Abstract
Background
Yes‐associated protein (YAP) overexpression is reported to be associated with risk of hepatocellular carcinoma (HCC) but current studies have not explored the relationship between YAP expression with HCC clinicopathological features.
Methods
To assess these associations, a meta‐analysis was performed which included four eligible studies including 391 HCC cases and 334 controls. There were eight eligible studies to investigate the association between YAP expression in HCC and clinicopathological features of liver cancer patients. Literature was obtained from PubMed, Embase, Wangfang and China National Knowledge Infrastructure.
Results
Analysis indicated that YAP expression in HCC was greater than in adjacent non‐tumour tissue (odds ratio [OR], 15.80, 95% confidence interval [CI], 10.53‐23.70, P<.00001; heterogeneity=.30). YAP overexpression in HCC was significantly associated with vascular invasion (OR, 2.21, 95% CI, 11.64‐2.97, P<.00001, heterogeneity=.10), less cellular differentiation (OR, 2.38, 95% CI, 1.61‐3.51, P<.00001, heterogeneity=.333), tumours larger than 5 cm (OR, 2.52, 95% CI, 1.75‐3.62, P<.00001; heterogeneity=.17) and TNM tumour stage I + II (OR, 0.44, 95% CI, 0.28‐0.75, P=.00003, heterogeneity=.12).
Conclusions
Overexpression of YAP contributes to HCC formation, and its overexpression is associated with vascular invasion, low cellular differentiation tumours larger than 5 cm and TNM tumour stage III + IV.
Keywords: clinicopathological feature, hepatocellular carcinoma, meta‐analysis, Yes‐associated protein
Abbreviations
- CI
confidence interval
- CNKI
China National Knowledge Infrastructure
- CTGF
connective tissue growth factor
- HCC
hepatocellular carcinoma
- ORs
odds ratios
- YAP
Yes‐associated protein
Key points.
The literature that describes Yes‐associated protein (YAP) overexpression and clinicopathological aspects of hepatocellular carcinoma (HCC) offer different results.
Yes‐associated protein overexpression contributes to hepatobiliary carcinoma.
We confirm consistencies between YAP overexpression and HCC clinicopathological features.
Yes‐associated protein overexpression is associated with vascular invasion, cellular differentiation, tumor size and TNM tumor stage.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common hepatic primary malignancy and the third leading cause of cancer‐related mortality worldwide.1 Although multiple therapeutic modalities are available to treat HCC, mortality is still unacceptably high. Recently, research on tumour signal transduction pathways have revealed important pathway proteins that could be exploited for treatment targets, and these approaches have increased patient survival.2, 3
The hippo signalling pathway regulates cell proliferation and apoptosis during normal development and the Yes‐associated protein (YAP) is a major effector of this pathway—YAP overexpression causes hepatocellular tumour cell transformation.4 YAP is more greatly expressed in HCC compared to non‐tumour specimens, and overexpression of YAP is associated with clinicopathological features of HCC,5, 6, 7 however, data have been inconsistent and heterogeneous. Therefore, this meta‐analysis was performed to evaluate the relationship between YAP overexpression and HCC.
2. Methods
2.1. Literature search strategy
A search was conducted with PubMed, EMBase, Wangfang and the Chinese National Knowledge Infrastructure (CNKI) for studies published from 1 January 1996 until 1 September 2016. Relevant studies were identified using the following terms: (“hepatocellular carcinoma” or “liver cancer” or “HCC”) and (“YAP”). Article languages were limited to English and Chinese. All searched studies were retrieved and references were reviewed to locate additional eligible studies. Authors were emailed for studies without sufficient data. We manually searched to ensure that all available studies were included in this meta‐analysis.
2.2. Inclusion and exclusion criteria
Studies included measured YAP expression in HCC using immunohistochemistry. HCC was diagnosed histopathologically. Controls were confirmed to be HCC‐free. Samples used were tumours and adjacent non‐tumour tissues. Only full‐text articles were included. Cases were excluded if they mentioned other cancer types, were case reports, letters, or reviews lacking original data or non‐full‐text papers. No article was duplicated in the analysis.
2.3. Assessment of study quality
Study quality was assessed using a Newscastle‐Ottawa Quality Assessment Scale.8 Two researchers appraised the methodological quality independently. As shown in Table 1, selection method, comparability and exposure assessment method of cases and controls were included in the assessment system. Study “stars” (1‐10) referred to their fitness for being incorporated into the meta‐analysis. Studies with <5 stars were considered to be “low‐quality” studies and were excluded.
