, and (iii) had a defect size sufficient for GBR. Defects eligible for GBR in the study had one or two walls missing and a DH of 3 mm up to 7 mm. Larger defects up to 10 mm were eligible if the defect width did not exceed 2 mm. All defect measurements were performed with a UNC15 periodontal probe.
Surgical protocol and postoperative care
Preoperative medications were given based on each clinic's routine protocol and included prophylactic broad‐spectrum antibiotics and analgesics. Following local anesthesia, the surgeon performed a full‐thickness mid‐crestal incision or a slightly palatal incision in the keratinized gingiva. For surgical access, one or two divergent vertical incisions were placed one tooth away at the opposite line angle from the surgical site. This technique avoids wound‐healing complications due to the presence of the underlying graft material or membrane. The alveolar ridge was thoroughly debrided of granulation tissue and extraction remnants, and implants (NobelReplace CC; Nobel Biocare) were placed at the planned position according to the manufacturer's protocol. Implant primary stability was assessed by hand testing. At this time, secondary inclusion criteria were applied. Defect measurements using a periodontal probe and photographic documentation from the buccal and occlusal views were collected. After implant placement, an independent evaluator measured the osseous defect of the eligible site as described earlier. Decortication holes were made in the planned bone augmentation area (e.g., using a 1‐mm round metal burr) to draw blood from the cancellous bone into the graft site. Autologous bone chips were then placed on the surface of the dental implant, and anorganic bovine bone mineral (Bio‐Oss; Geistlich) was placed on top of the bone chips for slower resorption according to the previously described sandwich technique (Fig. 2; Wang et al. 2004). After placement of the particulate bone graft, the collagen membrane (either CXP or BG) was trimmed, positioned, and rehydrated with sterile saline solution. In all cases, the membrane was fixed using either periosteal vertical mattress sutures or titanium cortical bone pins. To avoid high‐tension forces during flap closure, which increases the risk of wound dehiscence, horizontal periosteal release incisions were performed apical to the bone graft (Burkhardt & Lang 2010). Complete closure of the mucoperiosteal flap was performed according to the clinic's routine protocol. After the surgery, subjects were provided with instructions for home care, including performing a 0.1–0.2% chlorhexidine rinse for 2 weeks after implant insertion, and scheduled for post‐surgical visits on an individual basis. Patients were advised against brushing the surgical area before suture removal, and a soft‐bristled toothbrush was recommended after suture removal. Normal, thorough oral care was recommended for dentition outside the surgical area. Prophylactic antibiotic treatment was prescribed for 5 days. Analgesics were prescribed for 2 days post‐surgery and varied based on individual needs.
Figure 2.
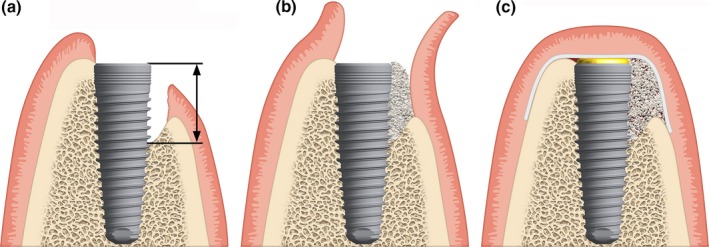
Schematic representation of the guided bone regeneration procedure: (a) place implant and measure defect height, (b) fill the defect with autologous bone chips on the implant surface and anorganic bovine bone matrix on top, (c) cover the defect and implant site with the collagen membrane.
Reentry protocol
Reentry surgery was performed 6 months post‐implantation. After raising mucoperiosteal flaps, the cover screw was removed, and a healing abutment was placed. At the same time, the bone defect size was remeasured by an independent evaluator. Defect measurements using a periodontal probe and photographic documentation from the buccal and occlusal views were collected. Flaps were adjusted to fit around the neck of the healing abutment and sutured with single interrupted sutures. If the soft tissue was compromised at the time of reentry, surgeons were allowed to perform soft tissue augmentation.
Statistical methods
The study was designed to test non‐inferiority when comparing two collagen membranes with respect to a DH change 6 months after implantation with a power of 80%. The margin of inferiority was set at 1 mm, and the standard deviation was assumed to be 0.94 mm (Jung et al. 2003). Accounting for 25% subject withdrawal rate, 40 subjects (20 per arm) were needed for the study.
To assess non‐inferiority of the CXP arm to the BG arm, a nonparametric two‐sided 95% confidence interval for the difference in arm means (CXP minus BG) was calculated. With its upper boundary less than 1 mm, the non‐inferiority would be demonstrated at a one‐sided significance level of α = 0.025.
