Abstract
Regular use of marijuana during adolescence enhances the risk of long‐lasting neurobiological changes in adulthood. The present study was aimed at assessing the effect of long‐term administration of the synthetic cannabinoid WIN55212.2 during adolescence in young adult mice. Adolescent mice aged 5 weeks were subjected daily to the pharmacological action of WIN55212.2 for 3 weeks and were then left undisturbed in their home cage for a 5‐week period and finally evaluated by behavioral testing. Mice that received the drug during adolescence showed memory impairment in the Morris water maze, as well as a dose‐dependent memory impairment in fear conditioning. In addition, the administration of 3 mg/kg WIN55212.2 in adolescence increased adult hippocampal AEA levels and promoted DNA hypermethylation at the intragenic region of the intracellular signaling modulator Rgs7, which was accompanied by a lower rate of mRNA transcription of this gene, suggesting a potential causal relation. Although the concrete mechanisms underlying the behavioral observations remain to be elucidated, we demonstrate that long‐term administration of 3 mg/kg of WIN during adolescence leads to increased endocannabinoid levels and altered Rgs7 expression in adulthood and establish a potential link to epigenetic changes.
Keywords: Adolescence, CA regions, DNA methylation and Rgs7, learning and memory, WIN55212.2
Introduction
Adolescence is the most important stage of postnatal neurodevelopment and covers distinct maturational mechanisms in the corticolimbic circuitries (Powell 2006; Schneider 2013) including neuronal plasticity, myelination, synaptic pruning, volumetric growth, programming of neurotrophic levels and the maturation of dopamine, glutamate and GABA neurotransmitters (Kilb, 2012; O'Donnell, 2010; Renard et al., 2014).
Clinical evidence indicates that the pathophysiology of neuropsychiatric disorders is associated with dysfunctions of the adolescent brain (Merikangas et al. 2009), and this fact is aggravated when teenagers regularly smoke marijuana (for review, see Hoch et al 2015).
Exposure to drugs of abuse during adolescence is particularly critical, and consumption during this time window has more dramatic consequences in later life when compared to adulthood or juvenile drug exposure (for a review of evidence in animals see Schneider 2013, for human data see Hoch et al 2015). Several studies suggest that abuse of the psychotropic component of marijuana—Δ9‐tetrahydrocannabinol (THC)—during adolescence enhances the risk of long‐lasting neurobiological changes in the adult brain (Abush and Akirav, 2012; Renard et al., 2013; Rubino et al., 2009). Long‐lasting cognitive impairments are in fact the most prominent effects after chronic THC consumption during adulthood in humans (for review, see Hoch et al 2015). Such impairments might be more serious following the use of synthetic cannabinoids, which are several fold more potent than THC in activating its receptors in the brain, have become very popular in recent years and whose consequences later in life have not been investigated yet (Brents et al., 2011; Schneir et al., 2012).
The endocannabinoid system (eCB), whose major active component in learning‐related areas such as the hippocampus is the G‐protein coupled receptor CB1, regulates axon innervation patterns during adolescence (Eggan et al., 2010; Renard et al., 2013) and specifically controls distinct neurodevelopmental processes, including the proliferation and differentiation of progenitor cells, neuronal migration, axonal guidance, fasciculation, positioning of cortical interneurons, neurite outgrowth and morphogenesis by acting at inhibitory and excitatory synapses in brain regions involved in emotional or non‐emotional processes (for review see Harkany et al., 2007, 2008).
Moreover, THC consumption can alter specific gene programs by epigenetic mechanisms such as DNA methylation, histone modifications and chromatin restructuring (for review, see D'Addario et al., 2013). Most studies to date that addressed the role of epigenetic mechanisms regarding the effects of exogenous cannabinoid drugs focused on adulthood (Tomasiewicz et al., 2012; Watson et al., 2015). We are the first to evaluate the effects of adolescent exposure to the synthetic cannabinoid agonist WIN55212.2 (WIN) during adulthood in relation with epigenetic alterations.
In particular, we focused on the effects of adolescent WIN55212.2 exposure on DNA methylation (DNAme). We chose this mark because it is a relatively stable modification that could persist during the withdrawal period and explain the remote behavioral effects that the synthetic cannabinoid WIN has after adolescent exposure. DNAme provides a stable and potentially heritable epigenetic component, moderates gene–environment interactions (Rotter et al., 2012) and participates in normal brain development (Wilson and Sengoku, 2013) and psychiatric disorders (Tuesta and Zhang, 2014; Watson et al., 2015). Most importantly, THC exposure has already been linked to DNAme changes (for review, see Szutorisz and Hurd, 2015), but the potential effects of DNAme alterations on remote cognitive performance after drug exposure have not been deeply explored.
Given that CB1 receptors are highly expressed in the hippocampal CA regions (Wang et al., 2003), which are essential for learning and short‐term as well as intermediate memory (Agis‐Balboa and Fischer, 2014), we focused on this region for the molecular characterization of the effects of adolescent exposure to WIN55212.2 on learning and memory during adulthood. Because the most prominent behavioral deficits reported in the current study were found in those animals chronically treated with either lowest or highest dose of the drug during adolescence, we directed further investigation on endocannabinoid content, epigenetic and transcriptional gene regulation level under these conditions. We studied Rgs7 as a candidate methylated gene for the following reasons: RGS proteins are active regulators of G protein‐coupled receptors signaling (Sjögren, 2011; Xie and Martemyanov, 2011), they are tightly regulated in response to non‐cannabinoid drugs of abuse (Holden et al., 2014; Kach et al., 2012; Sjögren, 2011) and also involved in hippocampus‐dependent learning and memory (Ostrovskaya et al., 2014). In brief, we report that 3 weeks of WIN exposure during adolescence are enough to impair hippocampus‐dependent memory in adulthood. Furthermore, we show that endocannabinoid levels remain altered in the adult hippocampus and also show that WIN exposure results in persistent DNAme alterations that could potentially be linked to transcriptional changes.
Materials and Methods
All the procedures performed with mice were approved by the Göttingen University Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the use of animals in research and the European Communities Council Directive (86/609/EEC).
Animals
A total of 60 C57Bl6/J male mice aged 4 weeks were obtained from Charles River Laboratories (Sulzfeld, Germany). On arrival, they were housed in groups of 5 and maintained under standard conditions (12‐hour light/dark cycle with 6:00/18:00 lights on/off, a room temperature of 21 ± 2 °C and food and water ad libitum). One week after the habituation period, they were subjected to the experiment (5 weeks of age).
Drugs
The CB1/CB2 receptor agonist WIN55212.2 (WIN) (Sigma‐Aldrich, Seelze, Germany) was dissolved in a vehicle solution consisting of 10 percent DMSO (Sigma‐Aldrich, Seelze, Germany) and 0.1 percent Tween80 (Sigma‐Aldrich, Seelze, Germany) in 0.9 percent saline and freshly prepared on the day of administration. The same volume of drug or vehicle (200 µl) was applied intraperitoneally. The drug was administered at 0.5, 1 or 3 mg/kg.
