Abstract
Aims
To evaluate the efficacy and safety of evogliptin, a newly developed dipeptidyl peptidase‐4 inhibitor, in patients with type 2 diabetes (T2D) inadequately controlled by diet and exercise.
Materials and Methods
In this randomized, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase III study, 160 patients with T2D were assigned to either evogliptin 5 mg or placebo for 24 weeks. The primary endpoint was the mean change in glycated haemoglobin (HbA1c) from baseline to week 24.
Results
The mean baseline HbA1c levels were similar in the evogliptin and the placebo groups (7.20% ± 0.56% vs 7.20% ± 0.63%, respectively). At week 24, evogliptin significantly reduced HbA1c levels from baseline compared with placebo (−0.23% vs 0.05%, respectively, P < .0001). Additionally, the proportion of patients achieving HbA1c <6.5% was significantly higher in the evogliptin group than in the placebo group (33.3% vs 15.2%; P = .008). The overall incidence of adverse events, including hypoglycaemia, was similar in the 2 groups.
Conclusions
In this 24‐week study, once‐daily evogliptin monotherapy significantly improved glycaemic control and was well tolerated in patients with T2D.
Keywords: antidiabetic drug, clinical trial, DPP‐4 inhibitor, phase III study, type 2 diabetes
1. INTRODUCTION
Treatment strategies for the management of type 2 diabetes (T2D) have been diversified since the late 2000s, and incretin‐based therapy is a representative example of these changes. Glucagon‐like peptide‐1 (GLP‐1), one of main incretin hormones, which stimulates insulin secretion and inhibits glucagon secretion from pancreas β cells, plays an important role in glucose homeostasis. Unfortunately, GLP‐1 has a very short physiological half‐life because it is rapidly degraded by dipeptidyl peptidase‐4 (DPP‐4). DPP‐4 inhibitors have therefore been developed to enhance insulin secretion by preventing GLP‐1 degradation.1 In real‐world clinical practice, DPP‐4 inhibitors have been reported to result in a significant and clinically meaningful reduction in glycated haemoglobin (HbA1c) with no augmentation of weight and a low incidence of hypoglycaemia in patients with T2D. These factors may explain the significant increase in prescriptions for DPP‐4 inhibitors.2, 3, 4
Evogliptin is a novel, oral, once‐daily, selective DPP‐4 inhibitor with a low potential for interaction with other simultaneously administered drugs.5 In a phase I clinical trial, plasma DPP‐4 activity was inhibited by >80% within 0.5 to 1.0 hours with each single dose of evogliptin 5, 10 or 20 mg. The 80% inhibition of plasma DPP‐4 activity was maintained over 24 hours after 10 days of evogliptin administration at these doses.6 Evogliptin does not interact with cytochrome P450 3A4 (CYP3A4) and <35% of evogliptin is excreted in the urine.7 Analysis of the pharmacokinetics and tolerability of evogliptin in patients with renal impairment showed that dose adjustment of evogliptin was not required.8 In a phase II clinical trial, treatment with evogliptin (2.5, 5 and 10 mg once daily for 12 weeks) significantly reduced HbA1c levels compared with placebo, and evogliptin 5 mg was selected for a phase III clinical trial because of its superior efficacy.9
Based on these findings, we conducted a phase III trial of evogliptin monotherapy. The aim of the present study was to evaluate the efficacy and safety of evogliptin 5 mg vs placebo for 24 weeks in patients with T2D inadequately controlled by diet and exercise.
2. MATERIALS AND METHODS
2.1. Study setting and design
The present study was a multicentre, randomized, double‐blind, placebo‐controlled, phase III trial. The trial was conducted at 25 sites in Korea (ClinicalTrials.gov Identifier: NCT02946541). The protocol was reviewed and approved by the institutional review board at each participating centre. This study was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent prior to participation.