For all other metrically scaled secondary endpoints, such as DW or DH at insertion, means ± standard deviation were calculated. Arm means were compared using two‐sided t‐tests. All other variables were scaled categorically and evaluated using contingency tables. For statistical comparisons, Pearson's chi‐squared or McNemar's tests were used, when appropriate. Soft tissue results were evaluated using Fisher's exact test (wound dehiscence, membrane exposure, and redness) and the chi‐squared test for linear association (swelling). Pain and OHIP scores were evaluated using the Mann–Whitney test. For all secondary endpoints, the two‐sided level of significance was α = 0.05. All statistical evaluations were carried out using the open‐source software R 3.1 (Team RC 2014) and IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA).
Results
Patient enrollment, characteristics, and follow‐up
All implant placement and bone augmentation procedures took place between December 17, 2013 and July 22, 2015. This interim report includes data available until May 20, 2016. The flowchart of the study is shown in Fig. 3. Of 67 enrolled patients, 49 met the inclusion criteria for bone defect size at the time of surgery, 17 did not meet the defect size inclusion criteria, and one withdrew before surgery. Patients were randomized into two treatment arms, with 24 patients in the CXP arm and 25 in the BG arm. Detailed patient and implant characteristics per study arm are provided in Table 2. Two patients were lost to follow‐up because they went to a different clinic for restoration, and the remaining 47 patients underwent reentry surgery. One patient from the BG arm received soft tissue augmentation at reentry. The overall implant survival rate at the time of reentry was 100%.
Figure 3.
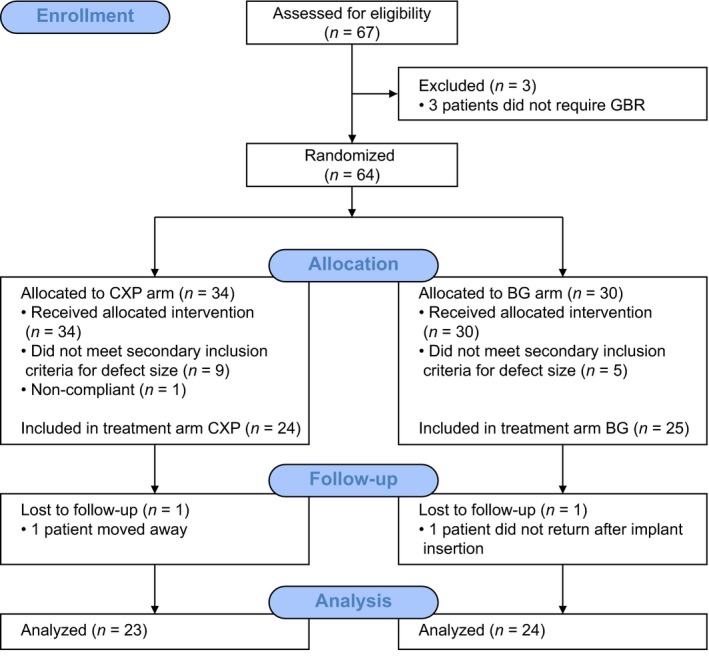
CONSORT 2010 flowchart of the study.
Table 2.
Patient and implant site characteristics
| Patient characteristics | CXP | BG |
|---|---|---|
| N | 24 | 25 |
| Gender | ||
| Female, n (%) | 11 (46) | 9 (36) |
| Male, n (%) | 13 (54) | 16 (64) |
| Age at surgery | ||
| Mean ± SD (years) | 38.6 ± 15.3 | 48.9 ± 17.0 |
| Smoking | ||
| Non‐smoking | 21 (88) | 17 (68) |
| Smoking 0–5 cigarettes/day, n (%) | 2 (8) | 2 (8) |
| Smoking 6–10 cigarettes/day, n (%) | 1 (4) | 6 (24) |
| History of periodontitis, n (%) | 2 (8) | 4 (16) |
| Treated diabetes | 0 (0) | 1 (4) |
| Implant site characteristics | ||
| Position, n (%) | ||
| Maxilla | 17 (71) | 18 (72) |
| Mandible | 7 (29) | 7 (28) |
| Type of site, n (%) | ||
| Healed, >6 months post‐extraction | 12 (50) | 8 (32) |
| Healed, >3 and <6 months post‐extraction | 9 (38) | 15 (60) |
| Other (agenesis) | 3 (13) | 2 (8) |
| Biotype, n (%) | ||
| Thin | 15 (63) | 9 (36) |
| Thick | 9 (38) | 16 (64) |
| Bone quality, n (%) | ||
| 1 | 3 (13) | 1 (4) |
| 2 | 14 (58) | 14 (56) |
| 3 | 7 (29) | 10 (49) |
| 4 | 0 | 0 |
| Bone quantity, n (%) | ||
| A | 5 (21) | 3 (12) |
| B | 11 (46) | 12 (48) |
| C | 6 (25) | 8 (32) |
| D | 2 (8) | 2 (8) |
| Implant insertion torque | ||
| Mean ± SD (years) | 39.6 ± 6.2 | 37.6 ± 9.5 |
| Implant position in the bone, n (%) | ||
| Subcrestal | 7 (29) | 4 (16) |
| Equicrestal | 17 (71) | 21 (84) |
| Defect morphology, n (%) | ||
| 1 wall missing | 22 (92) | 21 (84) |
| 2 walls missing | 2 (8) | 4 (16) |
CXP, creos xenoprotect membrane; BG, reference membrane; SD, standard deviation.