Experiment design and experimental groups
Mice were ascribed to one of four groups of 15 animals each: vehicle, 0.5 mg/kg (Low), 1 mg/kg and 3 mg/kg of WIN (High). Adolescent animals were subjected to daily injections of vehicle or WIN for 3 weeks (5–8 weeks of age). Then, animals were left undisturbed at their home cage for 5 weeks (withdrawal WIN treatment), and finally young adult mice were evaluated by behavioral testing (13 weeks of age). Animals were sacrificed immediately after the last behavioral paradigm. Animals were deeply anesthetized by intraperitoneal injection of 2,2,2‐tribromo‐ethanol (Sigma‐Aldrich, Hamburg, Germany) and transcardially perfused with cool 0.1 percent saline phosphate buffer (PBS). The hippocampal CA regions (≈ bregma −1.64 and interaural 2.16 mm) were freshly isolated and frozen in liquid nitrogen for LC‐MS, MEDIP‐qPCR and quantitative RT‐PCR (qRT‐PCR). See also Fig. S1.
Spatial memory
Morris water maze was performed in a circular pool of 1.2‐m diameter and filled with opaque water. The platform (11 × 11 cm) was placed in the center of the target quadrant below the water level. The swimming pattern was registered by a video camera device and tracked using the VideoMot2 software (TSE, Bad Homburg, Germany). For each training session, mice were subjected to four consecutive trials. Each trial was started from a different point of the pool, and the pattern was randomized for consecutive days. For each trial, animals were allowed to search the platform for 60 seconds. If the platform was not found, mice were guided to it and left on it for 15 seconds before the next trial. Twenty‐four hours after the last training trial, mice memory was evaluated (probe trial). In the probe trial, the platform was removed from the pool, and the mice were allowed to swim for 60 seconds during which the time spent in the target quadrant was registered as a measure of spatial memory. N = 15 mice/group.
Contextual fear memory
Associative learning was measured by use of NIR Video Fear Conditioning system (Med Associates Inc., St. Albans, USA). The training consisted of a single exposure to the context (3 minutes) followed by a tone [30 seconds, 10 kHz, 75‐dB sound pressure level (SPL)] and a foot shock (2 seconds, 0.7 mA, constant current). The context memory test was performed 24 hours later by re‐exposing the mice for 3 minutes to the conditioning context. For the cued memory test, mice were put into a novel context for 3 minutes and after an initial 30 seconds free of tone to evaluate context generalization, the same training tone was played for 3 minutes. Freezing was automatically recorded by use of a video camera device coupled to computer equipped with VideoFreeze software (Med Associates Inc., St. Albans, USA). N = 15 mice/group.
General motor activity and anhedonia behavior
A distinct cohort of animals (n = 15 mice/group) was subjected to the same experimental procedure and then consecutively tested by open field and tail suspension to elucidate plausible neurobiological effects of the long‐term administration of the cannabinoid agonist WIN on general motor activity and anhedonia behavior, respectively. In the open field test the spontaneous locomotor and exploratory activity of mice was monitored. The experiment was conducted in a Plexiglas arena (45 × 45 × 55 cm). Each animal was placed separately in the same start position in the arena. Mice were allowed to explore the open field for 10 minutes without habituation time. Parameters recorded were distance traveled, percent of time moving, time spent in the center of open field (defined as 70 percent of area), hyperactivity (forward movement with speed greater than 20 cm/second) and number of rearings. All parameters were analyzed automatically by the ActiMot software (TSE, Bad Homburg, Germany) as described before (Brzozka et al. 2011). Anhedonia was evaluated by tail suspension test as described (Hayase T, 2011). Briefly, a cube of 35 cm each side with an open front was used to perform the experiment. The mouse was suspended by its tail in the center of the upper surface using a tail hanger and non‐irritating adhesive tape. The cumulative immobility time during a 6‐minute period was manually registered by use of a timer.
Extraction and pre‐purification procedures and measurement of AEA, 2‐AG, OEA and PEA levels
The endocannabinoids AEA and 2‐AG, and the endocannabinoid‐related molecules N‐palmitoylethanolamine (PEA) and N‐oleoylethanolamine (OEA) were extracted from the hippocampal CA regions and then purified from other lipids and quantified as described elsewhere (Matias et al. 2008). First, tissues were dounce‐homogenized and extracted with chloroform/methanol/Tris‐HCl 50 mM pH 7.5 (2:1:1, v/v) containing internal deuterated standards for AEA, 2‐AG, PEA and OEA quantification by isotope dilution ([2H]8AEA, [2H]52AG, [2H]4 PEA, [2H]4 OEA) (Cayman Chemicals, MI, USA). The lipid‐containing organic phase was dried down, weighed and pre‐purified by open bed chromatography on silica gel. Fractions were obtained by eluting the column with 99:1, 90:10 and 50:50 (v/v) chloroform/methanol. The 90:10 fraction was used for AEA, 2‐AG, PEA and OEA quantification by liquid chromatography‐atmospheric pressure chemical ionization‐mass spectrometry (LC‐APCI‐MS), as previously described and using selected ion monitoring at M + 1 values for the four compounds and their deuterated homologues, as described in Bisogno et al. (2008). N = 4 mice/group.
DNA and RNA extraction. Immunoprecipitation of the methylated DNA
Total DNA and RNA were purified using the AllPrep DNA/RNA Mini Kit (Qiagen, Düsseldorf, Germany). DNA was sheared using a Bioruptor NGS (Diagenode) to have a size distribution of 100 to 600 bp. Subsequently, meDNA IP was carried out using Diagenode's kit (C02010020, Diagenode, Seraing, Belgium) according to the manufacturer's instructions.
RNA was digested with RNase‐Free DNase (Qiagen, Barcelona, Spain) and checked for integrity by capillary gel electrophoresis (Bioanalyzer, Agilent Technologies, Santa Clara, USA). The concentration of RNA was determined using a Nano‐Drop® ND‐1000 spectrophotometer (Thermo Scientific, Wilmington, USA).
MEDIP‐qPCR and quantitative RT‐PCR
MEDIP‐qPCR was performed using SYBR Green I Master mix with custom primers (Rgs7 forward 5′‐GTTCCACTCACCAGAGGTCTCAG‐3′ and reverse 5′‐TGCAGTACATAGGGAAGCAGAC‐3′) (all from Applied Biosystems, Darmstadt, Germany) on a CXF96TM Real‐Time PCR system (Bio‐Rad, Hercules, USA). Quantitative RT‐PCR was performed using 1‐µg RNA and was converted to cDNA using the High‐Capacity RNA‐to‐cDNA™ Kit (TermoFisher Scientific, Darmstadt, Germany). Messenger RNA (mRNA) expression was quantified by qRT‐PCR using the CXF96TM Real‐Time PCR system (Bio‐Rad, Hercules, USA). GAPDH mRNA was used as an endogenous control. TaqMan primers and probes for mouse Rgs7 cDNAs were obtained from validated and predesigned Assays‐on‐Demand (Applied Biosystems, Darmstadt, Germany) and used in real‐time PCR amplifications to detect the expression of Rgs7.