Table 1.
Newscastle‐Ottawa Quality Assessment Scale
| Scales for quality assessment criteria | Stars |
|---|---|
| Selection | |
| (1) Is the case definition adequate? | |
| a) Yes, with independent validation | ☆ |
| b) Yes, e.g. record linkage or based on self reports | |
| c) No description | |
| (2) Representativeness of the cases | |
| a) Consecutive or obviously representative series of cases | ☆ |
| b) Potential for selection biases or not stated | |
| (3) Selection of Controls | |
| a) Community controls | ☆ |
| b) Hospital controls | |
| c) No description | |
| (4) Definition of Controls | |
| a) No history of disease (endpoint) | ☆ |
| b) No description of source | |
| Comparability | |
| (1) Comparability of cases and controls on the basis of the design or analysis | |
| a) Study controls for _______________ (Select the most important factor.) | ☆ |
| b) Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor.) | ☆ |
| Exposure | |
| (1) Ascertainment of exposure | |
| a) Secure record (e.g. surgical records) | ☆ |
| b) Structured interview where blind to case/control status | ☆ |
| d) Written self report or medical record only | |
| e) No description | |
| (2) Same method of ascertainment for cases and controls | |
| a) Yes | ☆ |
| b) No | |
| (3) Non‐Response rate | |
| a) Same rate for both groups | ☆ |
| b) Non respondents described | |
| c) Rate different and no designation | |
2.4. Data extraction
Full‐texts of candidate articles were reviewed by two independent investigators and data were extracted and sorted. If results were in disagreement, a third investigator resolved the inconsistency. Data extracted from selected articles included the first author's name, publication year, country, numbers of cases and controls, YAP expression in HCC and adjacent normal tissue, and clinicopathological features.
2.5. Statistical analysis
The meta‐analysis was performed using STATA 12.0 (College Station, TX, USA). Association of YAP overexpression between HCC and adjacent non‐tumour tissues and any correlation with clinicopathological features in HCC were estimated using odds ratios (ORs) with 95% confidence intervals (95% CIs) and P<.05 was considered to indicate statistical significance. Study heterogeneity was investigated using a Chi‐square‐based Q‐test and quantified using I 2. P‐values <.05 and I 2 values exceeding 50% indicated significant heterogeneity. ORs were pooled according to a fixed effects model (Mantel‐Haenszel model). Otherwise, a random‐effects model (DerSimonian and Laird model) was used. Sensitivity analyses were used to assess stability of meta‐analysis results and sensitivity analysis was performed by removing a specific study from the meta‐analysis. Begg's funnel plots were used to evaluate publication bias and two reviewers analysed data independently and obtained the same conclusions.
3. Results
3.1. Study characteristics
A total of 454 records, 210 from PubMed, 110 from EMbase, 108 from the CNKI and 26 from the Wanfang Database were found using our search strategy. The complete literature selection process appears in Figure 1. Study characteristics of those chosen appear in Tables 2, 3, 4.
Figure 1.