Primary outcome measure
Overall, mean DH decreased from 5.0 ± 2.0 mm at insertion to 1.3 ± 1.7 mm at reentry 6 months later. The DH at insertion was comparable between the two treatment arms, with a mean value of 5.1 ± 2.1 mm for the CXP arm and 4.9 ± 1.9 mm for the BG arm (P = 0.832, Table 3). In the CXP arm, the defect height at implant insertion reduced at reentry to 1.0 ± 1.3 mm (n = 23), while in the BG arm, the defect height reduced at reentry to 1.7 ± 2.1 mm (n = 24). Table 3 lists the change in defect size, and Fig. 4 illustrates the difference in DH measurements between the CXP and the BG arms.
Table 3.
Defect size at the time of implant insertion and at re‐entry surgery performed after a 6‐month healing period
| Defect size at implant insertion | Defect size at re‐entry | Bone gain from implant insertion to re‐entry | % Bone gain from implant insertion to re‐entry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Defect height | Defect width | Defect depth | Infrabony defect height | Defect height | Defect width | Defect depth | Infrabony defect height | Defect height | Defect width | Defect depth | Infrabony defect height | Defect height | Defect width | Defect depth | Infrabony defect height | |
| Total | ||||||||||||||||
| Mean ± SD (mm) | 5.0 ± 2.0 | 3.3 ± 1.0 | 1.4 ± 1.6 | 0.1 ± 0.5 | 1.3 ± 1.7 | 2.1 ± 2.0 | 0.3 ± 0.6 | 0.0 ± 0.1 | 3.7 ± 2.5 | 1.1 ± 2.3 | 1.2 ± 1.7 | 0.1 ± 0.5 | 71 ± 47 | 27 ± 75 | 74 ± 54 | 100 ± 0 |
| Median | 4 | 3 | 1 | 0 | 1 | 2.5 | 0 | 0 | 3.5 | 1 | 1 | 0 | 86 | 25 | 100 | 100 |
| N | 49 | 49 | 49 | 49 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 29 | 4 |
| CXP | ||||||||||||||||
| Mean ± SD (mm) | 5.1 ± 2.1 | 3.3 ± 0.9 | 1.3 ± 1.5 | 0.2 ± 0.6 | 1.0 ± 1.3 | 1.7 ± 2.1 | 0.2 ± 0.5 | 0.0 ± 0.0 | 4.1 ± 2.2 | 1.5 ± 2.3 | 1.1 ± 1.5 | 0.2 ± 0.7 | 81 ± 24 | 44 ± 70 | 81 ± 36 | 100 ± 0 |
| Median | 4 | 3.25 | 1 | 0 | 0.5 | 1.5 | 0 | 0 | 3.5 | 2 | 1 | 0 | 88 | 60 | 100 | 100 |
| N | 24 | 24 | 24 | 24 | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 14 | 3 |
| BG | ||||||||||||||||
| Mean ± SD (mm) | 4.9 ± 1.9 | 3.2 ± 1.0 | 1.5 ± 1.7 | 0.0 ± 0.2 | 1.7 ± 2.1 | 2.5 ± 1.9 | 0.3 ± 0.6 | 0.0 ± 0.1 | 3.3 ± 2.8 | 0.6 ± 2.2 | 1.2 ± 1.8 | 0.0 ± 0.2 | 62 ± 61 | 11 ± 78 | 68 ± 67 | 100 ± na |
| Median | 4.5 | 3 | 1 | 0 | 1 | 3 | 0 | 0 | 3.0 | 1 | 1 | 0 | 78 | 25 | 100 | na |
| N | 25 | 25 | 25 | 25 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 15 | 1 |
| P‐value CXP vs. BG | 0.832 | 0.797 | 0.714 | 0.289 | 0.159 | 0.168 | 0.794 | 0.328 | 0.459 | 0.140 | 0.860 | 0.170 | 0.138 | 0.106 | 0.938 | 1.000 |
CXP, creos xenoprotect membrane; BG, reference membrane; SD, standard deviation; na, not applicable
Figure 4.