The reactions were performed in triplicate using 2 µl of cDNA in a 10‐µl reaction volume. Relative fold expression for each sample was calculated using the comparative cycle threshold (Ct) method with 2 − ΔΔCt, according to the manufacturer's instructions (Applied Biosystems, Darmstadt, Germany). Specific cDNAs were quantified relative to a ‘calibrator’ control sample serving as reference. The 2 − ΔΔCt for this ‘calibrator’ control sample was arbitrarily set to 1. N = 4 mice/group.
Statistical analysis
Statistical significance was evaluated by two‐way ANOVA or one‐way ANOVA when applicable; individual comparisons were done using the Student's t‐test. A Bonferroni post‐hoc test was applied for the comparison of multiple groups. A one sample t‐test was used to determine whether the sample mean was statistically different from a known chance level. Nonparametric Mann–Whitney U‐test was used to analyze discrete variables. Pearson correlation coefficients between behavioral data and differentially methylated gene expression were properly calculated. Significance values were set to p < 0.05. In all figures and text, data are represented as mean ± SEM. Significant effects were identified using Statistica (StatSoft Software, Oklahoma, USA).
Results
Cognitive impairment in mice chronically treated with WIN during adolescence
In order to evaluate the effect of cannabinoid exposure during adolescence in adulthood, we devised the following experimental paradigm (Fig. S1): adolescent mice were injected daily for 3 weeks with either vehicle or three increasing doses of WIN55212.2. They were then left undisturbed for 5 weeks and at 13 weeks of age, and they were tested for cognitive performance in the Morris water maze and finally fear conditioning task.
All groups performed similarly during the training phase of the water maze, as shown in the escape latency patterns (Fig. 1a). However, two‐way ANOVA analyses for the escape latency revealed a significant main effect for Drug (F(3, 112) = 3.68, p < 0.05), Time (F(7, 112) = 48.90, p < 0.001), but not for the Time × Drug interaction (F(21, 112) = 0.87, NS). Two‐way ANOVA also revealed no significant main effect for either Drug or Time × Drug interaction in the distance traveled (F(3, 112) = 1.55, NS; F(21, 112) = 1.12, NS, respectively) and swim speed (F(3, 112) = 2.12, NS; F(21, 112) = 0.83, NS, respectively), but a significant main effect for Time in both distance traveled and swim speed (F(7, 112) = 67.77, p < 0.001; F(7, 112) = 15.45, p < 0.001, respectively) (Fig. S2a). For the probe test, a one‐sample t‐test against a chance level of 25 percent indicated that control animals spent significantly more than 25 percent of the probe test time in the target quadrant (t(14) = 4.68, p < 0.001) which indicates robust memory for the location of the platform. In the probe test, one‐way ANOVA revealed significant WIN‐treatment effect for the number of target crossings (F(3, 54) = 3.17, p < 0.05) (Fig. 1b), swim speed (F(3, 54) = 4.73, p < 0.01) (Fig. 1c), distance traveled (F(3, 54) = 4.43, p < 0.01) (Fig. 1d), percentage of time in the target quadrant (F(3, 46) = 7,55, p < 0.01) and percentage of time spent in the rest of quadrants (F(3, 46) = 7,55, p < 0.01) (Fig. 1e). In fact, control mice outperformed mice treated with WIN in a dose‐dependent fashion, although the effect was only statistically significant for the 3 mg/kg group, as indicated by a significantly higher number of target crossings (U = 2.15, p < 0.05) (Fig. 1b). Furthermore, those animals subjected to the pharmacological action of either 0.5, 1 or 3 mg/kg of WIN displayed a decrease in the swim speed (cm/second) (t(26) = 3.42; t(28) = 3.09; t(28) = 2.52; p < 0.05, respectively) (Fig. 1c), distance traveled (t(26) = 3.25; t(28) = 3.08; t(28) = 2.52; p < 0.05, respectively) (Fig. 1d) and percent of time in target quadrant (t(21) = 4.22; t(21) = 4.03; t(22) = 5.04; p < 0.001, respectively) (Fig. 1e) in comparison to the control group.
Figure 1.
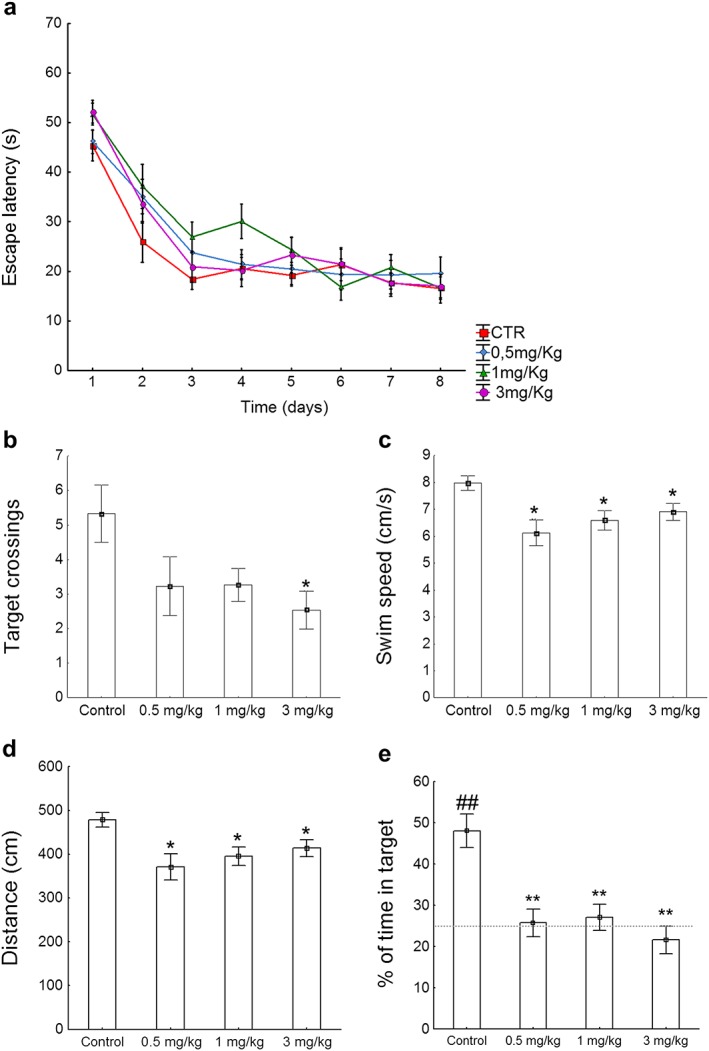
Morris water maze test. (a) Two‐way ANOVA analyses for the escape latency revealed a significant main effect for Drug (F(3, 112) = 3.68, p < 0.05), Time (F(7, 112) = 48.90, p < 0.001), but not for the Time × Drug interaction (F(21, 112) = 0.87, NS) during acquisition. (b–e) For the probe test, one‐way ANOVA revealed a significant main effect for the drug in the number of target crossings (F(3, 54) = 3.17, p < 0.05), swim speed (F(3, 54) = 4.73, p < 0.01), distance traveled (F(3, 54) = 4.43, p < 0.01) and percentage of time in target quadrant (F(3, 54) = 2.54, p < 0.05). (b) Control mice showed a significant number of crossings for the target quadrant when compared to animals treated with 3 mg/kg of WIN (U = 2.15, p < 0.05). Those animals subjected to the pharmacological action of either 0.5, 1 or 3 mg/kg of WIN displayed a decrease in (c) swim speed (cm/second) (t(26) = 3.42; t(28) = 3.09; t(28) = 2.52; p < 0.05, respectively), (d) distance traveled (t(26) = 3.25; t(28) = 3.08; t(28) = 2.52; p < 0.05, respectively) and (e) percent of time in target quadrant (t(21) = 4.22; t(21) = 4.03; t(22) = 5.04; p < 0.001, respectively) in comparison to the control group. One‐sample t‐test against a chance level of 25 percent indicated that control animals spent significantly more than 25 percent of the probe test time in the target quadrant (t(14) = 4.68, p < 0.001), which in turn, indicates robust memory for the location of the platform. Data are expressed as means ± SEM. P values were set as follows: *p < 0.05 and **p < 0.001. N = 15 mice/group
Animals treated with the drug exhibited slower swimming activity during the probe test, but this fact was not attributable to loss of interest (anhedonia) or deficits in general motor activity. Indeed, no obvious differences in the cumulative immobility time were found in the tail suspension test (F(3, 55) = 0.67, NS) (Fig. S3a). There were also no differences in the distance traveled (F(3, 54) = 0.79, NS), time spent in center (F(3, 54) = 0.33, NS), hyperactive behavior (F(3, 54) = 0.54, NS) and time moving (F(3, 54) = 1.04, NS) (Fig. S3b–e).
