Abstract
The prevention of chemotherapy‐induced nausea and vomiting was one of the most challenging supportive care issues in oncology, especially to highly emetogenic chemotherapy (HEC). A total of 645 patients were randomized into fosaprepitant group (fosaprepitant/placebo 150 mg d1 in combination with granisetron and dexamethasone) or aprepitant group (aprepitant/placebo 125 mg d1; 80 mg d2‐d3 plus granisetron and dexamethasone).The primary endpoint was the percentage of patients who had a complete response (CR) over the entire treatment course (0–120 hr, overall phase [OP]). It was assessed by using a non‐inferiority model, with a non‐inferiority margin of 10%. The difference of the CR rate was compared between two groups with chi‐square analysis. Six hundred and twenty‐six patients were included in the per protocol analysis. The percentage of patients with a CR in the fosaprepitant group was not inferior to that in the aprepitant group (90.85% versus 94.17%, p = .1302) during OP. Whether the cisplatin‐based chemotherapy or not, the CR rate of the fosaprepitant group was not inferior to that of the aprepitant group. Both regimens were well tolerated. The most common adverse event was constipation. Fosaprepitant provided effective and well‐tolerated control of nausea and vomiting associated with HEC in Chinese patients.
Keywords: aprepitant, chemotherapy‐induced nausea and vomiting, fosaprepitant
1. INTRODUCTION
Chemotherapy‐induced nausea and vomiting (CINV) is the most distressing symptoms experienced by patients with cancer. CINV has a negative impact on the life quality of patients by further diminishing cancer treatment adherence (Sommariva, Pongiglione, & Tarricone, 2016; Tageja & Groninger, 2016). Failing adequate antiemetic treatment, more than 90% of patients experience CINV due to highly emetogenic chemotherapy (HEC) (Hesketh, 2008).Thus, it is critical to prevent CINV, especially HEC.
There has been substantial progress in improving the control of CINV since the serotonin 5‐hydroxytryptamine (5‐HT3) receptor antagonists (RAs)were developed in the 1990s (Gralla et al., 1999). Nevertheless, patients who received HEC but are still suffering from CINV constitute a difficult‐to‐treat group. Therefore, new and effective antiemetic agents are needed in controlling CINV. The neurokinin‐1 receptor (NK‐1R) is closely involved in CINV, and substance P induces vomiting by binding to NK‐1R in the central nervous system. This anatomical localization has led to the successful clinical development of antagonists against NK‐1R in the treatment of CINV (Garcia‐Recio & Gascón, 2015).
Both fosaprepitant and aprepitant are potent and selective NK1R antagonists that can improve prevention of CINV in patients receiving moderately emetogenic chemotherapy (MEC) and HEC with the addition to a standard regimen of a 5‐HT3 RA and dexamethasone (Langford & Chrisp, 2010; Ruhlmann & Herrstedt, 2012). Current guidelines recommend this three‐drug combination in the control of CINV in patients receiving HEC (Basch et al., 2012; Jordan, Gralla, Jahn, & Molassiotis, 2014). Fosaprepitant is a water‐soluble, phosphorylated analog of aprepitant. When administered intravenously, fosaprepitant is rapidly converted to aprepitant. In contrast to aprepitant, fosaprepitant could offer potential benefits for patients who may be unable to tolerate oral administration of antiemetics during an episode of nausea or vomiting (Langford & Chrisp, 2010).
Fosaprepitant(EMEND®, Merck and Co. Inc.) has been approved by FDA as an alternative to oral aprepitant in 2008. The previous studies were carried out majorly in American, European and African population (Grunberg et al., 2011; Weinstein et al., 2016). However, only limited data pertaining to the use of fosaprepitant is currently available for Chinese and other Asian patients.
This is the first phase III study to evaluate the efficacy and tolerability of fosaprepitant (Chia Tai Tianqing Pharmaceutical Group Co., Ltd) in patients receiving HEC in China. The antiemetic regimens applied in both groups of the study were designed to be consistent with those applied in a previous trial (Grunberg et al., 2011).