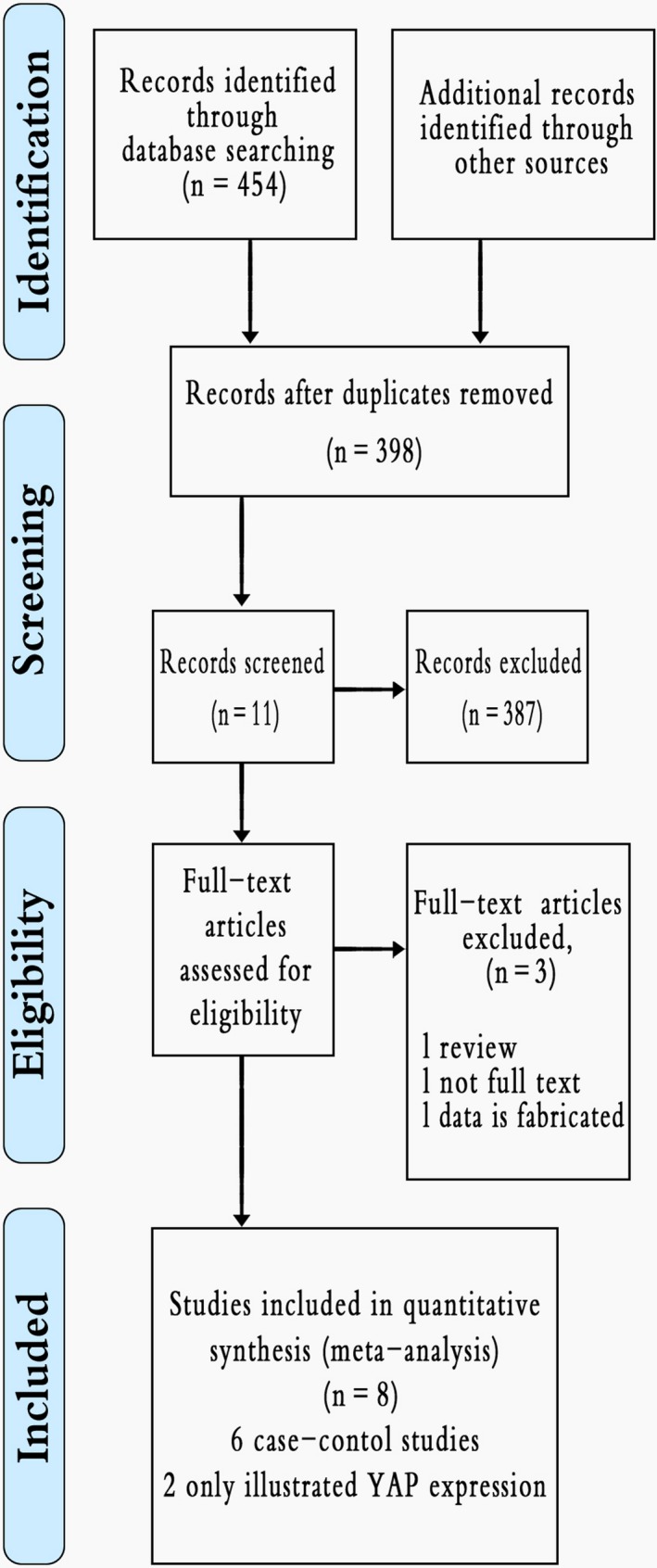
Flow chart for this study's selection process (Data S1)
Table 2.
Characteristics of included studies in meta‐analysis (correlation of Yes‐associated protein [YAP] expression between hepatocellular carcinoma and adjacent non‐tumour tissue)
Table 3.
Characteristics of studies included in meta‐analysis (correlation of Yes‐associated protein [YAP] expression in hepatocellular carcinoma with clinicopathological features)
| Study | YAP (+) | YAP (+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M/F) | Vascular invasion (Y) | Cellular differentiation (L) | Tumour Size (>5 cm) | TNM (I + II) | AFP (>400 ng/mL) | Tumour Number (>1) | Hepatitis (Y/N) | AJCC (I) | ||
| Lei (2015) | 42 | 38/4 | 25 | N | N | N | 40 | N | N | 17 |
| Li (2015)25 | 54 | 49/5 | 31 | 8 | 33 | N | 29 | 29 | 52 | 10 |
| Han (2014)5 | 27 | 25/2 | 3 | 12 | 15 | 10 | N | 1 | 23 | N |
| Xu (2009)11 | 110 | 90/20 | 59 | 21 | N | N | 57 | 24 | 69 | 42 |
| Wu (2016)23 | 78 | 67/11 | 28 | N | 61 | 19 | N | N | 74 | N |
| Wang (2016)7 | 59 | 56/3 | 11 | 30 | N | N | N | 35 | 55 | N |
| Xu (2013)9 | 129 | 108/21 | 57 | 47 | 65 | 88 | N | 21 | 126 | N |
| Liu (2015)12 | 49 | N | 33 | 15 | N | 32 | 43 | N | N | N |
Table 4.