In order to establish whether the effect of WIN on spatial memory is extensive to associative memory, we tested mice in the fear conditioning paradigm. A one‐way ANOVA revealed significant differences by administration of the drug during context (F(3, 54) = 2.86, p < 0.05) and tone presentation (F(3, 54) = 5.73, p < 0.01) (Fig. 2). Analogous to the effects seen on the Morris water maze, mice showed a dose‐dependent impairment in contextual freezing behavior as indicated: control versus 0.5 mg/kg, t(26) = 2.13, p < 0.05; control versus 1 mg/kg, t(26) = 2.03, p < 0.05; control versus 3 mg/kg, t(26) = 3.23, p < 0.001 (Fig. 2a). The response to the tone (cued fear conditioning) was similarly impaired in those animals treated with either 0.5 (t(26) = 2.65, p < 0.01), 1 (t(28) = 2.73, p < 0.01) or 3 mg/kg (t(28) = 3.76, p < 0.001) of the drug (Fig. 2b).
Figure 2.
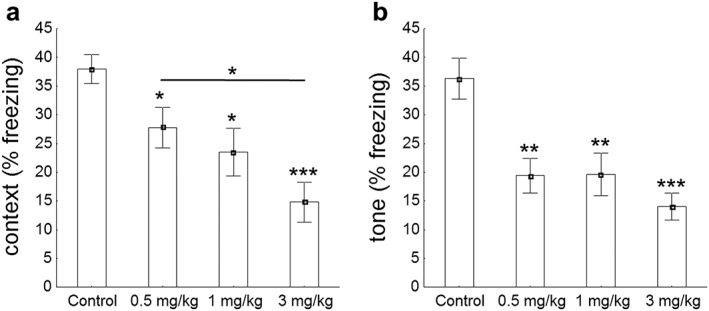
Pavlovian fear conditioning test. (a) Baseline freezing behavior followed a dose‐dependent manner during a contextual fear conditioning paradigm (F(3, 54) = 2.86, p < 0.05). In fact, controls exhibited elevated freezing behavior when compared to either 0.5 (t(26) = 2.13, p < 0.05), 1 (t(26) = 2.03, p < 0.05) or 3 mg/kg of WIN (t(26) = 3.23, p < 0.001). (b) During the tone presentation, ANOVA revealed a significant global effect of the drug (F(3, 54) = 5.73, p < 0.01). Control animals exhibited greater freezing behavior than mice exposed to either 0.5 (t(26) = 2.65, p < 0.01), 1 (t(28) = 2.73, p < 0.01) or 3 mg/kg of WIN (t(28) = 3.76, p < 0.001). Data are expressed as means ± SEM. P values were set as follows: *p < 0.05, **p < 0.01 and ***p < 0.001. N = 15 mice/group
The administration of WIN during adolescence causes a persistent increase in AEA levels in adulthood as well as epigenetic and transcriptional alterations
Next, we examined the levels of endocannabinoids in adult mice long after the exposure to WIN. One‐way ANOVA indicated that there were significant differences in AEA (F(2, 11) = 5.71, p < 0.05) but not in 2‐AG (F(2, 11) = 1.35, NS), PEA (F(2, 9) = 3.50, NS) and OEA (F(2, 10) = 0.25, NS) content (Fig. 3). We found significantly elevated AEA levels in the hippocampus of mice treated with 3 mg/kg of WIN in comparison to controls (t(7) = −2.28, p < 0.05) and 0.5 mg/kg of WIN (t(7) = −2.39, p < 0.05) (Fig. 3a).
Figure 3.
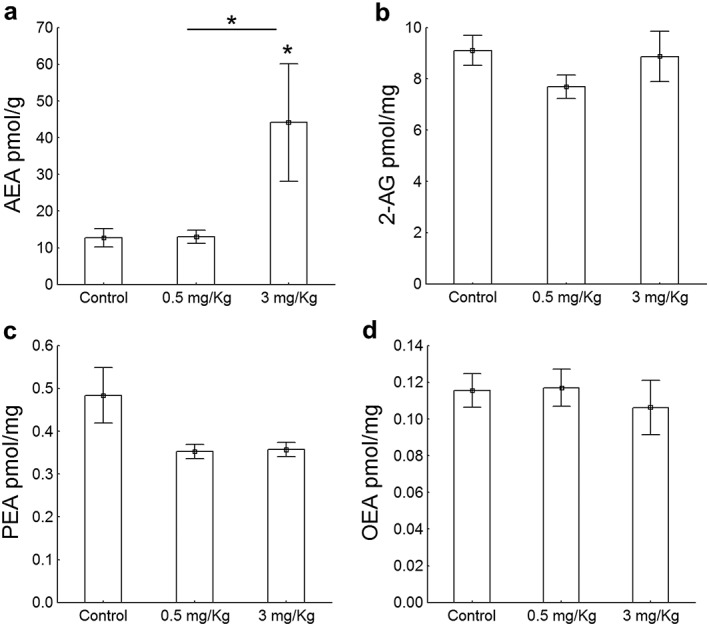
Endocannabinoid and endocannabinoid‐related molecules in hippocampal CA regions. (a) The administration of 3 mg/kg of the drug in adolescent animals increased hippocampal AEA levels in comparison to controls (t(7) = −2.28, p < 0.05) and mice exposed to 0.5 mg/kg of WIN (t(7) = −2.39, p < 0.05) whereas (b–d) the concentration of 2‐AG (F(2, 11) = 1.35, NS), PEA (F(2, 9) = 3.50, NS) and OEA (F(2, 10) = 0.25, NS) were similar in all groups. Data are expressed as means ± SEM. P value was set at 0.05. N = 4 mice/group
Finally, we argued that the remote behavioral and biochemical changes observed in adulthood 5 weeks after drug exposure could be partly because of epigenetic mechanisms. Such mechanisms have been reported in association with drugs of abuse before (for review, see Kalda and Zharkovsky, 2015) and are long‐lasting enough to explain the behavioral impairment described above. We focused on DNAme because it has largely been unexplored in the context of THC, especially as a potential mediator of long‐lasting effects after adolescence exposure. One‐way ANOVA revealed a significant main effect of the drug in the DNA methylation (F(2, 14) = 17.17, p < 0.001) and gene transcription of the candidate gene Rgs7 (F(2, 16) = 4.40, p < 0.05) (Fig. 4). We found an increase in DNA methylation in an intragenic region of Rgs7 gene in those animals treated with 3 mg/kg of WIN when compared to controls (t(10) = −6.08, p < 0.001) and animals treated with 0.5 mg/kg of WIN (t(10) = −4.59, p < 0.01), while animals exposed to low dose of WIN exhibited a similar DNA methylation profile to controls (t(10) = −1.65, NS) (Fig. 4a). Interestingly, for the 3 mg/kg treated animals, we also observed a small but significant decrease in the mRNA levels of Rgs7 (t(10) = 2.05, p < 0.05) indicating a potential causal link to DNA hypermethylation in this locus (Fig. 4b). Strikingly however, we detected a significant increase in Rgs7 mRNA levels in animals treated with the lowest drug dose (t(12) = −2.45, p < 0.05) (Fig. 4b), potentially indicating compensatory effects or independent effector mechanisms for the low and high dosage of the drug. None of the Person correlation coefficients revealed a significant association between behavioral data and differentially methylated gene expression (Fig. S4).