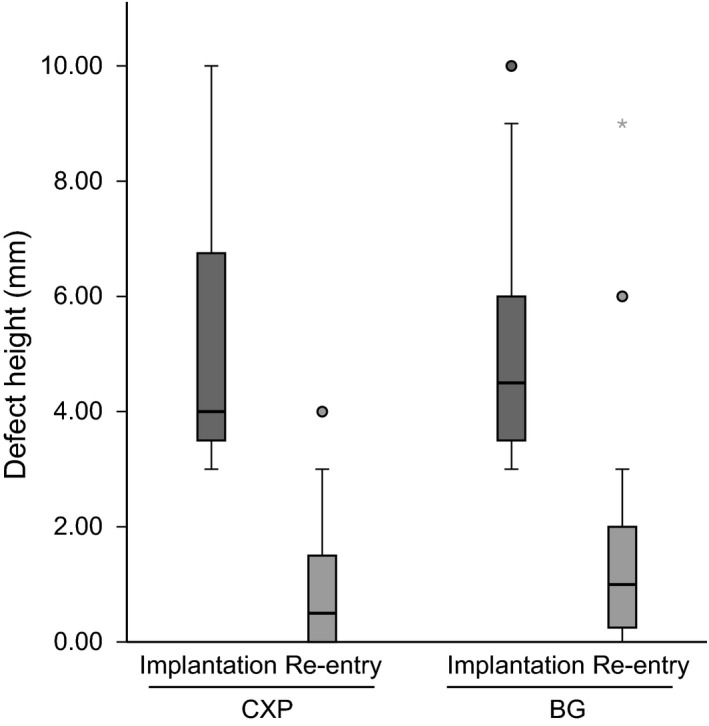
Changes in defect height from implantation to reentry surgery, performed after a 6‐month healing period, in the CXP and the BG arms. The boxes indicate quartile distributions, with the top of the box indicating the upper quartile (75%), the bottom of the box indicating the lower quartile (25%), and the middle line indicating the median. Circles denote outliers farther than 1.5 interquartile ranges but closer than 3 interquartile ranges. The star denotes the outlier farther than 3 interquartile ranges.
[Correction added on 8 April, 2017, after first online publication: Reviewer‐requested amendments to Table 3 were not incorporated at article acceptance. This has been rectified.]
At reentry, unexpected DHs were observed in two patients from the BG arm (9 and 6 mm) and one patient from the CXP arm (4 mm) (Fig. 4). Sample clinical case from the CXP arm is shown in Fig. 5.
Figure 5.
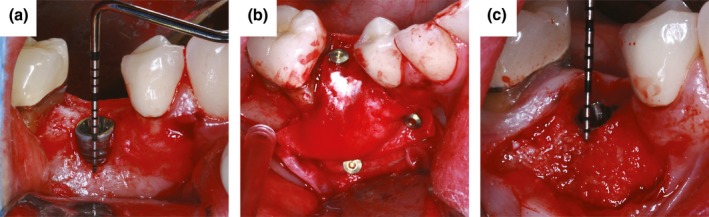
Clinical views of the GBR procedure with titanium pin fixation of CXP membrane. (a) 3‐mm vertical defect at implant in position 45, (b) CXP membrane fixed with cortical pins, (c) horizontal bone gain visible at reentry.
The mean bone height gain from implant insertion to reentry was 4.1 ± 2.2 mm (81%) in the CXP arm and 3.3 ± 2.8 mm (62%) in the BG arm (P = 0.459).
The nonparametric analog for the mean difference between DH at reentry for the CXP and the BG arm was −0.5 mm, and its nonparametric two‐sided 95% confidence interval was calculated to be −1.000, 0.000. Based on this result, the CXP arm is statistically non‐inferior to the BG arm at the one‐sided 2.5% level of significance (P < 0.001).
Secondary outcome measures
The defect size decreased in all tested dimensions in both treatment arms. In the CXP arm, the DW at implant insertion was 3.3 ± 0.9 mm (n = 24) and reduced at reentry by 44% to 1.7 ± 2.1 mm (n = 23). In the BG arm, the DW at implant insertion was 3.2 ± 1.0 mm (n = 25) and reduced at reentry by 11% to 2.5 ± 1.9 mm (n = 24). Overall, the DD at implant insertion was 1.4 ± 1.6 mm (n = 49) and reduced at reentry by 74% to 0.3 ± 0.6 mm (n = 47). The differences between the two arms were not statistically significant for any of the dimensions (Table 3).
Overall, soft tissue healing parameters included swelling (33 patients), redness (19 patients), wound dehiscence (11 patients), and membrane exposure (six patients). Wound dehiscence was observed in four patients in the CXP arm and in seven patients in the BG arm, while membrane exposure was recorded in two CXP patients and four BG patients. The occurrence of wound dehiscence and membrane exposure was highest at weeks 3 and 6. No infection or other complication occurred due to the wound or membrane dehiscence. Table 4 lists the soft tissue healing parameters and their assessment results by time point and by membrane. No statistically significant difference was observed for any of the analyzed parameters at any of the time points.
Table 4.