The study consisted of the following three periods: a 2‐week screening period; a 2‐week single‐blind placebo period; and a 24‐week double‐blind, randomized treatment period. During the screening period, the patients were provided with counselling on diet and exercise. After the screening period, HbA1c and safety laboratory measurements were performed to confirm study eligibility at week −2. Eligible patients were enrolled in the single‐blind placebo period. After the single‐blind placebo period, the patients underwent baseline evaluations and were assigned randomly in a 1:1 ratio to receive once‐daily evogliptin 5 mg or placebo for 24 weeks at week 0. The randomization was stratified by HbA1c (<8.5% or ≥8.5%) measured at week −2. Diet and exercise were reinforced during the entire study period.
2.2. Study participants
Eligible patients were aged ≥18 years and had not received any antidiabetic agents for 6 weeks prior to the screening. The patients were required to have fasting plasma glucose (FPG) levels <15.0 mmol/L and HbA1c levels of 6.5% to 10% at the screening and at week −2. Patients were excluded if they met one or more of the following criteria: type 1 diabetes; gestational diabetes; myocardial infarction or a stroke within 6 months prior to the study screening; impaired hepatic function; New York Heart Association class III or IV congestive heart failure; a prescription for insulin, GLP‐1 analogues, thiazolidinediones or DPP‐4 inhibitors within 6 months prior to the study screening; a history of coronary artery bypass surgery or gastrointestinal surgery; serum creatinine ≥124 μmol/L in women or >133 μmol/L in men; triglycerides ≥4.52 mmol/L; a history of illegal drug or alcohol abuse in the 2 months prior to the study screening; and treatment with systemic glucocorticoids, CYP3A4 inducers or inhibitors. Additionally, patients were excluded if they were pregnant, lactating, planning a pregnancy, or had a body mass index (BMI) <20 or >40 kg/m2.
2.3. Study endpoints
The primary efficacy endpoint was the mean change in HbA1c from baseline to week 24. The secondary efficacy endpoints assessed at week 24 included the proportion of patients achieving HbA1c <6.5% and the change in FPG from baseline. Exploratory endpoints included the following: changes in body weight and body fat, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, free fatty acids, fasting C‐peptide, insulin, proinsulin, GLP‐1 and gastric inhibitory polypeptide (GIP); changes in homeostasis model assessment of β‐cell function (HOMA‐β) index, homeostasis model assessment of insulin resistance (HOMA‐IR) index, quantitative insulin sensitivity check index (QUICKI), proinsulin/insulin ratio and insulinogenic index10; and changes in 2‐hour glucose, C‐peptide, insulin, proinsulin, GLP‐1 and GIP during an oral glucose tolerance test (OGTT). Changes in the area under the curve (AUC)0‐2h were also calculated for variables from the OGTT.
Safety was evaluated throughout the study via the assessment of hypoglycaemic episodes, vital signs, physical examinations, safety‐related laboratory variables, and 12‐lead ECGs. Treatment‐emergent adverse events (AEs) were summarized for the safety analysis and coded using the Medical Dictionary for Regulatory Activities (MedDRA; version 15.0).
Laboratory analyses for efficacy were performed at a central laboratory (Seoul Clinical Laboratories, Seoul, Korea). HbA1c levels were measured using a Cobas Integra Tina‐Quant G2 instrument (Roche, Rotkreuz, Switzerland). Body fat was measured using dual‐energy X‐ray absorptiometry. Hypoglycaemia was defined as signs and/or symptoms consistent with hypoglycaemia with or without a documented glucose measurement, or as plasma glucose level ≤3.9 mmol/L without signs or symptoms.