2. MATERIALS AND METHODS
2.1. Patients
Cancer patients who were administered HEC (according to NCCN Clinical Practice Guidelines in Oncology: Antiemesis version 1.2014) were eligible for enrolment in the study. The major emetogenic drugs were used for one day. The required dose of cisplatin was 60 –80 mg/m2 lever in the cisplatin‐contained regimen. The primary exclusion criteria included uncontrolled nausea (≥grade 2) and vomiting within 72 hr before chemotherapy initiation and/or HEC given within 2 weeks.
2.2. Study design
This was a randomized, active control, double‐blind, parallel‐group study, conducted at 21 centres in China. The primary objective was to demonstrate the efficacy of fosaprepitant compared to aprepitant in Chinese patients. Secondary objectives included assessment of the safety and tolerability of the fosaprepitant.
Patients were randomized in a 1:1 ratio using a central randomization system to receive fosaprepitant (fosaprepitant/placebo 150 mg d1 in combination with granisetron and dexamethasone) or aprepitant (aprepitant/placebo 125 mg d1; 80 mg d2‐d3 plus granisetron and dexamethasone) (Table 1).
Table 1.
Study drug schedule
| Regimen | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Fosaprepitant | 150 mg IV plus granisetron 3 mg IV plus dexamethasone 6 mg orally or IV | Dexamethasone 3.75 mg orally | Dexamethasone 3.75 mg orally every 12 hr | Dexamethasone 3.75 mg orally every 12 hr |
| Aprepitant | 125 mg orally plus granisetron 3 mg IV plus dexamethasone 6 mg orally or IV | 80 mg orally plus dexamethasone 3.75 mg orally | 80 mg orally plus dexamethasone 3.75 mg orally | Dexamethasone 3.75 mg orally |
Chemotherapy regimen was required to include high‐risk emetogenic drugs and the duration for 1 day. All patients provided written informed consent before enrolment into the study. The study protocol was approved by the Ethics Committee Review Board at each participating centre and was registered with www.chinadrugtrials.org.cn (identifier, CTR20140900).
2.3. Efficacy parameters
The primary efficacy endpoint was complete response (CR) during the 120 hr after initiation of chemotherapy (overall phase, OP), defined as no vomiting and/or retching with no use of rescue medication.
Secondary efficacy endpoints included the proportions of subjects who achieved CR during the acute phase and delayed phase (0–24 and 25–120 hr after chemotherapy initiation, respectively), the time to the first vomiting episode and the frequency of vomiting per day; the time to the first rescue therapy from the chemotherapy initiation (hours) and the proportion of patients receiving rescue therapy; the proportion of patients without significant nausea and the proportion of patients without nausea; the change in ECOG. ECOG was compared per day by Wilcoxon rank sum test between the groups.
2.4. Study visits and evaluation
Assessments of efficacy, tolerability and safety variables were performed for 5 days after the start of chemotherapy (0–120 hr), including the acute and delayed phase. Global satisfaction in control of nausea was evaluated by patients themselves using 100‐mm Visual Analog Scale (VAS; 101 point scale, in which 0 represents a condition without nausea and 100 represents a condition with the worst conceivable nausea). No nausea (VAS score, <5mm) and no significant nausea (VAS score, <25mm).
2.5. Statistical analysis
The primary efficacy endpoint was assessed using a non‐inferiority model with a non‐inferiority margin of 10%. Assuming 75% CR with aprepitant and a difference between treatment groups of ≤10%, 588 patients were required to ensure 80% power of a test. Assuming a 5% dropout rate, 620 patients needed to be enrolled.
Statistical analyses were performed in the safety set (SS; all patients who received at least one dose of study treatment), the full analysis set (FAS; all SS patients who had ≥1 efficacy assessment) and the per protocol set (PPS; all FAS patients who had no protocol violations that directly affected the primary endpoint). The primary and secondary efficacy endpoints were evaluated with FAS and PPS.