Analysis of soft tissue healing at different time points
| Complications | Week 1 | Week 3 | Week 6 | Week 12 | Re‐entry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CXP | BG | P value | CXP | BG | P value | CXP | BG | P value | CXP | BG | P value | CXP | BG | P value | |
| Wound dehiscence, n (%) | 1 (4) | 2 (8) | 1.000 | 1 (4) | 6 (26) | 0.096 | 0 | 4 (18) | 0.108 | 1 (5) | 2 (9) | 1.000 | 0 | 2 (8) | 0.491 |
| Membrane exposure, n (%) | 1 (4) | 2 (8) | 1.000 | 1 (4) | 4 (17) | 0.346 | 0 | 1 (5) | 1.000 | 0 | 1 (4) | 1.000 | 0 | 0 | |
| Redness, n (%) | 9 (38) | 7 (29) | 0.760 | 1 (4) | 0 | 1.000 | 1 (5) | 0 | 0.488 | 2 (9) | 1 (4) | 0.608 | 1 (5) | 0 | 0.468 |
| Swelling, n (%) | 28 (78) | 15 (63) | 0.558 | 1 (4) | 3 (13) | 0.483 | 0 | 0 | 1 (5) | 1 (4) | 1.000 | 0 | 0 | ||
| Total n a | 23 | 25 | 23 | 23 | 21 | 22 | 22 | 23 | 23 | 24 | |||||
Not all visits were attended by all patients.
CXP, creos xenoprotect membrane; BG, reference membrane; SD, standard deviation.
Self‐assessment showed that patients reported some pain in the beginning of the healing period, with a mean score of 2.3 of 10 at 1 week post‐surgery, and the feeling of pain ceased with time, with mean values of 0.3 at week 3, 0.2 at week 6, and 0 at week 12 and reentry. Patient quality of life evaluated according to the OHIP‐14 questionnaire showed that overall patient discomfort was 7.0 at pretreatment, 7.3 at implant insertion, peaked at one week post‐surgery with a mean score of 10.2, and from then on continued to decrease to 6.8 at week 3, 5.0 at week 6, 3.7 at week 12, and 3.9 at reentry. There were no significant differences between the two treatment arms with regard to pain or quality of life assessment during the healing phase (all P > 0.05).
Discussion
The aim of this randomized controlled clinical trial was to compare the clinical performance of a new native non‐cross‐linked collagen membrane with a reference membrane for treatment of dehisced implant sites using GBR. This manuscript evaluates bone augmentation results, soft tissue healing, and implant survival rate.
This study was designed to test non‐inferiority of the CXP membrane to the reference membrane by comparing their mean difference in DH at reentry 6 months after implant placement and bone augmentation. Based on the calculated 95% confidence interval of that difference, it can be concluded that the CXP membrane is non‐inferior to the reference BG membrane.
The recent systematic review and meta‐analysis by Sanz‐Sanchez et al. (2015) have shown that the mean defect height gain in bone augmentation using particulate autologous bone, xenograft, and a bioabsorbable membrane was 3.491 mm, a value that is comparable to the results reported in this study. In both arms, the amount of newly regenerated bone was similar to that of other studies investigating GBR using BG at dehisced implant sites that reported collagen membrane fixation (Zitzmann et al. 1997; Hammerle & Lang 2001; Jung et al. 2009). In this study, bone gain results for both membranes were superior to those of studies investigating the same indication that did not fix collagen membranes, especially with respect to DH values (Becker et al. 2009; Friedmann et al. 2011).
The goal of grafting with membrane fixation at dehisced implant sites is to provide more space in the occluso‐buccal corner of the implant. This space is critical, because the sutured flap can apply pressure, pushing some of the bone graft material out into surrounding areas, which can result in poorer bone regeneration. We hypothesize that fixing collagen membranes to the underlying bone can immobilize particulate graft material, preventing the previously described problem. This hypothesis is supported by an in vitro study by Mir‐Mari et al. (2016), in which particulate graft materials migrated to the surrounding tissues during wound closure and suturing when no membrane fixation was performed. In this study, the collagen membranes were fixed using either periosteal vertical mattress sutures (Urban et al. 2016) or titanium cortical bone pins (Carpio et al. 2000) to immobilize the bone graft at the desired position.
The membrane exposure rate was 8.3% (two patients) for CXP and 16.7% (four patients) for BG. While both rates were below the rate reported for previous randomized controlled trials with the BG membrane, the membrane exposure rate for BG was still twofold higher than that for CXP. The low membrane exposure rate for CXP is consistent with that reported in the only prior clinical study investigating the CXP membrane (Nemcovsky & Artzi 2002; Jung et al. 2009; Wessing et al. 2016).
In this study, the simultaneous implant placement and GBR were performed as a closed healing procedure using mobilized full‐thickness flaps to achieve tension‐free primary wound closure. This technique resulted in small wound dehiscence and membrane exposure rates and consequently, a high early implant survival rate of 100% at reentry.