2.4. Statistical analysis
The study was designed with 85% power to show the superiority of evogliptin vs placebo on the mean change in HbA1c from baseline to week 24 using a two‐sided α value of 0.05. A mean difference in HbA1c change of 0.5% with evogliptin vs placebo (standard deviation [s.d.] of 0.9) was assumed. Under this assumption, the sample size was set at 104 patients in the evogliptin group and 52 patients in the placebo group by considering a 15% dropout rate. Because of an unintended treatment allocation coding error, 80 patients were assigned to each group, resulting in 89% power at a two‐sided α of 0.05 under the assumption described above. The efficacy endpoints were analysed using the full analysis set of data, which refers to all randomized participants treated with at least 1 dose of the study medication and who had HbA1c measured at baseline and at least once during the study period. The last observation carried forward method was used for imputing missing data for all efficacy endpoints. Baseline demographics, characteristics and efficacy endpoints are expressed as mean ± s.d. for continuous variables and number (percentage) for categorical variables. The primary efficacy endpoint was analysed using a Wilcoxon rank sum test. The chi‐squared test was used to compare the percentages of patients who attained the target HbA1c between the 2 groups. Continuous efficacy variables were analysed using a 2‐sample t‐test or a Wilcoxon rank sum test, as appropriate, for comparisons between the 2 groups. Formal statistical inferences on the secondary efficacy endpoints were evaluated when statistical significance in the primary endpoint was achieved. To adjust for type 1 error inflation regarding the two secondary endpoints, Bonferroni correction was applied. A P value ≤.05 was taken to indicate statistical significance for the primary endpoint. For the secondary endpoints, a P‐value ≤.025 (.05/2) was considered statistically significant. For the exploratory endpoints, no adjustment for multiplicity was made and all reported P values were nominal. Safety was assessed in patients who received at least 1 dose of the study drug. For each AE, including hypoglycaemia, the chi‐squared test or Fisher's exact test was performed for comparisons between the 2 groups. The statistical software SAS (Ver. 9.3, SAS Institute, Cary, North Carolina) was used for all statistical analyses.
3. RESULTS
3.1. Patient disposition
Of the 222 patients screened, 160 eligible patients were randomized for treatment as follows: 80 patients received evogliptin and 80 patients received placebo. After excluding three patients without efficacy data among the randomized patients, a total of 157 patients were included in the full analysis set. In total, 147 patients (72 patients in the evogliptin group and 75 patients in the placebo group) completed the 24‐week treatment (Figure 1). The baseline demographics and clinical characteristics of the patients at randomization were similar in the 2 groups (Table 1). The mean HbA1c at baseline was 7.2% in both treatment groups. Approximately 96% of the patients had HbA1c levels <8.5%.
Figure 1.
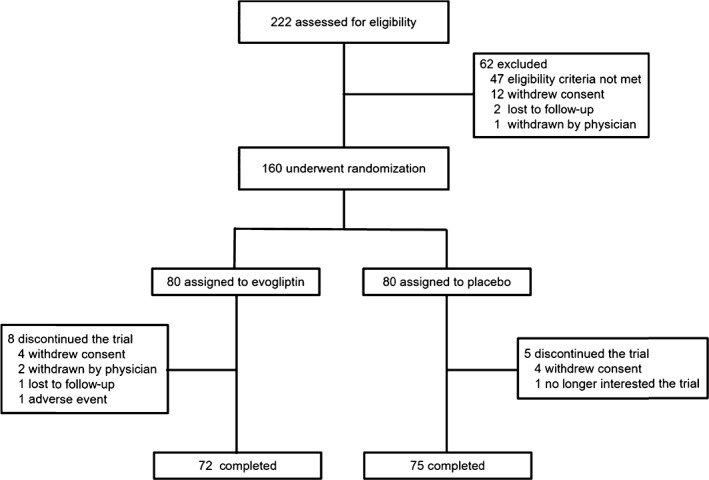
Flow diagram: screening, randomization and follow up
Table 1.