Figure 4.
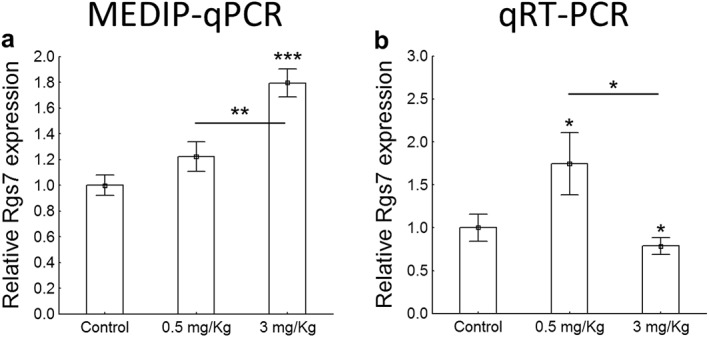
MEDIP‐qPCR and qRT‐PCR. To characterize the role of methylated DNA (DNAme) in CA regions, we examined changes in DNAme for Rgs7 shortly after the last behavioral paradigm. The one‐way ANOVA revealed a significant main effect of the drug in the DNA methylation (F(2, 14) = 17.17, p < 0.001) and gene transcription of the candidate gene Rgs7 (F(2, 16) = 4.40, p < 0.05). (a) We found the DNA hypermethylation at the intragenic Rgs7 region in mice treated with 3 mg/kg of WIN when compared to controls (t(10) = −6.08, p < 0.001) and mice exposed to 0.5 mg/kg of WIN (t(10) = −4.59, p < 0.01), whereas mice exposed to the lowest dose of WIN exhibited a similar DNA methylation profile (t(10) = −1.65, NS) when compared to controls. (b) The administration of 3 mg/kg of WIN decreased the expression of Rgs7 when compared to controls (t(10) = 2.05, p < 0.05). In contrast, mice subjected to 0.5 mg/kg displayed an increase in Rgs7 expression when compared to either controls (t(12) = −2.45, p < 0.05), potentially indicating compensatory effects or independent effector mechanisms for the low and high dosage of the drug. Data are expressed as means ± SEM. P values were set as follows: *p < 0.05, **p < 0.01 and ***p < 0.001. N = 4 mice/group. Rgs7, regulator of G protein signaling 7
Discussion
In mice, postnatal days 35–56 correspond to human adolescent development (Dinieri and Hurd, 2012). Adolescence covers a developmental period of psychosocial, social and biological changes. Under normal physiological conditions in adult mice, CB1 receptors and the levels of endocannabinoid AEA and 2‐AG are abundant in the hippocampus (Di Marzo et al., 2000), a crucial structure involved in learning and memory (for review, see Zanettini et al., 2011).
The regular use of drugs of abuse during adolescence has been associated with alterations in brain structure, function and neurocognition. In fact, teenagers who frequently smoke marijuana suffer dysfunctions in synaptic pruning processes and display worse performance in learning tests, cognitive flexibility, visual scanning, error commission and working memory (Medina et al., 2007a, 2007b; Nagel et al., 2005). Sometimes, the sum of these mostly cognitive changes is named ‘Amotivational Syndrome’, and it can be the result of chronic THC intoxication or long lasting effects of the abuse of THC for longer periods of time, with unknown pathophysiological mechanisms. Therefore, consumption of THC during adolescence has adverse consequences in later life in both human (Rubino and Parolaro, 2008) and animal models (Jacobus and Tapert, 2014; O'Tuathaigh et al., 2010; Rubino et al, 2009; Spear, 2013; Tantra et al., 2014). Adolescent rodents display slower desensitization of CB1 receptors following chronic treatment with WIN than adults (Abush and Akirav, 2012), and this fact might confer vulnerability to turn into long‐lasting effects. The current use of more potent CB1 agonists, such as the many synthetic cannabinoids found in the so‐called spice drugs (Brents et al., 2011; Schneir et al., 2012), which have potency and efficacy higher than THC and comparable to WIN, the drug used in the present study, might lead to even more dramatic consequences.
In line with the present findings, long‐term administration of THC during adolescence causes persistent impairments in short‐term memory in rats (Abush and Akirav, 2012) and deficits in fear conditioning during adulthood in mice (Gleason et al., 2012). The administration of marijuana‐derived compounds interferes with hippocampal inhibitory synapses and causes dysfunctions in cognition and recall (Wilson and Nicoll 2002). In particular, the eCB system is involved in memory acquisition, consolidation and retrieval of fear (Ruehle et al., 2012). The elevation of the content of AEA in the hippocampus is also associated with deficits in learning and memory (Basavarajappa et al., 2014). In agreement with this data, we found that animals exposed to 3 mg/kg of WIN displayed impairment in both spatial and associative memory with a concomitant increase in the levels of AEA in the CA hippocampal subregion. However, none of the Pearson's comparisons revealed a significant associative effect between behavioral and differentially methylated gene expression. In order to validate the consequences of the drug WIN at 3 mg/kg on both spatial and associative memory, further investigation is required in the model. The fact that we observe differences in the probe trial but not during acquisition underpins a specific effect of the drug on memory recall as previously described (Wilson and Nicoll 2002).