The main limitation of this study is that it was restricted to bone augmentation of dehiscence defects. Further studies are necessary to investigate the benefits of the CXP membrane for larger augmentation procedures.
Conclusion
Both collagen membranes resulted in safe bone augmentation of dehiscence defects. The membrane exposure rate during the healing phase was lower in the CXP arm, and the mean bone gain after 6 months of healing achieved in defects protected with CXP was higher than with the BG membrane, although these differences did not reach statistical significance. The observed trend toward lower exposure rate and higher mean bone gain with CXP compared to BG should be further investigated.
Conflict of interest
The authors declare no other conflict of interests.
Author contributions
B. W. made substantial contributions to study conception and design, acquisition, analysis, and interpretation of data, as well as drafted and critically reviewed the article. I. U. made substantial contributions to study conception and design, acquisition, analysis, and interpretation of data, as well as critically reviewed the article. W. Z., G. P., and M. S. made substantial contributions to study conception and design, acquisition of data, as well as critically reviewed the article. J. A., N. A., S. M., E. M., and M. H. contributed to data acquisition and critically reviewed the article. All authors approved the final version of the article.
Supporting information
Appendix S1. CONSORT 2010 checklist of information to include when reporting a randomised trial.
Acknowledgement
The authors thank Dr Konrad Neumann for statistical evaluation. This study was supported by Nobel Biocare Services AG (grant number T‐186).
Wessing B, Urban I, Montero E, Zechner W, Hof M, Alández Chamorro J, Alández Martin N, Polizzi G, Meloni S, Sanz M. A multicenter randomized controlled clinical trial using a new resorbable non‐cross‐linked collagen membrane for guided bone regeneration at dehisced single implant sites: interim results of a bone augmentation procedure. Clin. Oral Impl. Res. 28, 2017, e218–e226
References
- Aghaloo, T.L. & Moy, P.K. (2007) Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? International Journal of Oral & Maxillofacial Implants 22 (Suppl):49–70. [PubMed] [Google Scholar]
- Araújo, M.G. & Lindhe, J. (2005) Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology 32: 212–218. [DOI] [PubMed] [Google Scholar]
- Araújo, M.G. , Wennström, J.L. & Lindhe, J. (2006) Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clinical Oral Implants Research 17: 606–614. [DOI] [PubMed] [Google Scholar]
- Becker, J. , Al‐Nawas, B. , Klein, M.O. , Schliephake, H. , Terheyden, H. & Schwarz, F. (2009) Use of a new cross‐linked collagen membrane for the treatment of dehiscence‐type defects at titanium implants: a prospective, randomized‐controlled double‐blinded clinical multicenter study. Clinical Oral Implants Research 20: 742–749. [DOI] [PubMed] [Google Scholar]
- Becker, W. , Dahlin, C. , Becker, B.E. , Lekholm, U. , van Steenberghe, D. , Higuchi, K. & Kultje, C . (1994) The use of e‐PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: a prospective multicenter study. International Journal of Oral and Maxillofacial Implants 9: 31–40. [PubMed] [Google Scholar]
- Bozkurt, A. , Apel, C. , Sellhaus, B. , van Neerven, S. , Wessing, B. , Hilgers, R.D. & Pallua, N . (2014) Differences in degradation behavior of two non‐cross‐linked collagen barrier membranes: an in vitro and in vivo study. Clinical Oral Implants Research 25: 1403–1411. [DOI] [PubMed] [Google Scholar]
- Branemark, P.I. , Engstrand, P. , Ohrnell, L.O. , Grondahl, K. , Nilsson, P. , Hagberg, K. , Darle, C. & Lekholm, U. (1999) Branemark Novum: a new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow‐up study. Clinical Implant Dentistry and Related Research 1: 2–16. [DOI] [PubMed] [Google Scholar]
- Branemark, P.I. , Hansson, B.O. , Adell, R. , Breine, U. , Lindstrom, J. , Hallen, O. & Ohman, A . (1977) Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scandinavian Journal of Plastic and Reconstructive Surgery Supplementum 16: 1–132. [PubMed] [Google Scholar]
- Burkhardt, R. & Lang, N. (2010) Role of flap tension in primary wound closure of mucoperiosteal flaps: a prospective cohort study. Clinical Oral Implants Research 21: 50–54. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Bragger, U. , Lang, N.P. & Nyman, S. (1990) Regeneration and enlargement of jaw bone using guided tissue regeneration. Clinical Oral Implants Research 1: 22–32. [DOI] [PubMed] [Google Scholar]