Baseline demographics and characteristics of the study participants
| Placebo (n = 80) | Evogliptin (n = 80) | |
|---|---|---|
| Age, years | 56.8 ± 9.8 | 57.6 ± 11.0 |
| Men, n (%) | 46 (57.5) | 39 (48.8) |
| Weight, kg | 67.2 ± 10.8 | 67.6 ± 11.5 |
| BMI, kg/m2 | 25.4 ± 3.4 | 25.6 ± 3.2 |
| eGFRa, mL/min/1.73 m2 | 88.6 ± 18.6 | 88.6 ± 21.5 |
| Diabetes durationb, years | 4.25 ± 4.10 | 4.74 ± 3.81 |
| ≤4 weeks, n (%) | 28 (35) | 20 (25) |
| >4 weeks, n (%) | 52 (65) | 60 (75) |
| HbA1c, % | 7.20 ± 0.63 | 7.21 ± 0.56 |
| HbA1c < 8.5%, n (%) | 75 (93.8) | 78 (97.5) |
| HbA1c ≥ 8.5%, n (%) | 5 (6.3) | 2 (2.5) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin. Values are expressed as mean ± s.d.
eGFR estimated from serum creatinine using Modification of Diet in Renal Disease Study equation.
Duration of diabetes was calculated as the number of years between the day of consent and the day of diagnosis of T2D.
3.2. Efficacy
The mean HbA1c significantly decreased from 7.20% at baseline to 6.97% at week 24 in the evogliptin group. By contrast, the mean HbA1c level increased modestly in the placebo group. As a result, the mean change in HbA1c from baseline to week 24 was significantly greater in the evogliptin group than in the placebo group (−0.23% vs 0.05%; P < .0001[Figure 2A]).
Figure 2.
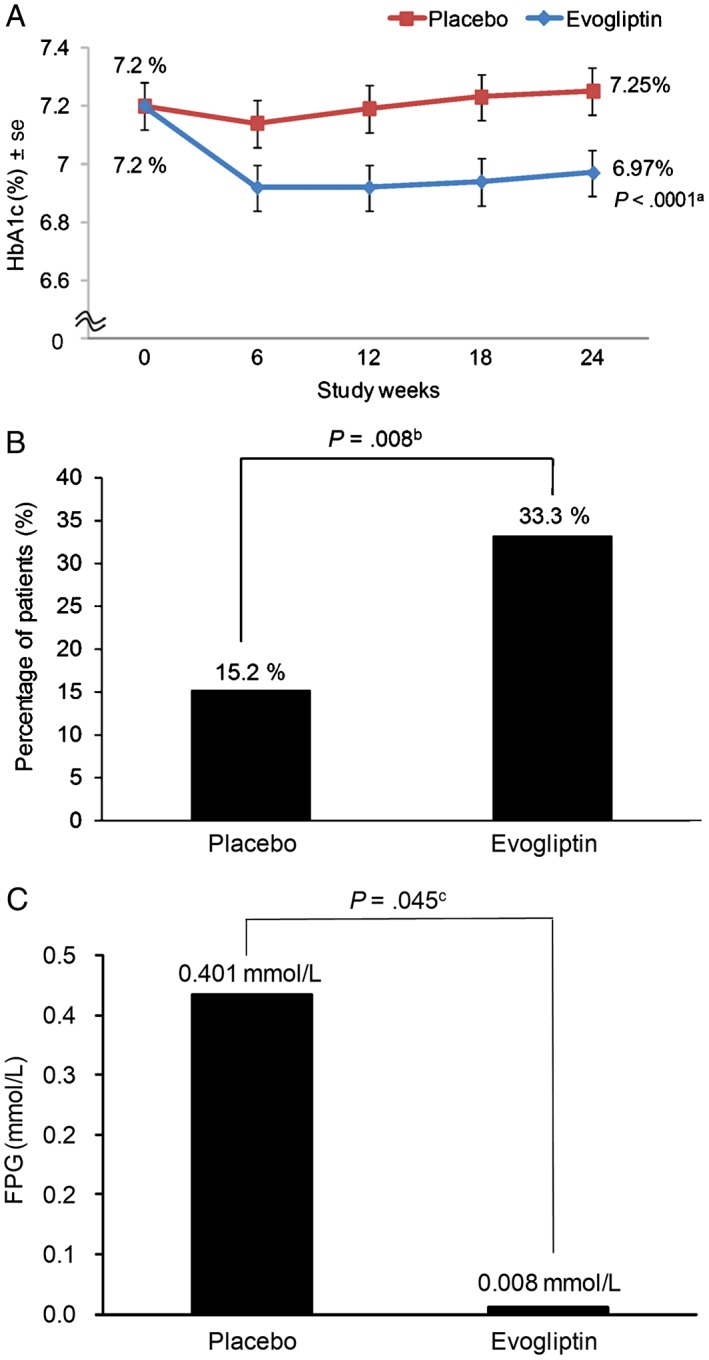
Primary and secondary efficacy outcomes. A, Mean changes in HbA1c from baseline to week 24. B, Percentage of patients with HbA1c level <6.5% at week 24. C, Mean changes in FPG from baseline to week 24. se, standard error. aUsing Wilcoxon rank sum test. bUsing chi‐squared test with Bonferroni correction (level of significance P < .025). cUsing Wilcoxon rank sum test with Bonferroni correction (level of significance P < .025)
The proportion of patients achieving HbA1c <6.5% at the end of the study was significantly higher in the evogliptin group than in the placebo group (33.3% vs 15.2%; P = .008 [Figure 2B]). Although the mean change in FPG from baseline to week 24 increased in both groups, a numerically greater increase was seen with placebo compared with evogliptin (0.008 mmol/L in the evogliptin group and 0.401 mmol/L in the placebo group [Figure 2C]).