Chi‐square was used for statistical analysis. A two‐sided p < .05 was considered statistically significant. All analyses were conducted using SAS version 9.3.
3. RESULTS
Between November 2014 and July 2015, 645 patients were randomized to the fosaprepitant group (n = 328) or aprepitant group (n = 317). Of these patients, 645 were in the SS, 645 were in the FAS, and 626 were in the PPS (Figure 1).
Figure 1.
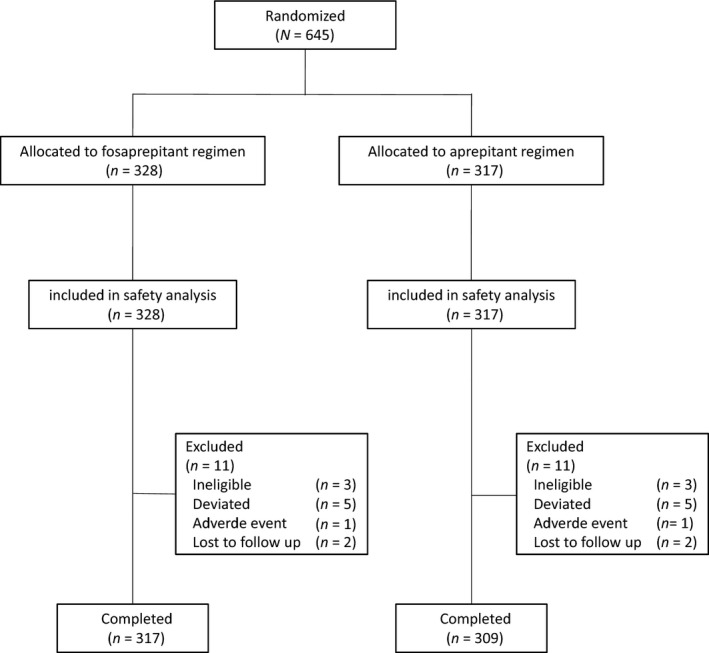
Study flow chart
The baseline demographic, medical characteristics and chemotherapy regimen were comparable between the two groups (Table 2). The prior chemotherapy included the cisplatin‐contained regimen, ABVD, AC, etc. The incidence of CINV in the prior chemotherapy was 34.2% (137/401). In the prior cisplatin‐contained regimen, the incidence of CINV was 37.1% (89/240), whereas that of the prior non‐cisplatin‐contained regimen was 29.8% (48/161). In the current chemotherapy, the median cisplatin dose was 73.3 mg/m2 in the fosaprepitant group and 66.7 mg/m2 in the aprepitant group. Other non‐cisplatin HEC regimens were similar between the groups, which included AP, CAP, NP, etc.
Table 2.
Patient baseline characteristics and therapy history: FAS
| Fosaprepitant group (n = 328) | Aprepitant group (n = 317) | p value | |
|---|---|---|---|
| Age, median (range) | 55 (20–79) | 53 (18–74) | .084 |
| Sex | |||
| Male | 163 (49.70) | 163 (51.42) | .694 |
| Female | 165 (50.30) | 154 (48.58) | |
| ECOGa | |||
| 0 | 64 (19.57) | 64 (20.19) | .939 |
| 1 | 251 (76.76) | 238 (75.08) | |
| 2 | 12 (3.67) | 15 (4.73) | |
| Prior chemotherapy | |||
| No | 121 (36.89) | 123 (38.80) | .627 |
| Yes | 207 (63.11) | 194 (61.20) | |
| Prior radiotherapy | |||
| No | 244 (74.39) | 256 (80.76) | .059 |
| Yes | 84 (25.61) | 61 (19.24) | |
| Cisplatin‐contained | |||
| No | 65 (19.8) | 83 (26.2) | .055 |
| Yes | 263 (80.2) | 234 (73.8) | |
FAS, full analysis set.