Repeated exposure to psychostimulants induces epigenetic changes including histone modification, DNA methylation and noncoding RNAs and concomitantly behavioral dysfunctions (for review, see Kalda and Zharkovsky, 2015). RGS proteins are potent negative regulators of neurotransmitter signaling via G protein‐coupled receptors (Sjögren, 2011; Xie and Martemyanov, 2011). Among the G‐protein coupled receptors, the cannabinoid CB1 receptor is highly expressed in hippocampal CA regions (Wang et al., 2003). Furthermore, several RGS proteins are regulated in response to a different stimulus, e.g. stress, drug exposure, changes in neurotransmitter signaling, etc. (Holden et al., 2014; Kach et al., 2012). In particular, RGS7 plays key roles in the regulation of several biological processes such as vision, memory, motor control, reward behavior and nociception (Anderson et al., 2009; Orlandi et al., 2015). Although the mechanisms of this regulation remain to be elucidated, we demonstrated that long‐term administration of 3 mg/kg of WIN during mouse adolescence induces DNA hypermethylation at the intragenic Rgs7 region and concomitantly decreases the rate of transcription, potentially contributing at least in part to the learning and memory deficits as depicted in Fig. 5. The differential dose‐dependent response reported in this manuscript is in line with our previous results (Tomas‐Roig et al., 2016). In brief, Tomas‐Roig and colleagues (2016) found that low dose of synthetic cannabinoid agonist possesses a neuroprotective like effect while high dose of the drug has a deleterious effect in the murine model of demyelination by cuprizone feeding.
Figure 5.
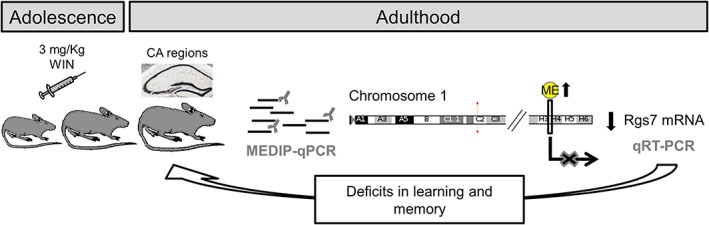
Working hypothesis. Long‐term administration of 3 mg/kg of WIN during mouse adolescence induces DNA hypermethylation at the intragenic Rgs7 region and concomitantly decreases the rate of transcription, potentially contributing at least in part to the learning and memory deficits in young adult animals. ME, DNA methylation; Rgs7, regulator of G protein signaling 7
Ostrovskaya and colleagues (2014) determined that Rgs7−/− mice exhibited augmented GABABR signaling in the hippocampus which resulted in selective deficits in LTD and depotentiation, with normal LTP. LTP, LTD and depotentiation are distinct forms of synaptic plasticity implicated in hippocampal learning and memory‐related processes (Martin et al., 2000). Indeed, Rgs7 controls synaptic plasticity while the lack of Rgs7 leads to a disruption of learning and memory (Ostrovskaya et al., 2014). In line with this evidence, we observed deficits in hippocampus‐dependent learning and memory processes attributable potentially in part to declined gene expression levels of Rgs7 in young adults exposed to 3 mg/kg of WIN during adolescence. Because all animals, including vehicle‐treated animals, were subjected to the same behavioral battery and were handled exactly like drug‐injected animals, we are confident that the effects on endocannabinoid and DNAme are associated with the drug treatment.
Overall, we have shown that treatment with the synthetic cannabinoid WIN during adolescence causes persistent alterations in two behavioral paradigms during adulthood: the Morris water maze and the fear conditioning test. We have also shown that these behavioral abnormalities are accompanied by a persistent increase in AEA levels in the CA region of the hippocampus. Finally, we found DNA hypermethylation at the Rgs7 locus, alongside underregulation of the mRNA levels for the same gene. Although the matter begs for further investigation, we hypothesize that at least part of the persistent effects of the drug may be driven by changes in DNA methylation at specific loci within the hippocampus.
Supporting information
Figure S1. Schematic drawing of the experiment. Adolescent mice aged 5 weeks were single daily subjected to vehicle (control) or WIN55212.2 for 3 weeks (treated), then left undisturbed at their home cage for a 5‐week period (Withdrawal of WIN treatment) and finally evaluated in Morris water maze and Pavlovian fear conditioning tests (n = 15 mice/group). After behavioral testing, the hippocampal CA regions (≈ bregma −1.64 and interaural 2.16 mm) were freshly isolated and frozen in liquid nitrogen for LC‐MS, MEDIP‐qPCR and quantitative RT‐PCR (qRT‐PCR). N = 4 mice/group
Figure S2. Acquisition and memory phase in Morris Water Maze paradigm. The locomotor activity during acquisition was measured by the distance traveled and swim speed while percent of time in target was recorded during probe phase as a proof of learning memory. Briefly, all mice were subjected to contextual‐associative learning for 8 days (acquisition) and 24 hours after the last training session, mice memory was tested (probe test). Two‐way ANOVA revealed no significant main effect for either Drug or Time × Drug interaction in (a) the distance traveled (F(3, 112) = 1.55, NS; F(21, 112) = 1.12, NS, respectively) and (b) swim speed (F(3, 112) = 2.12, NS; F(21, 112) = 0.83, NS, respectively) during acquisition phase. (c) For the probe test, one‐way ANOVA indicates a significant global effect of the drug in the percentage of time spent in the target quadrant and also in the remaining quadrants (F(3, 46) = 7,55, p < 0.01). In fact, those animals subjected to the pharmacological action of either 0.5, 1 or 3 mg/kg of WIN displayed a decrease in the percent of time in target quadrant (t(21) = 4.22; t(21) = 4.03; t(22) = 5.04; p < 0.001, respectively) when compared to controls. Furthermore, one‐sample t‐test against a chance level of 25 percent indicated that control animals spent significantly more than 25 percent of the probe test time in the target quadrant (t(14) = 4.68, p < 0.001) which in turn, indicates robust memory for the location of the platform. An * indicates significant differences in the percentage of time in target between WIN‐treated groups and their respective controls. Level of significance in one‐sample t‐test against a chance level of 25 percent is represented by #. The significant differences in the percentage of time spent in other quadrants between WIN‐treated animals and the control group are indicated by a +. Two symbols P value indicated p < 0.001. Data are expressed as means ± SEM. N = 15 mice/group
Figure S3. General motor activity and anhedonia behavior. A distinct cohort of mice were subjected to identical experimental procedure and then evaluated by tail suspension (TS) and open field (OF) paradigm. (a) The loss of mice interest (anhedonia) was assessed by the cumulative immobility time in the TS. (b–e) Mice were allowed to explore the open field arena for 10 minutes without habituation while locomotor and exploratory activities were registered. One‐way ANOVA did not reveal significant global effects for WIN‐treatment in (a) the cumulative time immobile (F(3, 55) = 0.67, NS), (b) total distance traveled in center (F(3, 54) = 0.79, NS), (c) time spent in center (F(3, 54) = 0.33, NS), (d) hyperactivity (F(3, 54) = 0.54, NS) and (e) time moving (F(3, 54) = 1.04, NS). Data are expressed as means ± SEM. N = 15 mice/group
Figure S4. Scatter plot and correlation analysis. The degree of association between behavioral and diferentialy methylated Rgs7 gene expression was determined by use of Pearson's correlation coefficient. (a) Scatter plot was used to illustrate the correlation between the variables. (b) Contextual and cued fear conditioning‐related variables as well as (c) general motor activity and anhedonia behavior data were correlated with differentialy methylated Rgs7 rate. None of the comparisons analysed by Pearson's correlation test were significant (See panel b and c). CTR, control; Low, mice treated with 0.5 mg/kg of WIN; High, mice treated with 3 mg/kg of WIN. N = 15 mice/group
Sup info item
Sup info item
Sup info item
Sup info item
Acknowledgements
The authors would like to thank Dr. Rashi Halder (German Center for Neurodegenerative Diseases, Göttingen, Germany) for excellent technical advice.