The results from the exploratory efficacy endpoints are provided in Table S1 and Figure S1. A numerically greater improvement was observed in the HOMA‐β index with evogliptin after 24 weeks compared with placebo (4.51 vs −5.41, respectively, nominal P = .030). The effects of evogliptin on fasting C‐peptide, insulin, proinsulin, GLP‐1, GIP, HOMA‐IR, QUICKI, proinsulin/insulin ratio, or insulinogenic index were not different from those of placebo. At baseline, an OGTT was performed in 35 patients (19 patients in the evogliptin group and 16 patients in the placebo group), while the data for 13 patients in the evogliptin group and 10 in the placebo group were available at week 24. The glucose AUC0‐2h reductions were numerically greater in the evogliptin group compared with the placebo group (−3.65 vs 0.69 mmol/L, respectively; nominal P = .033). The AUC0‐2h for insulin, C‐peptide and active GIP increased more with evogliptin than with placebo. There were no between‐group differences in fasting lipid variables, such as total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides and free fatty acids after 24 weeks. No evident changes in body weight and body fat were observed in the evogliptin group from baseline to week 24.
Pre‐planned analyses were performed for gender, age (≥65 years or <65 years), BMI (>25 kg/m2 or ≤25 kg/m2), and the use of any previous antidiabetic drug. The mean reductions in HbA1c from baseline to week 24 were greater in the evogliptin group than in the placebo group (data not shown).
3.3. Safety
Treatment‐emergent AEs were reported by 33.3% of the patients in the evogliptin group and 35.0% of the patients in the placebo group (Table 2). Four adverse drug reactions (constipation, diarrhoea, hypersensitivity and hyperglycaemia) were suspected to be related to evogliptin, but the overall incidence of adverse drug reactions was low and similar between the 2 groups. No serious AEs occurred with evogliptin, and one serious AE (tendon rupture) was reported in one placebo recipient. The most common AEs in patients who received evogliptin included nasopharyngitis, arthralgia and various mild gastrointestinal disorders during the treatment period. One asymptomatic hypoglycaemic episode was reported in the evogliptin group, but this episode was not suspected to be related to treatment. No clinically meaningful findings emerged regarding the laboratory analyses or vital signs.
Table 2.