- Buser, D. , Mericske‐Stern, R. , Bernard, J.P. , Behneke, A. , Behneke, N. , Hirt, H.P. , Belser, U.C . & Lang, N.P . (1997) Long‐term evaluation of non‐submerged ITI implants. Part 1: 8‐year life table analysis of a prospective multi‐center study with 2359 implants. Clinical Oral Implants Research 8: 161–172. [DOI] [PubMed] [Google Scholar]
- Carpio, L. , Loza, J. , Lynch, S. & Genco, R. (2000) Guided bone regeneration around endosseous implants with anorganic bovine bone mineral. A randomized controlled trial comparing bioabsorbable versus non‐resorbable barriers. Journal of periodontology 71: 1743–1749. [DOI] [PubMed] [Google Scholar]
- Dahlin, C. , Linde, A. , Gottlow, J. & Nyman, S. (1988) Healing of bone defects by guided tissue regeneration. Plastic and Reconstructive Surgery 81: 672–676. [DOI] [PubMed] [Google Scholar]
- Donos, N. , Mardas, N. & Chadha, V. (2008) Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). Journal of Clinical Periodontology 35: 173–202. [DOI] [PubMed] [Google Scholar]
- Friedmann, A. , Gissel, K. , Soudan, M. , Kleber, B.M. , Pitaru, S. & Dietrich, T. (2011) Randomized controlled trial on lateral augmentation using two collagen membranes: morphometric results on mineralized tissue compound. Journal of Clinical Periodontology 38: 677–685. [DOI] [PubMed] [Google Scholar]
- Garber, D.A. & Belser, U.C. (1995). Restoration‐driven implant placement with restoration‐generated site development. Compendium of Continuing Education in Dentistry 16: 796, 798–802, 804. [PubMed] [Google Scholar]
- Gottlow, J. , Nyman, S. , Karring, T. & Lindhe, J. (1984) New attachment formation as the result of controlled tissue regeneration. Journal of Clinical Periodontology 11: 494–503. [DOI] [PubMed] [Google Scholar]
- Hammerle, C.H. & Lang, N.P. (2001) Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clinical Oral Implants Research 12: 9–18. [DOI] [PubMed] [Google Scholar]
- den Hartog, L. , Slater, J.J. , Vissink, A. , Meijer, H.J. & Raghoebar, G.M. (2008) Treatment outcome of immediate, early and conventional single‐tooth implants in the aesthetic zone: a systematic review to survival, bone level, soft‐tissue, aesthetics and patient satisfaction. Journal of Clinical Periodontology 35: 1073–1086. [DOI] [PubMed] [Google Scholar]
- Hof, M. , Pommer, B. , Ambros, H. , Jesch, P. , Vogl, S. & Zechner, W. (2015) Does timing of implant placement affect implant therapy outcome in the Aesthetic Zone? A clinical, radiological, aesthetic, and patient‐based evaluation. Clinical Implant Dentistry and Related Research 17: 1188–1199. [DOI] [PubMed] [Google Scholar]
- Hof, M. , Pommer, B. , Strbac, G.D. , Suto, D. , Watzek, G. & Zechner, W. (2013) Esthetic evaluation of single‐tooth implants in the anterior maxilla following autologous bone augmentation. Clinical Oral Implants Research 24: 88–93. [DOI] [PubMed] [Google Scholar]
- Jung, R.E. , Glauser, R. , Scharer, P. , Hammerle, C.H. , Sailer, H.F. & Weber, F.E. (2003) Effect of rhBMP‐2 on guided bone regeneration in humans. Clinical Oral Implants Research 14: 556–568. [DOI] [PubMed] [Google Scholar]
- Jung, R.E. , Halg, G.A. , Thoma, D.S. & Hammerle, C.H. (2009) A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clinical Oral Implants Research 20: 162–168. [DOI] [PubMed] [Google Scholar]
- Khzam, N. , Arora, H. , Kim, P. , Fisher, A. , Mattheos, N. & Ivanovski, S. (2015) A systematic review of soft tissue alterations and aesthetic outcomes following immediate implant placement and restoration of single implants in the anterior maxilla. Journal of Periodontology 86: 1321–1330. [DOI] [PubMed] [Google Scholar]
- Lang, N.P. , Hammerle, C.H. , Bragger, U. , Lehmann, B. & Nyman, S.R. (1994) Guided tissue regeneration in jawbone defects prior to implant placement. Clinical Oral Implants Research 5: 92–97. [DOI] [PubMed] [Google Scholar]
- Lekholm, U. , Gunne, J. , Henry, P. , Higuchi, K. , Linden, U. , Bergstrom, C. & van Steenberghe, D. (1999) Survival of the Branemark implant in partially edentulous jaws: a 10‐year prospective multicenter study. International Journal of Oral and Maxillofacial Implants 14: 639–645. [PubMed] [Google Scholar]
- Lindhe, J. , Karring, T. & Lang, N.P. (2003) Clinical periodontology and implant dentistry, Vol. 4 Copenhagen: Blackwell Munksgaard. [Google Scholar]