One data missing in Fosaprepitant group.
3.1. Primary efficacy analysis
The study met its predefined primary endpoint as fosaprepitant was non‐inferior to aprepitant. In FAS, CR rate was achieved by 293 (89.33%) patients in the fosaprepitant group and 294 (92.74%) patients in the aprepitant group in the OP. There was no significant difference between the two groups (p = .1330). During the AP, 95.73% of patients in the fosaprepitant group reported CR compared with 95.90% in the aprepitant group (p = 1.0000). During the delay phase (DP), 91.16% of patients in the fosaprepitant group reported CR compared with 93.38% in the aprepitant group (p = .3065). The same conclusion was also drawn from PPS analyses (Figures 2 and 3).
Figure 2.
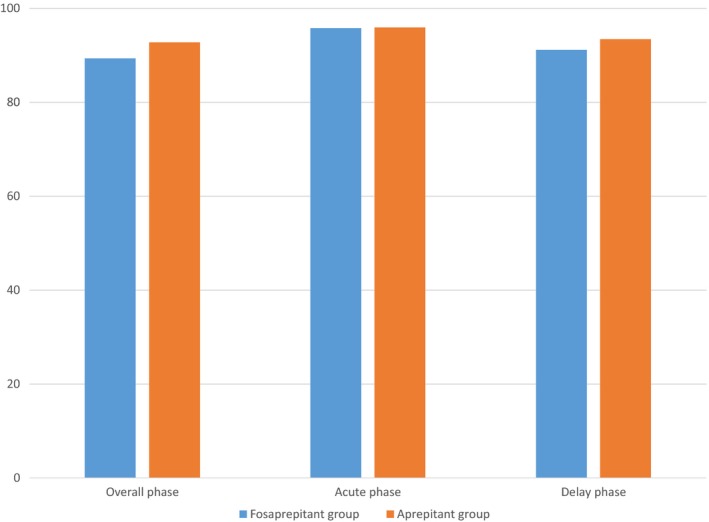
Percentages of patients achieving complete response (no emesis and no use of rescue therapy) (full analysis set)
Figure 3.
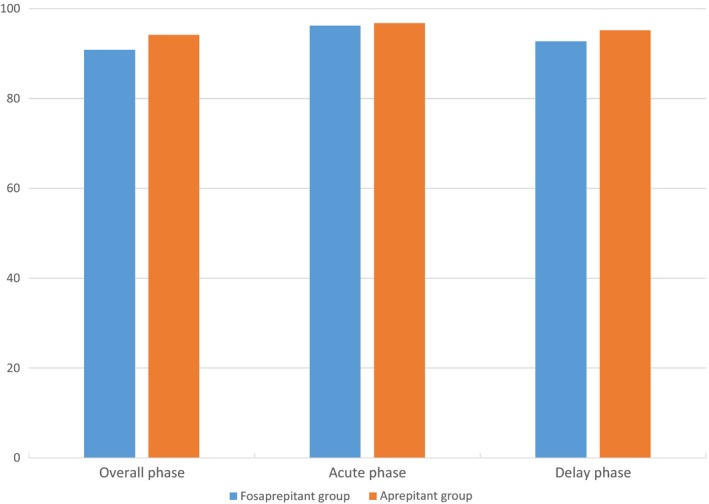
Percentages of patients achieving complete response (no emesis and no use of rescue therapy) (per protocol set). Overall phase was 0–120 hr after initation of chemotherapy. Acute phase was 0–24 hr after initiation of chemotherapy. Delayed phase was 25–120 hr after initiation of chemotherapy
In FAS, fosaprepitant was not inferior to aprepitant in the time to rescue therapy (92.73 hr versus 104.02 hr, p = .0458) and the incidence of rescue therapy (6.40% versus 2.84%, p = .0389). For the secondary efficacy endpoints of the incidence of vomiting and nausea, there was no statistical difference (Table 3). ECOG of per day was compared between the groups and no statistical difference was observed.