Incidence of adverse events (AEs) reported in the 2 groups during 24 weeks of treatment
| Placebo (n = 80) | Evogliptin (n = 78) | |
|---|---|---|
| Number of patients with AEs, n (%) | 28 (35.0) | 26 (33.3) |
| Drug‐related AEs, n (%) | 0 (0.0) | 4 (5.1) |
| Serious AEs, n (%) | 1 (1.3) | 0 (0.0) |
| Drug‐related serious AEs, n (%) | 0 (0.0) | 0 (0.0) |
| AEs reported in ≥ 3% | ||
| Nasopharyngitis, n (%) | 5 (6.3) | 5 (6.4) |
| Arthralgia, n (%) | 0 (0.0) | 3 (3.8) |
| Gastrointestinal disorders, n (%) | 7 (8.8) | 4 (5.1) |
4. DISCUSSION
Evogliptin 5 mg once‐daily monotherapy for 24 weeks significantly improved HbA1c compared with placebo in patients with T2D. The proportion of patients achieving HbA1c <6.5% was also significantly greater in the evogliptin group than in the placebo group. Additionally, we observed a numerically greater increase in FPG levels in the placebo group at 24 weeks compared with the evogliptin group. The incidence of treatment‐emergent AEs was similar to the incidence in the placebo group.
According to previous clinical trials with DPP‐4 inhibitors as monotherapy for 24 weeks, DPP‐4 inhibitors have been reported to reduce HbA1c level by 0.5% to 0.8% from baseline.11, 12, 13, 14, 15, 16, 17 Based on these findings, the 0.23% reduction in HbA1c from baseline may indicate that evogliptin is less effective than other DPP‐4 inhibitors; however, considering that the mean baseline HbA1c levels in the phase III clinical trials of other DPP‐4 inhibitors as monotherapy were relatively high (7.8%‐8.4%) and that higher levels of baseline HbA1c were associated with greater declines in HbA1c with therapeutic intervention,18, 19, 20 the results from the present study should be interpreted with caution, because baseline HbA1c level was 7.2%. In a phase II trial of evogliptin in patients with T2D whose mean baseline HbA1c level was 7.6%, evogliptin 5 mg/d for 12 weeks had a significant effect on HbA1c reduction compared with the placebo group (−0.70% vs −0.13%, respectively; P = .0002).9 The effect of HbA1c reduction in the phase II trial appears to be similar to the effect of other DPP‐4 inhibitors.
Regarding the effect of DPP‐4 inhibitors on reaching the target HbA1c <6.5%, 16% of the patients who received gemigliptin 50 mg monotherapy and 21% of the patients who received alogliptin 25 mg monotherapy were reported to exhibit HbA1c <6.5% in previous phase III trials.13, 14 A statistical significance was observed only in the former compared with the placebo group (16% in the gemigliptin 50 mg group vs 3% in the placebo group; P < .005). The present study also showed that a significantly greater proportion of patients receiving evogliptin 5 mg monotherapy achieved target HbA1c <6.5% compared with the patients receiving placebo (33% vs 15%, respectively; P = .008). Although a lower baseline HbA1c is associated with a higher likelihood of attaining the target HbA1c, the finding that a significantly higher proportion of patients in the evogliptin group achieved the target HbA1c level of <6.5% in this study supports the significant HbA1c‐lowering effect of evogliptin treatment.
Given the trend of an increased prevalence of T2D worldwide and the decreased proportion of undiagnosed cases attributable to early detection of T2D with improvements in screening programmes,21, 22, 23 now it is not unusual for clinicians to see patients with newly diagnosed diabetes with modestly elevated HbA1c levels (~7%) in daily clinical practice more than they ever did before; therefore, the results from the present trial showing the efficacy and safety of evogliptin 5 mg in patients with a mean baseline HbA1c of 7.2% and the finding that 25% of the evogliptin recipients had a T2D duration of <4 weeks suggest that evogliptin may be a suitable choice for patients with modest hyperglycaemia.
