Abstract
Dolutegravir (DTG) is approved in the United States to treat HIV‐1‐infected patients weighing ≥30 kg. A dispersible DTG tablet formulation was recently developed for pediatric patients. This study compares the pharmacokinetics (PK) of the dispersible tablet with that of a previously evaluated granule formulation. In this randomized, open‐label, crossover study, 15 healthy adults received single oral doses of DTG 20 mg every 7 days across 5 treatment arms: granules consumed immediately after mixture with purified water, dispersible DTG consumed immediately after reconstitution in low‐mineral‐content (LMC) or high‐mineral‐content (HMC) water, and dispersible DTG consumed 30 minutes after dispersal in LMC or HMC water. Primary endpoints were bioavailability of immediately consumed dispersible tablet in LMC water relative to granule formulation reconstituted in purified water and PK of the dispersible tablet. Secondary endpoints included tolerability and palatability. The DTG dispersible tablet showed equivalent exposures to the granule formulation with geometric least‐squares mean treatment ratios of 1.06 and 1.12 for AUC0‐∞ and Cmax, respectively. DTG PK parameters were unaffected by mineral content or the 30‐minute delay. Adverse events were mild; only nausea (n = 1) was considered drug related. DTG exposure observed with the dispersible tablet supports evaluation of this formulation for further development.
Keywords: bioavailability, dispersible tablet, dolutegravir, granule, pediatric
The World Health Organization reports that 2.6 million children less than 15 years of age were living with HIV‐1 in 2014.1 Although therapeutic options for this patient population have improved, additional pediatric formulations of antiretroviral agents are greatly needed.2 Dolutegravir (DTG; Tivicay®; ViiV Healthcare, Research Triangle Park, North Carolina) is an integrase strand transfer inhibitor (INSTI) approved for the treatment of HIV‐1–infected individuals weighing at least 30 kg in the United States, with applications pending in other countries.3 DTG has a high barrier to the development of viral resistance, low to moderate pharmacokinetic (PK) variability, and a 14‐hour half‐life that supports once‐daily dosing without the need of a boosting agent.4 Studies determining the efficacy and safety of DTG in HIV‐infected infants and young children are currently ongoing (International Maternal Pediatric Adolescent AIDS Clinical Trials Network [IMPAACT] protocol P1093), and alternative DTG formulations are in development with the aim of improving the ease of administration in younger patients with HIV‐1.5, 6
The PK and safety of a pediatric granule formulation of DTG were previously studied in an adult relative bioavailability study7 and are currently being evaluated in pediatric patients in Study P1093 (ClinicalTrials.gov, NCT01302847). An alternative option for DTG administration with the potential for improved palatability in children may include a dispersible tablet formulation that can be dispersed in water prior to administration. The objective of the present study was to compare the PK and relative bioavailability of a newly developed dispersible tablet formulation with the former pediatric granule formulation. Mechanistically, INSTI drugs such as DTG exert their antiviral activity by chelating magnesium ions required for the enzymatic insertion of HIV viral DNA into the host genome.8, 9 Previous drug interaction studies have demonstrated that concomitant administration of INSTIs with divalent or trivalent metal cation‐containing products, such as antacids, calcium, and iron supplements, result in a significant reduction in INSTI plasma exposure as a result of metal chelation and reduced absorption.9, 10, 11, 12 Chemically, DTG is a weak acid and is formulated as a sodium salt for improved solubility in water. When dispersed in liquid, DTG sodium can dissociate to the corresponding free acid form over time, possibly lowering DTG bioavailability. Therefore, this study was conducted to evaluate the PK of the dispersible tablet when dispersed for varying lengths of time in low‐mineral‐content water (LMC) or high‐mineral‐content (HMC) water to encompass the levels of minerals seen in the majority of water types readily available either as potable or bottled supply.
Subjects and Methods
Study Population
Adults (ages 18‐65 years) were eligible to enroll in the study if they were determined to be healthy based on a physical examination, medical history, laboratory testing, and cardiac monitoring. Participating females were of nonchildbearing potential, had same‐sex partners, or agreed to use one of the approved contraception methods prior to dosing and 5 days after their last dose. Key exclusion criteria included a positive test for HIV antibody, hepatitis C antibody, or hepatitis B surface antigen; a positive illicit drug result, regular tobacco use, or alcohol consumption; current or chronic history of liver disease; and use of any prescription or nonprescription drugs, including vitamins or herbal products, within 7 to 14 days before the first dose and throughout the study (with the exception of acetaminophen at doses of ≤2 g/day). Pregnant or lactating females were also excluded from the study. Use of antacids, vitamins, and calcium or iron supplements was not allowed from 24 hours prior to the first dose of study medication and for the duration of the study.