Characteristics of studies included in the meta‐analysis (correlation of Yes‐associated protein [YAP] expression in hepatocellular carcinoma with clinicopathological features)
| Study | YAP (−) | YAP (−) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M/W) | Vascular invasion (Y) | Cellular differentiation (L) | Tumour Size (>5 cm) | TNM (I + II) | AFP (>400 ng/mL) | Tumour number (>1) | Hepatitis (Y/N) | AJCC (I) | ||
| Lei (2015) | 10 | 8/2 | 4 | N | N | N | 5 | N | N | 1 |
| Li (2015)25 | 51 | 49/2 | 14 | 5 | 21 | N | 18 | 21 | 51 | 19 |
| Han (2014)5 | 12 | 10/2 | 3 | 1 | 5 | 5 | N | 2 | 7 | N |
| Xu (2009)11 | 67 | 53/14 | 31 | 9 | N | N | 13 | 17 | 46 | 29 |
| Wu (2016)23 | 59 | 50/9 | 12 | N | 24 | 18 | N | N | 56 | N |
| Wang (2016)7 | 25 | 22/3 | 3 | 6 | N | N | N | 15 | 23 | N |
| Xu (2013)9 | 98 | 90/8 | 24 | 23 | 34 | 83 | N | 20 | 97 | N |
| Liu (2015)12 | 29 | N | 7 | 1 | N | 28 | 14 | N | N | N |
3.2. Heterogeneity and sensitivity analysis
Analysis of the correlation of YAP expression between HCC and adjacent non‐tumour tissue revealed that two studies5, 9 had substantial influence over the data and overall estimates remained stable. Based on Q‐test and I 2 statistics, there was no evidence of significant heterogeneity (χ2=3.70, P=.30, I 2=19%), therefore the fixed effects model was used for OR calculations. Analysis of correlations of YAP overexpression with clinicopathological features in HCC revealed that heterogeneity and sensitivity analysis were performed in the same way.
3.3. Publication bias
Funnel plots were symmetrical, and Egger's and Begg's tests suggested no obvious publication bias in the analysis of correlations between YAP overexpression in HCC and clinicopathological features (Figure 2B, Table 5).
Figure 2.
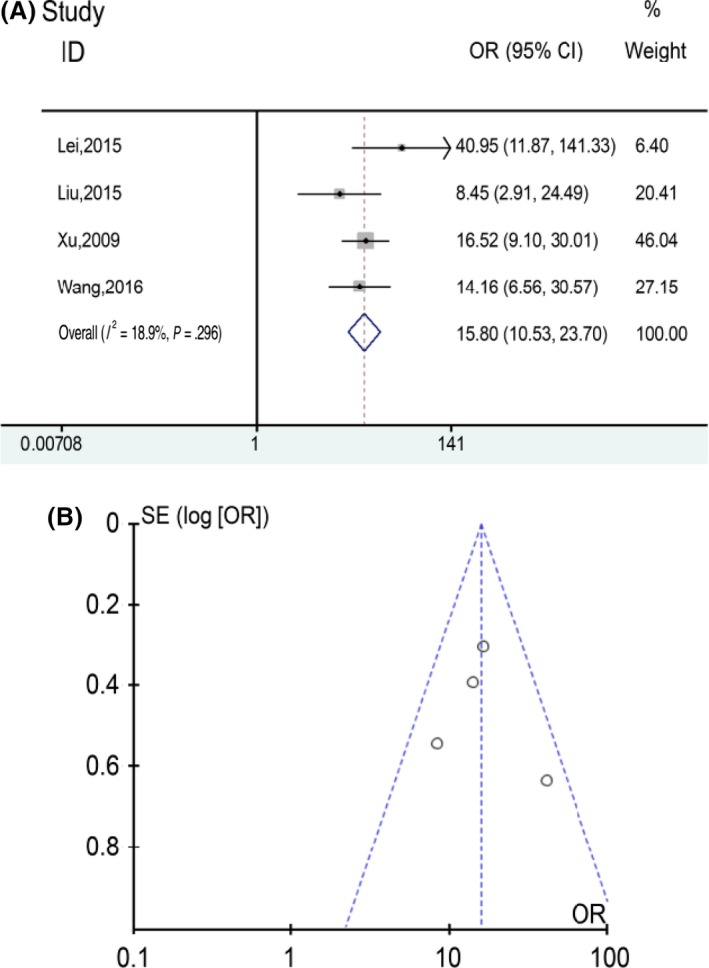
Analysis of Yes‐associated protein (YAP) expression between hepatocellular carcinoma (HCC) and adjacent non‐tumour tissue. (A) Forest plot depicting YAP expression between HCC and adjacent non‐tumour tissue. (B) funnel plot depicting YAP expression between HCC and adjacent non‐tumour tissue
Table 5.