Table 3.
Secondary efficacy endpoint comparison
| Fosaprepitant group (n = 328) | Aprepitant group (n = 317) | p value | |
|---|---|---|---|
| The percentage of vomiting | 9.76 | 7.26 | .2647 |
| Complete control of vomiting | 70.73 | 73.50 | .4825 |
| The time to the first vomiting episode (hr) | 94.07 | 112.23 | .2647 |
| The time to rescue therapy (hr) | 92.73 | 104.02 | .0458 |
| The percentage of rescue therapy | 6.40 | 2.84 | .0389 |
| The percentage of no nausea | 39.94 | 40.69 | .8726 |
3.2. Subgroup analysis
The predefined subgroup analyses included chemotherapy history, sex, age, ECOG, and cisplatin‐contained regimen. There was no significant difference in terms of CR rate in OP (p > .05) (Table 4).
Table 4.
Subgroup analysis of complete response (CR) in overall phase (OP)
| Subgroup | Fosaprepitant group | Aprepitant group | p value | ||
|---|---|---|---|---|---|
| Response (n) | CR (%) | Response (n) | CR (%) | ||
| Prior chemotherapy | |||||
| No | 102 | 84.30 | 112 | 91.06 | .1217 |
| Yes | 191 | 92.27 | 182 | 93.81 | .5636 |
| Sex | |||||
| Male | 150 | 92.02 | 154 | 94.48 | .5086 |
| Female | 143 | 86.67 | 140 | 90.91 | .2885 |
| Age | |||||
| <65 | 246 | 90.11 | 246 | 92.48 | .3619 |
| ≥65 | 47 | 85.45 | 48 | 94.12 | .2052 |
| ECOG | |||||
| 0 | 53 | 82.81 | 59 | 92.19 | .1802 |
| 1, 2 | 239 | 90.87 | 235 | 92.89 | .4251 |
| Cisplatin‐contained | |||||
| No | 57 | 87.69 | 78 | 93.98 | .2437 |
| Yes | 236 | 89.73 | 216 | 92.31 | .3501 |
3.3. Safety analysis
A total of 645 patients were included in the SS, of whom 319 experienced adverse events (AEs; fosaprepitant: 169/328; aprepitant: 150/317). The study drug‐related AE for fosaprepitant was similar to that of aprepitant (6.10% versus 7.26%, p > .05). The most common study drug‐related AE was constipation, which was reported less frequently in patients receiving fosaprepitant than those receiving aprepitant (3.35% versus 3.15%). The AEs are summarized in Table 5. All AEs were of mild to moderate severity and tolerable. The incidence of SAE was lower, which only one occurred acute pancreatitis in the aprepitant group, not related to study treatments.
Table 5.
Adverse events (SS)
| Adverse events | Fosaprepitant group (n = 328) | Aprepitant group (n = 317) | p value |
|---|---|---|---|
| Constipation | 11 (3.35) | 10 (3.15) | 1.0000 |
| Diarrhoea | 1 (0.30) | 0 (0) | 1.0000 |
| Distension | 1 (0.30) | 0 (0) | 1.0000 |
| Headache | 1 (0.30) | 0 (0) | 1.0000 |
| Anaphylaxis | 0 (0.00) | 1 (0.32) | .4915 |
| Hiccup | 2 (0.61) | 2 (0.63) | 1.0000 |
| Laboratory test and ECG examination | |||
| White cell count | 2 (0.61) | 1 (0.32) | 1.000 |
| ALT increasing | 1 (0.30) | 0 (0.00) | 1.0000 |
| QT interval prolongation | 0 (0.00) | 2 (0.63) | .2412 |
SS, safety set.
Values are expressed as n (%).