- Machtei, E.E. (2001) The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta‐analysis. Journal of Periodontology 72: 512–516. [DOI] [PubMed] [Google Scholar]
- Mir‐Mari, J. , Wui, H. , Jung, R.E. , Hammerle, C.H. & Benic, G.I. (2016) Influence of blinded wound closure on the volume stability of different GBR materials: an in vitro cone‐beam computed tomographic examination. Clinical Oral Implants Research 27: 258–265. [DOI] [PubMed] [Google Scholar]
- Moses, O. , Pitaru, S. , Artzi, Z. & Nemcovsky, C.E. (2005) Healing of dehiscence‐type defects in implants placed together with different barrier membranes: a comparative clinical study. Clinical Oral Implants Research 16: 210–219. [DOI] [PubMed] [Google Scholar]
- Nemcovsky, C.E. & Artzi, Z. (2002) Comparative study of buccal dehiscence defects in immediate, delayed, and late maxillary implant placement with collagen membranes: clinical healing between placement and second‐stage surgery. Journal of Periodontology 73: 754–761. [DOI] [PubMed] [Google Scholar]
- Nisand, D. , Picard, N. & Rocchietta, I. (2015) Short implants compared to implants in vertically augmented bone: a systematic review. Clinical Oral Implants Research 26: 170–179. [DOI] [PubMed] [Google Scholar]
- Nyman, S. & Karring, T. (1979) Regeneration of surgically removed buccal alveolar bone in dogs. Journal of Periodontal Research 14: 86–92. [DOI] [PubMed] [Google Scholar]
- Pjetursson, B.E. , Thoma, D. , Jung, R. , Zwahlen, M. & Zembic, A. (2012) A systematic review of the survival and complication rates of implant‐supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clinical Oral Implants Research 23: 22–38. [DOI] [PubMed] [Google Scholar]
- Sanz‐Sanchez, I. , Ortiz‐Vigon, A. , Sanz‐Martin, I. , Figuero, E. & Sanz, M. (2015) Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta‐analysis. Journal of Dental Research 94: S128–S142. [DOI] [PubMed] [Google Scholar]
- Schropp, L. , Wenzel, A. , Kostopoulos, L. & Karring, T. (2003) Bone healing and soft tissue contour changes following single‐tooth extraction: a clinical and radiographic 12‐month prospective study. The International Journal of Periodontics & Restorative Dentistry 23: 313–323. [PubMed] [Google Scholar]
- Schulz, K.F. , Altman, D.G. & Moher, D. (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 152: 726–732. [DOI] [PubMed] [Google Scholar]
- Simion, M. , Baldoni, M. , Rassi, P. & Zaffe, D. (1994) A comparative study of the effectiveness of e‐PTFE membranes with and without early exposure during the healing period. International Journal of Periodontics & Restorative Dentistry 14: 166–180. [PubMed] [Google Scholar]
- Slade, G.D. (1997) Derivation and validation of a short‐form oral health impact profile. Community Dentistry and Oral Epidemiology 25: 284–290. [DOI] [PubMed] [Google Scholar]
- Tal, H. , Kozlovsky, A. , Artzi, Z. , Nemcovsky, C.E. & Moses, O. (2008) Long‐term bio‐degradation of cross‐linked and non‐cross‐linked collagen barriers in human guided bone regeneration. Clinical Oral Implants Research 19: 295–302. [DOI] [PubMed] [Google Scholar]
- Team RC (2014) R: A Language and Environment for Statistical Computing. R Vienna: Foundation for Statistical Computing. ISBN 3‐900051‐07‐0. [Google Scholar]
- Urban, I.A. , Lozada, J.L. , Wessing, B. , Suarez‐Lopez Del Amo, F. & Wang, H.L. (2016) Vertical bone grafting and periosteal vertical mattress suture for the fixation of resorbable membranes and stabilization of particulate grafts in horizontal guided bone regeneration to achieve more predictable results: a technical report. The International Journal of Periodontics & Restorative Dentistry 36: 153–159. [DOI] [PubMed] [Google Scholar]
- Wang, H.L. , Misch, C. & Neiva, R.F. (2004) “Sandwich” bone augmentation technique: rationale and report of pilot cases. The International Journal of Periodontics & Restorative Dentistry 24: 232–245. [PubMed] [Google Scholar]
- Wessing, B. , Emmerich, M. & Bozkurt, A. (2016) Horizontal ridge augmentation with a novel resorbable collagen membrane: a retrospective analysis of 36 consecutive patients. The International Journal of Periodontics & Restorative Dentistry 36: 179–187. [DOI] [PubMed] [Google Scholar]
- Zitzmann, N.U. , Naef, R. & Scharer, P. (1997) Resorbable versus nonresorbable membranes in combination with Bio‐Oss for guided bone regeneration. International Journal of Oral and Maxillofacial Implants 12: 844–852. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. CONSORT 2010 checklist of information to include when reporting a randomised trial.