Although the exact mechanisms of DPP‐4 inhibitors are less clear, the accumulated evidence shows that DPP‐4 inhibitors generally work by increasing incretin (GLP‐1 and GIP) levels in the physiological range and improving pancreatic β‐cell function.24 In the present study, evogliptin also improved the HOMA‐β index at week 24 and increased the C‐peptide AUC0–2h and insulin AUC0‐2h levels. Conversely, the glucose AUC0‐2h level was reduced during the OGTT. These data, showing that evogliptin regulated postprandial glucose through the stimulation of insulin secretion, were consistent with the previously reported results on other DPP‐4 inhibitors.25, 26 Regarding the insulinotropic activity by DPP‐4 inhibitors, the increased active GLP‐1 AUC0‐2h and active GIP AUC0‐2h levels by evogliptin, despite the decreased levels of total GLP‐1 AUC0–2h and total GIP AUC0‐2h, suggest that evogliptin enhances glucose metabolism by inhibiting degradation of the active form of GLP‐1 and GIP in addition to possibly affecting an inhibitory feedback mechanism.27
The most frequently reported AEs were similar to the adverse events typically observed with other DPP‐4 inhibitors and were mild in severity.11, 12, 13, 14, 15, 16, 17 Although one mild and asymptomatic case of hypoglycaemia was observed in the evogliptin group, this event was determined to be unrelated to exposure to the study drug. Pancreatitis was also not reported in this study.
Many DPP‐4 inhibitors have been approved for use in the USA, Europe and some countries in Asia, and are being widely used for the effective treatment of T2D worldwide. Although these clinically approved DPP‐4 inhibitors exhibit broadly similar HbA1c‐lowering efficacy and safety profiles, each DPP‐4 inhibitor clearly differs in terms of its chemical structure, selectivity for DPP‐4, duration of action, metabolism and elimination. We consider that evogliptin may be included as one molecule for the current DPP‐4 inhibitors, but that understanding characterization of each one is needed.
In conclusion, evogliptin 5 mg monotherapy significantly decreased HbA1c and was well tolerated in patients with T2D inadequately controlled on diet and exercise. The results from the present study suggest that patients with T2D with modest hyperglycaemia may be good candidates for evogliptin monotherapy. Evogliptin 5 mg once daily provides a new option for clinicians in the management of T2D.
Supporting information
Table S1. Mean changes from baseline to week 24 in exploratory efficacy parameters.
Figure S1. Mean concentration‐time profiles during the oral glucose tolerance test for A, glucose; B, insulin; C, C‐peptide; D, proinsulin; E, total GLP‐1; F, active GLP‐1; G, total GIP; H, and active GIP measured at baseline and week 24.
ACKNOWLEDGEMENTS
This study was supported by Dong‐A ST Co., Ltd, Seoul, Korea. The sponsor participated in the study design, data collection and analysis of the data, but had no role in the writing of the manuscript or in the decision to submit the manuscript for publication. We sincerely thank Seung Sik Hwang, MD, PhD (Professor, Department of Public Health Sciences, Seoul National University) and Jin‐Young Jeong, PhD (Professor, Department of Social and Preventive Medicine, Hallym University College of Medicine) for their valuable guidance and assistance in the statistical analysis and writing.
Conflict of interest
N. Y. Kwon is an employee of Dong‐A ST Co., Ltd. The other authors have no conflicts of interest related to this work.
Author contributions
S. W. Park, K. H. Yoon, S. R. Kim, K. J. Ahn and D. M. Kim designed the study; J. Park, S. W. Park, K. H. Yoon, S. R. Kim, K. J. Ahn, J. H. Lee, J. O. Mok, C. H. Chung, K. A. Han, G. P. Koh, J. G. Kang, C.B. Lee and D. M. Kim performed the study; J. Park, N. Y. Kwon and D. M. Kim analyzed the data; J. Park, S. H. Kim and D. M. Kim drafted and finalized the full manuscript.
Park J, Park SW, Yoon KH, et al. Efficacy and safety of evogliptin monotherapy in patients with type 2 diabetes and moderately elevated glycated haemoglobin levels after diet and exercise. Diabetes Obes Metab. 2017;19:1681–1687. https://doi.org/10.1111/dom.12987
Funding information This study was supported by Dong‐A ST Co., Ltd, Seoul, Korea.