Funding and Disclosure
The work was supported by DFG Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB) and the German‐Research‐Foundation (DFG) grant CNMPB C1‐6 to UHR. RCAB was supported by a German‐Research‐Foundation (DFG) grant (DFG 179/1‐1) and by a Ramón y Cajal grant (RYC‐2014‐15246).
Conflicts of Interest
The author reports no potential conflicts of interest.
Author Contribution
JTR initiated the study and designed the experiments. RCAB technically supported JTR in animal testing. FP and VDM contributed to LC‐MS data analysis. EB and JTR performed MEDIP‐qPCR. JTR and SHF performed quantitative RT‐PCR. JTR and EB were responsible for the analysis of the data and wrote the manuscript. JTR, EB, RCAB, FP, SHY, VDM and UHR contributed to discussion and reviewed/edited manuscript.
Tomas‐Roig, J. , Benito, E. , Agis‐Balboa, R. C. , Piscitelli, F. , Hoyer‐Fender, S. , Di Marzo, V. , and Havemann‐Reinecke, U. (2017) Chronic exposure to cannabinoids during adolescence causes long‐lasting behavioral deficits in adult mice. Addiction Biology, 22: 1778–1789. doi: 10.1111/adb.12446.
References
- Abush H, Akirav I (2012) Short‐ and long‐term cognitive effects of chronic cannabinoids administration in late‐adolescence rats. PLoS One 7(2):e31731. DOI: 10.1371/journal.pone.0031731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis‐Balboa RC, Fischer A (2014) Generating new neurons to circumvent your fears: the role of IGF signaling. Cellular and Molecular Life Sciences 71:21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Posokhova E, Martemyanov KA (2009) The R7 RGS protein family: multi‐subunit regulators of neuronal G protein signaling. Cell Biochemistry and Biophysics 54:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Nagre NN, Xie S, Subbanna S (2014) Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus 24:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Piscitelli F, Marzo V Di (2008). Lipidomic methodologies applicable to the study of endocannabinoids and related compounds: endocannabinoidomics. 111: 53. DOI: 10.1002/ejlt.200800233
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL (2011) Phase I hydroxylated metabolites of the k2 synthetic cannabinoid jwh‐018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 6(7):e21917. DOI: 10.1371/journal.pone.0021917. [Epub 2011 Jul 6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozka MM, Fischer A, Falkai P, Havemann‐Reinecke U (2011) Acute treatment with cannabinoid receptor agonist WIN55212.2 improves prepulse inhibition in psychosocially stressed mice. Behavioural Brain Research 218:280–7. [DOI] [PubMed] [Google Scholar]
- D'Addario C, Francesco AD, Pucci M, Finazzi Agrò A, MacCarrone M (2013) Epigenetic mechanisms and endocannabinoid signalling. FEBS Journal 280:1905–1917. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, et al (2000) Levels, metabolism, and pharmacological activity of anandamide in CB 1 cannabinoid receptor knockout mice: actions of anandamide in mouse brain. Journal of Neurochemistry 75:2434–2444. [DOI] [PubMed] [Google Scholar]
- Dinieri JA, Hurd YL (2012) Rat models of prenatal and adolescent cannabis exposure. Methods in Molecular Biology 829:231–242. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA (2010) Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cerebral Cortex 20:1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason K a, Birnbaum SG, Shukla a, Ghose S (2012) Susceptibility of the adolescent brain to cannabinoids: long‐term hippocampal effects and relevance to schizophrenia. Translational Psychiatry 2:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T (2011) Depression‐related anhedonic behaviors caused by immobilization stress: a comparison with nicotine‐induced depression‐like behavioral alterations and effects of nicotine and/or “antidepressant” drugs. Journal of Toxicological Sciences 36:31–41. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve‐Roperh I, Berghuis P, Devi LA, Mackie K (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences 28:83–92. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabás K, Mulder J (2008) Endocannabinoid functions controlling neuronal specification during brain development. Molecular and Cellular Endocrinology 286(1–2 Suppl 1):S84–90. DOI: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Hoch E, Bonnetn U, Thomasius R, Ganzer F, Havemann‐Reinecke U, Preuss UW (2015) Risks associated with the non‐medicinal use of cannabis. Deutsches Ärzteblatt International 112:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden NS, George T, Rider CF, Chandrasekhar A, Shah S, Kaur M, et al (2014) Induction of regulator of G‐protein signaling 2 expression by long‐acting b 2‐adrenoceptor agonists and glucocorticoids in human airway epithelial cells s. J Pharmacol Exp Ther J Pharmacol Exp Ther 348:12–24. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF (2014) Effects of cannabis on the adolescent brain. Current Pharmaceutical Design 20:2186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kach J, Sethakorn N, Dulin NO (2012) A finer tuning of G‐protein signaling through regulated control of RGS proteins. AJP Hear Circ Physiol 303:H19–H35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalda A, Zharkovsky A (2015) Epigenetic mechanisms of psychostimulant‐induced addiction. International Review of Neurobiology 120:85–105. [DOI] [PubMed] [Google Scholar]
- Kilb W (2012) Development of the GABAergic system from birth to adolescence. Neurosci 18:613–630. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annual Review of Neuroscience 649–711. DOI: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Marzo VD (2008) Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Molecular and Cellular Endocrinology 286(1–2 Suppl 1): S66–78. DOI: 10.1016/j.mce.2008.01.026 [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen‐Zion M, Nagel BJ, Tapert SF (2007a) Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society 13:807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen‐Zion M, Nagel BJ, Tapert SF (2007b) Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology 29:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Nakamura EF, Kessler RC (2009) Epidemiology of mental disorders in children and adolescents. Dialogues in Clinical Neuroscience 11:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF (2005) Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res—Neuroimaging 139:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P (2010) Adolescent maturation of cortical dopamine. Neurotoxicity Research 18:306–312. [DOI] [PubMed] [Google Scholar]
- Orlandi C, Xie K, Masuho I, Fajardo‐Serrano A, Lujan R, Martemyanov K a (2015) Orphan receptor GPR158 is an allosteric modulator of RGS7 catalytic activity with an essential role in dictating its expression and localization in the brain. Journal of Biological Chemistry 290:13622–13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovskaya O, Xie K, Masuho I, Fajardo‐Serrano A, Lujan R, Wickman K, et al (2014) RGS7/Gβ5/R7BP complex regulates synaptic plasticity and memory by modulating Hippocampal GABABR‐GIRK signaling. Elife 3:e02053. DOI: 10.7554/eLife.02053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K (2006) Neurodevelopment: how does the teenage brain work? Nature 442:865–867. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Jay TM, Pen GL (2013) Long‐term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: a strain comparison. Psychopharmacology 225:781–790. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Pen GL, Jay TM (2014) Long‐term consequences of adolescent cannabinoid exposure in adult psychopathology. Frontiers in Neuroscience 8:361 DOI: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A, Bayerlein K, Hansbauer M, Weiland J, Sperling W, Kornhuber J, et al (2012) CB1 and cb2 receptor expression and promoter methylation in patients with cannabis dependence. European Addiction Research 19:13–20. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2008) Long lasting consequences of cannabis exposure in adolescence. Molecular and Cellular Endocrinology 286:. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, et al (2009) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19:763–772. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Rey AA, Remmers F, Lutz B (2012) The endocannabinoid system in anxiety, fear memory and habituation. Journal of Psychopharmacology 26:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M (2013) Adolescence as a vulnerable period to alter rodent behavior. Cell and Tissue Research 354:99–106. [DOI] [PubMed] [Google Scholar]