Study Design
This was a phase 1, single‐center, randomized, open‐label, 5‐period crossover study to evaluate the PK and relative bioavailability of the dispersible DTG 5‐mg tablet formulation compared with the pediatric granule formulation. To evaluate each preparation, a Latin square design was applied, with participants randomly allocated to 1 of 5 blocks containing 5 single‐dose DTG 20 mg treatments as a (A) pediatric granule formulation reconstituted with purified water for immediate consumption, (B) dispersible tablet formulation dispersed in LMC water for immediate consumption, (C) dispersible tablet formulation dispersed in HMC water for immediate consumption, (D) dispersible tablet formulation dispersed in LMC water for consumption following a 30‐minute hold and resuspension, or (E) dispersible tablet formulation dispersed in HMC water for consumption following a 30‐minute hold and resuspension (Table 1). The DTG granule suspension (treatment A) is available as a 1.6‐mg/mL dose and was provided as a dose of 12.5 mL, equivalent to 20 mg. The DTG 5‐mg dispersible tablet is designed to be dispersed in 2 to 5 mL of water per tablet. Each 20‐mg dose was given with a total of 12.5 mL of either LMC or HMC water (treatments B‐D). Contrex® water (Nestlé Waters, Noisiel, France) containing high levels of calcium and magnesium, was used for HMC water; LMC water consisted of 5% Contrex in purified water. All treatments were orally administered on an empty stomach in the morning; food intake was prohibited for 4 hours following administration. Each treatment arm was separated by at least a 7‐day washout period. Serial PK samples were collected within 48 hours of study drug. The total study duration was approximately 10 weeks, including screening and follow‐up.
Table 1.
Five DTG Treatments
| Treatment | Formulation | Solvent | Consumption |
|---|---|---|---|
| A | DTG pediatric granule | Purified water | Immediate |
| B | DTG dispersible tablet | LMC water | Immediate |
| C | DTG dispersible tablet | HMC water | Immediate |
| D | DTG dispersible tablet | LMC water | 30‐minute delay |
| E | DTG dispersible tablet | HMC water | 30‐minute delay |
DTG, dolutegravir; HMC, high mineral content; LMC, low mineral content.
The study was performed at Quintiles Phase One Services (Overland Park, Kansas) in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants, and the protocol was approved by the MidLands Independent Review Board (Overland Park, Kansas). The trial is registered on ClinicalTrials.gov (NCT02185300).
Study Assessments
The primary endpoints of this study were (1) bioavailability of DTG 20 mg administered as 4 5‐mg dispersible tablets in LMC water (immediate ingestion) relative to the pediatric granule reconstituted with purified water, (2) single‐dose PK of the dispersible DTG as 4 5‐mg tablets dispersed in either HMC or LMC water, and (3) single‐dose PK of the dispersible DTG as 4 5‐mg tablets dispersed with LMC water and consumed after 30 minutes compared with the same dose consumed immediately after dispersal. Key secondary endpoints included safety, tolerability, and palatability of the oral DTG dispersible formulation.
PK parameters and tolerability were assessed in all study subjects. PK samples were collected predose and 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 12, 24, and 48 hours postdose in each dosing period. Plasma DTG area under the plasma concentration‐time curve from time of dose extrapolated to infinity (AUC0‐∞), maximum observed concentration (Cmax), and apparent oral clearance (CL/F) were primary PK parameters. Secondary PK parameters included plasma DTG area under the plasma concentration‐time curve from time of dose administration to time of last quantifiable postdose sample (AUC0‐τ), observed concentration at 24 hours postdose (C24), terminal elimination phase half‐life (t1/2), lag time for absorption (tlag), and time to maximum observed concentration (tmax). DTG was measured in plasma samples using a validated analytical method based on protein precipitation, followed by high‐performance liquid chromatography–tandem mass spectrometry analysis.4
Adverse events (AEs), clinical laboratory tests, vital signs, electrocardiogram results, and physical examinations were included in the tolerability assessments. Adverse events and serious AEs (SAEs) were assessed from the start of study treatment and for the duration of the study. A follow‐up visit occurred at least 7 days after the last dose of the study drug.
Taste was assessed with a palatability questionnaire. Questionnaires addressed bitterness, sweetness, color, mouth feel, and overall taste and were administered to volunteers within 10 minutes after dosing. Data were summarized descriptively.