REFERENCES
- 1. Ahrén B, Landin‐Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase‐4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(5):2078‐2084. [DOI] [PubMed] [Google Scholar]
- 2. Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125(3):302.e1‐302.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oishi M, Yamazaki K, Okuguchi F, et al. Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002–2011 in Japan (JDDM32). J Diabetes Investig. 2014;5(5):581‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clemens KK, Shariff S, Liu K, et al. Trends in antihyperglycemic medication prescriptions and hypoglycemia in older adults: 2002–2013. PLoS One. 2015;10(9):e0137596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCormack PL. Evogliptin: first global approval. Drugs. 2015;75(17):2045‐2049. [DOI] [PubMed] [Google Scholar]
- 6. Gu N, Park MK, Kim TE, et al. Multiple‐dose pharmacokinetics and pharmacodynamics of evogliptin (DA‐1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des Devel Ther. 2014;8:1709‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan X, Hu J. Evogliptin: a new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2016;17(9):1285‐1293. [DOI] [PubMed] [Google Scholar]
- 8. Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase‐4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18(4):333‐347. [DOI] [PubMed] [Google Scholar]
- 9. Jung CH, Park CY, Ahn KJ, et al. A randomized, double‐blind, placebo‐controlled, phase II clinical trial to investigate the efficacy and safety of oral DA‐1229 in patients with type 2 diabetes mellitus who have inadequate glycaemic control with diet and exercise. Diabetes Metab Res Rev. 2015;31(3):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta‐cell function. Best Pract Res Clin Endocrinol Metab. 2003;17(3):305‐322. [DOI] [PubMed] [Google Scholar]
- 11. Nishio S, Abe M, Ito H. Anagliptin in the treatment of type 2 diabetes: safety, efficacy, and patient acceptability. Diabetes Metab Syndr Obes. 2015;8:163‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632‐2637. [DOI] [PubMed] [Google Scholar]
- 13. DeFronzo RA, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 010 Group . Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2008;31(12):2315‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang SJ, Min KW, Gupta SK, et al. A multicentre, multinational, randomized, placebo‐controlled, double‐blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15‐0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013;1(5):410‐416. [DOI] [PubMed] [Google Scholar]
- 15. Pan CY, Yang W, Tou C, Gause‐Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug‐naïve Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev. 2012;28(3):268‐275. [DOI] [PubMed] [Google Scholar]
- 16. Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β‐cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258‐267. [DOI] [PubMed] [Google Scholar]
- 17. Pi‐Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug‐naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):132‐138. [DOI] [PubMed] [Google Scholar]
- 18. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose‐lowering efficacy: a meta‐regression analysis. Diabetes Care. 2006;29(9):2137‐2139. [DOI] [PubMed] [Google Scholar]
- 19. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1c levels: a systematic review and meta‐analysis. Diabetes Care. 2010;33(8):1859‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapell R, Gould AL, Alexander CM. Baseline differences in A1C explain apparent differences in efficacy of sitagliptin, rosiglitazone and pioglitazone. Diabetes Obes Metab. 2009;11(11):1009‐1016. [DOI] [PubMed] [Google Scholar]
- 21. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care. 2013;36(8):2286‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pivovarov R, Albers DJ, Hripcsak G, Sepulveda JL, Elhadad N. Temporal trends of hemoglobin A1c testing. J Am Med Inform Assoc. 2014;21(6):1038‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase‐4 inhibitors. Endocr Rev. 2014;35(6):992‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mari A, Sallas WM, He YL, et al. Vildagliptin, a dipeptidyl peptidase‐IV inhibitor, improves model‐assessed beta‐cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(8):4888‐4894. [DOI] [PubMed] [Google Scholar]
- 26. Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91(11):4612‐4619. [DOI] [PubMed] [Google Scholar]
- 27. Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal‐induced incretin secretion in dogs. J Endocrinol. 2002;172(2):355‐362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean changes from baseline to week 24 in exploratory efficacy parameters.
Figure S1. Mean concentration‐time profiles during the oral glucose tolerance test for A, glucose; B, insulin; C, C‐peptide; D, proinsulin; E, total GLP‐1; F, active GLP‐1; G, total GIP; H, and active GIP measured at baseline and week 24.