Analysis of correlation of Yes‐associated protein expression in hepatocellular carcinoma with clinicopathological features using a fixed effects model
| Clinicopathological features feature | OR (95% CI) | P‐value (Z test) | I 2 (%) | Heterogeneity | Egger's test P‐value | Begg's test P‐value |
|---|---|---|---|---|---|---|
| Sexa | 0.93 (0.61, 1.42) | .74 | 18 | .29 | .371 | .453 |
| Tumour numberb | 1.17 (0.82, 1.67) | .38 | 55 | .06 | .524 | .624 |
| Cellular differentiationc | 2.38 (1.61, 3.51) | <.00001 | 13 | .33 | .05 | .039 |
| Serum AFPd | 4.09 (2.59, 6.45) | <.00001 | 55 | .08 | .230 | .174 |
| Venous infiltratione | 2.21 (1.64, 2.97) | <.00001 | 42 | .11 | .791 | .621 |
| Hepatitisf | 0.9 (0.55, 1.47) | .66 | 5 | .38 | .933 | .573 |
| AJCC tumour stageg | 0.77 (0.48, 1.23) | .27 | 66 | .05 | .612 | .602 |
| TNM tumour stageh | 0.44 (0.28, 0.69) | .0003 | 48 | .12 | .586 | .497 |
| Tumour sizei | 2.52 (1.75, 3.62) | <.00001 | 40 | .17 | .899 | 1.000 |
Male/female.
Multiple/single.
Low differentiation/middle or high differentiation.
>400 ng/mL/<400 μg/mL.
Y/N.
Y/N.
I/II + III.
I + II/III + IV.
>5 cm/<5 cm.
3.4. Meta‐analysis results
Within four records7, 10, 11, 12 with 391 cases and 334 controls, YAP overexpression was correlated with HCC but not with adjacent non‐tumour tissue. Figure 2 shows that YAP expression in HCC was greater than in adjacent non‐tumour tissue. The pooled OR was 15.80 (95% CI: 10.53‐23.70, P<.00001).
Odds ratios from eight records1, 5, 6, 9, 10, 11, 12, 13 for YAP overexpression and HCC clinicopathological features (Figure 3 and Table 5) show that YAP overexpression in HCC was significantly associated with more vascular invasion, poor cellular differentiation, tumours larger than 5 cm and TNM tumor stage IIII + IV. Pooled ORs appear in Figure 3 and show that YAP overexpression increased vascular invasion, more poor differentiation, larger tumours and higher staged tumours in HCC.
Figure 3.
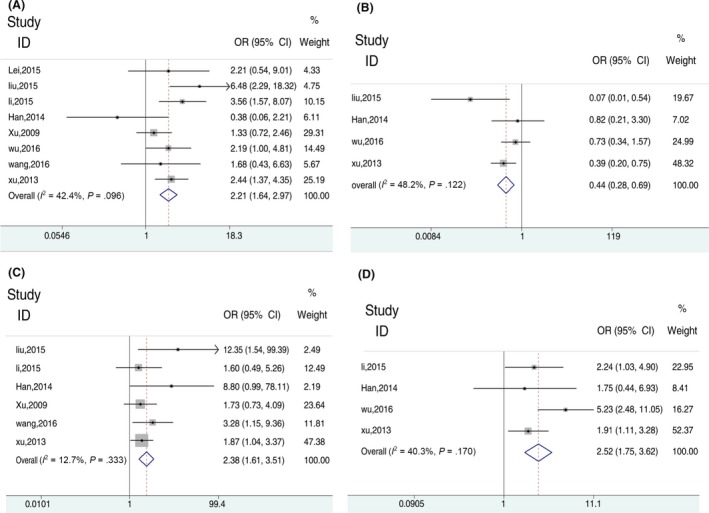
The relationship between Yes‐associated protein (YAP) expression and clinicopathological feature. (A) Forest plots depicting correlation between YAP expression in hepatocellular carcinoma (HCC) and vascular invasion. (B) HCC and TNM tumour stage. (C) Cellular differentiation. (D) Tumour size
4. Discussion
Hepatocellular carcinoma is a common malignant, highly invasive tumour with poor prognosis14 To better treat HCC, novel targets are being investigated such as HIPPO signalling pathways. YAP is a major effector in this pathway and is involved in numerous malignant tumours,15, 16, 17, 18 Tschahargane19 found that YAP activated the Notch signalling pathway by upregulating Jagged1 expression, thereby accelerating proliferation of hepatoma cells. Urtasun's group20 found that YAP expression was not only in HCC but also in primary human hepatocytes; they also demonstrated that YAP could upregulate the connective tissue growth factor (CTGF) expression, while CTGF could downregulate the tumour necrosis factor‐related apoptosis‐inducing ligand receptor 2 expression which affected HCC cells apoptosis, In other words, it is further proved that the abnormal expression of YAP may be related to the occurrence and development of HCC. Other researchers21, 22, 23 indicate that YAP overexpression occurs in HCC tissues and confirms a relationship between YAP overexpression and HCC. Our meta‐analysis confirmed the view—expression of YAP in HCC tissues may be higher than in cirrhotic liver samples and healthy livers. Because of limited studies, no subgroup analysis was made for the presence or absence of cirrhosis in non‐tumoral (NT) tissues and no studies included had subgroup analysis.