- Schneir AB, Baumbacher T (2012) Convulsions associated with the use of a synthetic cannabinoid product. Journal of Medical Toxicology 8:62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B (2011) Regulator of G protein signaling proteins as drug targets: current state and future possibilities. Advances in Pharmacology 62:315–347. DOI: 10.1016/B978-0-12-385952-5.00002-6 [DOI] [PubMed] [Google Scholar]
- Spear LP (2013) Adolescent neurodevelopment. J Adolesc Heal 52(2 Suppl 2):S7–13. DOI: 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Hurd YL (2015) Epigenetic effects of cannabis exposure. Biological Psychiatry 79(7):586–594. DOI: 10.1016/j.biopsych.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantra M, Kröcher T, Papiol S, Winkler D, Röckle I, Jatho J, et al (2014) St8sia2 deficiency plus juvenile cannabis exposure in mice synergistically affect higher cognition in adulthood. Behavioural Brain Research 275:166–175. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL (2012) Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological Psychiatry 72:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas‐Roig J, Wirths O, Salinas‐Riester G, Havemann‐Reinecke U (2016) The cannabinoid CB1/CB2 agonist WIN55212.2 promotes oligodendrocyte differentiation in vitro and neuroprotection during the cuprizone‐induced central nervous system demyelination. CNS Neuroscience and Therapeutics DOI: 10.1111/cns.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta L, Zhang Y (2014) Mechanisms of epigenetic memory and addiction. EMBO Journal 33:1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dow‐Edwards D, Keller E, Hurd YL (2003) Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118:681–694. [DOI] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, et al (2015) Genome‐wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross‐generational effects of adolescent THC exposure. Neuropsychopharmacology 40:2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–82. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Sengoku T (2013) Developmental regulation of neuronal genes by DNA methylation: environmental influences. International Journal of Developmental Neuroscience 31:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Martemyanov K (2011) Control of striatal signaling by g protein regulators. Frontiers in Neuroanatomy 5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S (2011) Effects of endocannabinoid system modulation on cognitive and emotional behavior. Frontiers in Behavioral Neuroscience 5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic drawing of the experiment. Adolescent mice aged 5 weeks were single daily subjected to vehicle (control) or WIN55212.2 for 3 weeks (treated), then left undisturbed at their home cage for a 5‐week period (Withdrawal of WIN treatment) and finally evaluated in Morris water maze and Pavlovian fear conditioning tests (n = 15 mice/group). After behavioral testing, the hippocampal CA regions (≈ bregma −1.64 and interaural 2.16 mm) were freshly isolated and frozen in liquid nitrogen for LC‐MS, MEDIP‐qPCR and quantitative RT‐PCR (qRT‐PCR). N = 4 mice/group
Figure S2. Acquisition and memory phase in Morris Water Maze paradigm. The locomotor activity during acquisition was measured by the distance traveled and swim speed while percent of time in target was recorded during probe phase as a proof of learning memory. Briefly, all mice were subjected to contextual‐associative learning for 8 days (acquisition) and 24 hours after the last training session, mice memory was tested (probe test). Two‐way ANOVA revealed no significant main effect for either Drug or Time × Drug interaction in (a) the distance traveled (F(3, 112) = 1.55, NS; F(21, 112) = 1.12, NS, respectively) and (b) swim speed (F(3, 112) = 2.12, NS; F(21, 112) = 0.83, NS, respectively) during acquisition phase. (c) For the probe test, one‐way ANOVA indicates a significant global effect of the drug in the percentage of time spent in the target quadrant and also in the remaining quadrants (F(3, 46) = 7,55, p < 0.01). In fact, those animals subjected to the pharmacological action of either 0.5, 1 or 3 mg/kg of WIN displayed a decrease in the percent of time in target quadrant (t(21) = 4.22; t(21) = 4.03; t(22) = 5.04; p < 0.001, respectively) when compared to controls. Furthermore, one‐sample t‐test against a chance level of 25 percent indicated that control animals spent significantly more than 25 percent of the probe test time in the target quadrant (t(14) = 4.68, p < 0.001) which in turn, indicates robust memory for the location of the platform. An * indicates significant differences in the percentage of time in target between WIN‐treated groups and their respective controls. Level of significance in one‐sample t‐test against a chance level of 25 percent is represented by #. The significant differences in the percentage of time spent in other quadrants between WIN‐treated animals and the control group are indicated by a +. Two symbols P value indicated p < 0.001. Data are expressed as means ± SEM. N = 15 mice/group
Figure S3. General motor activity and anhedonia behavior. A distinct cohort of mice were subjected to identical experimental procedure and then evaluated by tail suspension (TS) and open field (OF) paradigm. (a) The loss of mice interest (anhedonia) was assessed by the cumulative immobility time in the TS. (b–e) Mice were allowed to explore the open field arena for 10 minutes without habituation while locomotor and exploratory activities were registered. One‐way ANOVA did not reveal significant global effects for WIN‐treatment in (a) the cumulative time immobile (F(3, 55) = 0.67, NS), (b) total distance traveled in center (F(3, 54) = 0.79, NS), (c) time spent in center (F(3, 54) = 0.33, NS), (d) hyperactivity (F(3, 54) = 0.54, NS) and (e) time moving (F(3, 54) = 1.04, NS). Data are expressed as means ± SEM. N = 15 mice/group
Figure S4. Scatter plot and correlation analysis. The degree of association between behavioral and diferentialy methylated Rgs7 gene expression was determined by use of Pearson's correlation coefficient. (a) Scatter plot was used to illustrate the correlation between the variables. (b) Contextual and cued fear conditioning‐related variables as well as (c) general motor activity and anhedonia behavior data were correlated with differentialy methylated Rgs7 rate. None of the comparisons analysed by Pearson's correlation test were significant (See panel b and c). CTR, control; Low, mice treated with 0.5 mg/kg of WIN; High, mice treated with 3 mg/kg of WIN. N = 15 mice/group
Sup info item
Sup info item
Sup info item
Sup info item