4. DISCUSSION
Multiple clinical guidelines including Chinese antiemetic guidelines (2014 version) have recommended antiemetic therapy for HEC consisting of NK‐1R triple regimen (Gralla et al., 1999; Shiying, Jiliang, & Shukui, 2014; Tageja & Groninger, 2016). Aprepitant was the first potent and selective NK1 RA and was also approved for the prevention of CINV by CFDA in China. Compared to 3‐day oral aprepitant, single‐dose fosaprepitant was not inferior in terms of efficacy (Grunberg et al., 2011). However, the fosaprepitant regimen greatly simplified the antiemetic regimen which offers more convenient and alternative administration. To our knowledge, no studies have been conducted to directly compare the antiemetic effect of aprepitant with fosaprepitant in Chinese patients.
This is the first study to provide efficacy and safety data on a single 150 mg dose of fosaprepitant added to a 5‐HT3RA and a corticosteroid in the control of CINV in Chinese patients receiving HEC. On the whole, this single‐day, triple‐antiemetic fosaprepitant regimen was not inferior to a standard 3‐day oral aprepitant regimen in the control of HEC‐associated CINV.
In the present phase 3 trials, the CR rate of fosaprepitant in the OP was not inferior to the aprepitant (89.33% versus 92.74%, p = .1330). Especially in the DP, fosaprepitant has also showed good efficacy as it was not inferior to aprepitant in the control of delayed symptoms. The current findings in the Chinese population were consistent with previous reports, demonstrating that fosaprepitant 150‐mg regimen is not inferior to the aprepitant 3‐day regimen in the control of CINV in Chinese patients receiving HEC (Grunberg et al., 2011). In contrast to the findings of the EASE study, CR rates in this study were different whether in the OP, AP or DP (Grunberg et al., 2011). However, our results were similar to results of a Japanese study in which the CR rates were achieved in patients receiving cisplatin ≥60 mg/m2 (Ando et al., 2016). The major reason for the difference was possibly the cisplatin dose. In the EASE study, the CR was achieved in the target population receiving cisplatin ≥70 mg/m2. While the patients received non‐cisplatin HEC regimen or cisplatin ≥60 mg/m2 which is classified as HEC under treatment guidelines in the current study. In addition, only 25.9% of Asian patients were included in the aforementioned trial. Those results also suggested that differences were present between Asian patients and non‐Asian patients in the control of CINV, which was possibly related to heterogeneous populations.
In the present study, for the secondary efficacy endpoints, fosaprepitant was also not inferior to aprepitant in the time to rescue therapy and the incidence of rescue therapy. However, there was no statistical significance in the incidence of nausea between the groups. To our knowledge, nausea is more difficult to control compared to vomiting. At the same time, the incidence of nausea is often underestimated. Though no difference was found in the control of nausea in both groups, more attention should be given to the control of nausea.
The currently recommended antiemetic therapy is merely based on the emetogenic level of chemotherapy, regardless of the patient's individual risk factors (Hu et al., 2016). No significant difference were found in sex, age and cisplatin‐contained regimen in terms of CR rate of subgroup analysis in the present study. It still need to develop an approach for personalized management of CINV in the era of precision medicine.
Fosaprepitant was generally well tolerated, with acceptable AEs. The incidence of AEs related to the study drug was very low—6.10% in the fosaprepitant group and 7.26% in aprepitant group. Constipation was the most common AE. The study showed a similar safety profile when compared with previous studies.
In summary, fosaprepitant is effective and safe in the control of CINV in Chinese patients receiving HEC. It might be a more alternative option as antiemetic therapy.
Yang LQ, Sun XC, Qin SK, et al. Efficacy and safety of fosaprepitant in the prevention of nausea and vomiting following highly emetogenic chemotherapy in Chinese people: A randomized, double‐blind, phase III study. Eur J Cancer Care. 2017;26:e12668 https://doi.org/10.1111/ecc.12668
Funding information
This work was sponsored by Chia Tai Tianqing Pharmaceutical Group Co., Ltd.