We used the Newscastle‐Ottawa Scale to assess study quality, selection method, comparability and exposure; the assessment method was also comprehensively evaluated. Article “stars” were at least 7, indicating that all included studies were of high quality. The Egger's and Begg's tests indicated no significant publication bias.
Overall, eight studies were included and six contained data regarding YAP expression and HCC and adjacent non‐tumor tissues, suggesting that YAP expression between HCC and adjacent tissues differed. Funnel plots were symmetrical. There was evidence of significant heterogeneity before excluding work by Han and Xu.5, 9 Sensitivity analysis indicated that two studies5, 9 affected meta‐analysis stability, and after eliminating these two studies, heterogeneity significantly improved.
Work by Han and Xu5, 9 described YAP expression in HCC and non‐tumorous tissue and reported P‐values <.01, which supports our meta‐analysis that YAP expression in HCC was significantly higher than in adjacent tissues, and YAP overexpression contributed to the occurrence of HCC.
We also noted that YAP overexpression is related to poor differentiation, more venous infiltration, TNM stage III‐IV, and tumours larger than 5 cm. Liu12 and Wang's groups7 reported that YAP expression was related to HCC differentiation, and Liu12 and Xu et al.9 indicated that YAP expression was related to venous infiltration and tumour TNM stage. Few studies6, 9, 24 suggested that YAP expression was related to tumour size. Thus, YAP overexpression may be a risk factor for the formation of HCC.
As shown in Table 4, heterogeneity analysis of the relationship between YAP expression and AFP indicated no significant heterogeneity among the studies. YAP is involved in many signalling pathways associated with HCC,19, 25, 26, 27, 28 but its actual role is unclear and worthy of investigation, especially because Simile's group29 reported that upregulation of YAP is related to HCC prognosis.
This meta‐analysis was limited by few manuscripts with possibly biased results. Also, studies were from China and within each study, ethnicity was not identified. More studies with larger samples and different ethnicities are required to confirm an association between YAP overexpression and HCC.
Conflicts of Interest
The authors disclose no potential conflicts of interest.
Supporting information
Lin C, Hu Z, Lei B, et al. Overexpression of Yes‐associated protein and its association with clinicopathological features of hepatocellular carcinoma: A meta‐analysis. Liver Int. 2017;37:1675–1681. https://doi.org/10.1111/liv.13428
Funding information
This work was supported in part by The National Natural Science Foundation of China (no. 81430014 and no. 31370917), the Natural Science Foundation of Guangxi Province (2015GXNSFFA139004 and 2014GXNSFDA118019), the Bagui Scholarship Foundation, and the Guangxi Health and Family Planning Commission “139” Leading Talents Training Plan.
Chengjie Lin and Zhigao Hu contributed equally to this work.
Handling Editor: Carmen Berasain
References
- 1. Huo X, Zhang Q, Liu AM, et al. Overexpression of Yes‐associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29:840‐846. [DOI] [PubMed] [Google Scholar]
- 2. Thillai K, Ross P, Sarker D. Molecularly targeted therapy for advanced hepatocellular carcinoma—a drug development crisis? World J Gastrointest Oncol. 2016;8:173‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. el Tazi M, Essadi I, M'Rabti H, Touyar A, Errihani PH. Systemic treatment and targeted therapy in patients with advanced hepatocellular carcinoma. N Am J Med Sci. 2011;3:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell. 2007;130:1120‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han SX, Bai E, Jin GH, et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014;2014:261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Wang S, Wang G, et al. Yes‐associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Dig Surg. 2014;31:468‐478. [DOI] [PubMed] [Google Scholar]
- 7. Wang C, Zhu ZM, Liu CL, et al. Yes‐associated protein in hepatocellular carcinoma is associated with tumor differentiation and patient age at diagnosis, but not markers of HBV infection. Clin Lab. 2016;62:365‐371. [DOI] [PubMed] [Google Scholar]
- 8. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 February 2016.