REFERENCES
- Ando, Y. , Hayashi, T. , Ito, K. , Suzuki, E. , Mine, N. , Miyamoto, A. , … Yamada, S. (2016). Comparison between 5‐day aprepitant and single‐dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin‐based chemotherapy. Supportive Care in Cancer, 24(2), 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch, E. , Prestrud, A. A. , Hesketh, P. J. , Kris, M. G. , Somerfield, M. R. , & Lyman, G. H. (2012). Antiemetic use in oncology: Updated guideline recommendations from ASCO. American Society of Clinical Oncology Educational Book, 532–540. doi: 10.14694/EdBook_AM.2012.32.532 [DOI] [PubMed] [Google Scholar]
- Garcia‐Recio, S. , & Gascón, P. (2015). Biological and pharmacological aspects of the NK1‐receptor. BioMed Research International, 2015, 495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla, R. J. , Osoba, D. , Kris, M. G. , Kirkbride, P. , Hesketh, P. J. , Chinnery, L. W. , … Pfister, D. G. (1999). Recommendations for the use of antiemetics: Evidence‐based, clinical practice guidelines. American Society of Clinical Oncology. Journal of Clinical Oncology, 17(9), 2971–2994. [DOI] [PubMed] [Google Scholar]
- Grunberg, S. , Chua, D. , Maru, A. , Dinis, J. , DeVandry, S. , Boice, J. A. , … Herrstedt, J. (2011). Single‐dose fosaprepitant for the prevention of chemotherapy‐induced nausea and vomiting associated with cisplatin therapy: Randomized, double‐blind study protocol–EASE. Journal of Clinical Oncology, 29(11), 1495–1501. [DOI] [PubMed] [Google Scholar]
- Hesketh, P. J. (2008). Chemotherapy‐induced nausea and vomiting. New England Journal of Medicine, 358, 2482–2494. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Liang, W. , Yang, Y. , Keefe, D. , Ma, Y. , Zhao, Y. , … Zhang, L. (2016). Personalized estimate of chemotherapy‐induced nausea and vomiting: Development and external validation of a nomogram in cancer patients receiving highly/moderately emetogenic chemotherapy. Medicine (Baltimore), 95(2), e2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, K. , Gralla, R. , Jahn, F. , & Molassiotis, A. (2014). International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): Content and implementation in daily routine practice. European Journal of Pharmacology, 722, 197–202. [DOI] [PubMed] [Google Scholar]
- Langford, P. , & Chrisp, P. (2010). Fosaprepitant and aprepitant: An update of the evidence for their place in the prevention of chemotherapy‐induced nausea and vomiting. Core Evid, 5, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlmann, C. H. , & Herrstedt, J. (2012). Fosaprepitant for the prevention of chemotherapy‐induced nausea and vomiting. Expert Review of Anticancer Therapy, 12(2), 139–150. [DOI] [PubMed] [Google Scholar]
- Shiying, Y. , Jiliang, Y. , & Shukui, Q. (2014). Antiemetic guidelines on chemotherapy induced vomiting in China. Version 2014. Chinese Clinical Oncology, 3(19), 263–273. [Google Scholar]
- Sommariva, S. , Pongiglione, B. , & Tarricone, R. (2016). Impact of chemotherapy‐induced nausea and vomiting on health‐related quality of life and resource utilization: A systematic review. Critical Reviews in Oncology/Hematology, 99, 13–36. [DOI] [PubMed] [Google Scholar]
- Tageja, N. , & Groninger, H. (2016). Chemotherapy‐induced nausea and vomiting: An overview and comparison of three consensus guidelines. Postgraduate Medical Journal, 92(1083), 34–40. [DOI] [PubMed] [Google Scholar]
- Weinstein, C. , Jordan, K. , Green, S. A. , Camacho, E. , Khanani, S. , Beckford‐Brathwaite, E. , … Rapoport, B. L. (2016). Single‐dose fosaprepitant for the prevention of chemotherapy‐induced nausea and vomiting associated with moderately emetogenic chemotherapy: Results of a randomized, double‐blind phase III trial. Annals of Oncology, 27(1), 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]