- 9. Xu XN. The Clinical Significance of YAP Protein Expression in Hepatocellular Carcinoma and its Relationship with Hepatic Stellate Cells. Shanghai: Fudan University; 2013. [Google Scholar]
- 10. Lei CJ, Li L, Long HC, et al. The significance of the expression of Yes protein in human hepatocellular carcinoma. J Chin Pract Diagnosis Therapy. 2015;29:244‐246. [Google Scholar]
- 11. Xu MZ, Yao TJ, Lee NPY, et al. Yes‐associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576‐4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu YG. Study on the relationship between the expression of YAP and P‐YAP in hepatocellular carcinoma and its clinical biological behavior. Hebei: Hebei Medical University; 2015. [Google Scholar]
- 13. Wang YP, Tang DX. Expression of Yes‐associated protein in liver cancer and its correlation with clinicopathological features and prognosis of liver cancer patients. Int J Clin Exp Med. 2015;8:1080‐1086. [PMC free article] [PubMed] [Google Scholar]
- 14. Jang SY, Park SY, Lee HW, et al. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liv. 2016;10:948‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HM, Jung WH, Koo JS. Expression of Yes‐associated protein (YAP) in metastatic breast cancer. Int J Clin Exp Pathol. 2015;8:11248‐11257. [PMC free article] [PubMed] [Google Scholar]
- 16. Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta‐catenin signaling regulates Yes‐associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730‐11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moroishi T, Park HW, Qin B, et al. A YAP/TAZ‐induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015;29:1271‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vlug EJ, van de Ven RA, Vermeulen JF, et al. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol (Dordr). 2013;36:375‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tschaharganeh DF, Chen X, Latzko P, et al. Yes‐associated protein up‐regulates Jagged‐1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530.e1512‐1542.e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urtasun R, Latasa MU, Demartis MI, et al. Connective tissue growth factor autocriny in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes‐associated protein‐mediated activation. Hepatology. 2011;54:2149‐2158. [DOI] [PubMed] [Google Scholar]
- 21. Zhou TY, Zhuang LH, Hu Y, et al. Inactivation of hypoxia‐induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci Rep. 2016;6:30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaQuaglia MJ, Grijalva JL, Mueller KA, et al. YAP subcellular localization and hippo pathway transcriptome analysis in pediatric hepatocellular carcinoma. Sci Rep. 2016;6:30238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu D, Liu G, Liu Y, et al. Zinc finger protein 191 inhibits hepatocellular carcinoma metastasis through discs large 1‐mediated yes‐associated protein inactivation. Hepatology. 2016;64:1148‐1162. [DOI] [PubMed] [Google Scholar]
- 24. Wu H, Liu Y, Jiang XW, et al. Clinicopathological and prognostic significance of Yes‐associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumor Biol. 2016;10:13499‐13508. [DOI] [PubMed] [Google Scholar]
- 25. Li X, Tao J, Cigliano A, et al. Co‐activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget. 2015;6:10102‐10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valero V 3rd, Pawlik TM, Anders RA. Emerging role of Hpo signaling and YAP in hepatocellular carcinoma. J Hepatocell Carcinoma. 2015;2:69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Z, Hu T, Xu Z, et al. Targeting Hippo pathway by specific interruption of YAP‐TEAD interaction using cyclic YAP‐like peptides. FASEB J. 2015;29:724‐732. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Wang J, Zhang Y, et al. MEK1 promotes YAP and their interaction is critical for tumorigenesis in liver cancer. FEBS Lett. 2013;587:3921‐3927. [DOI] [PubMed] [Google Scholar]
- 29. Simile MM, Latte G, Demartis MI, et al. Post‐translational deregulation of YAP1 is genetically controlled in rat liver cancer and determines the fate and stem‐like behavior of the human disease. Oncotarget. 2016;7:49194‐49216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