Bioanalytical Methods
Plasma samples were analyzed for DTG by PPD Bioanalytical Laboratory (Middleton, Wisconsin) using a previously published validated liquid chromatography and tandem mass spectrometric method with 2 modifications: the mobile phase consisted of 40% acetonitrile (vs 39%) and the internal standard masses monitored were 428 to 277.13 The analytical runs for this study met all predefined run acceptance criteria. The bias for the analysis of plasma was −3.98% to 2.24% and precision was ≤14.5%.
Statistical Analysis
No formal hypothesis was tested. The sample size of 15 subjects to obtain at least 10 evaluable subjects was chosen based on the expected withdrawal rate and the within‐subject variability of DTG. In a previous study the within‐subject variability of DTG granules AUC and Cmax ranged from 15% to 16.2%; therefore, sample size calculation was based on the conservative estimate of 16.2%.7 Plasma DTG concentration‐time data were analyzed using noncompartmental methods with Phoenix WinNonlin (Certara USA, Inc, Princeton, New Jersey); geometric least‐squares (GLS) mean ratios (test/reference) and 95% confidence intervals (CIs) were generated by the mixed‐effects model for treatment comparisons. Values below the limit of quantification were considered 0 for calculation of means. Descriptive summaries were used for continuous variables, and number of patients and percentage were used as summary statistics for categorical variables, unless otherwise stated.
Results
Baseline Demographics
A total of 15 subjects were enrolled in the study (Table 2). The mean age of subjects was 39.8 years (standard deviation, 12.5 years). The majority of subjects were male (73%) and white (80%). The mean body mass index was 26.0 kg/m2 (standard deviation 2.6 kg/m2).
Table 2.
Summary of Subject Demographics
| Demographic | Total (N=15) |
|---|---|
| Age, y, mean ± SD | 39.8 ± 12.5 |
| Male, n (%) | 11 (73) |
| BMI, kg/m2, mean ± SD | 26.0 ± 2.6 |
| Height, cm, mean ± SD | 174.6 ± 13.1 |
| Weight, kg, mean ± SD | 79.6 ± 14.0 |
| Race, n (%) | |
| White/European | 11 (73) |
| African American/black | 2 (13) |
| Japanese/East Asian/Southeast Asian | 1 (7) |
| White/North African | 1 (7) |
BMI, body mass index; SD, standard deviation.
Pharmacokinetics
The plasma concentration‐time profiles of DTG after administration are presented in Figure 1, and a summary of the PK parameters is shown in Table 3.
Figure 1.
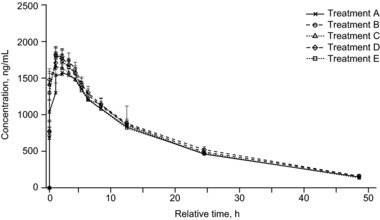
Arithmetic mean (SEM) plasma DTG concentration over time. Treatment A, DTG pediatric granules in purified water and immediately consumed; treatment B, DTG dispersible tablet dispersed in LMC water and immediately consumed; treatment C, DTG dispersible tablet dispersed in HMC water and immediately consumed; treatment D, DTG dispersible tablet dispersed in LMC water with a 30‐minute delay before consumption; treatment E, DTG dispersible tablet dispersed in HMC water with a 30‐minute delay before consumption. DTG, dolutegravir; HMC, high mineral content; LMC, low mineral content; SEM, standard error of the mean.
Table 3.
Summary of Plasma DTG PK Parameters
| Treatment Armsa | |||||
|---|---|---|---|---|---|
| PK Parameterb | Treatment A DTG 20‐mg Pediatric Granule Purified, 0 min (n=15) | Treatment B DTG 20‐mg Dispersible Tablet LMC Water, 0 Min (n=15) | Treatment C DTG 20‐mg Dispersible Tablet HMC Water, 0 Min (n=15) | Treatment D DTG 20‐mg Dispersible Tablet LMC Water, 30 Min (n=15) | Treatment E DTG 20‐mg Dispersible Tablet HMC Water, 30 Min (n=15) |
| AUC0‐∞ (μg·h/mL) | 31.65 (8.11) | 33.82 (9.15) | 31.89 (8.51) | 34.86 (10.08) | 33.23 (8.38) |
| AUC0‐τ | 28.41 (6.48) | 30.36 (7.42) | 28.78 (7.42) | 30.17 (8.20) | 29.79 (6.99) |
| Cmax (μg/mL) | 1.80 (0.38) | 2.04 (0.46) | 1.87 (0.44) | 2.01 (0.44) | 1.95 (0.39) |
| C24 (μg/mL) | 0.47 (0.13) | 0.49 (0.16) | 0.47 (0.15) | 0.52 (0.15) | 0.47 (0.14) |
| CL/F (L/h) | 0.67 (0.16) | 0.63 (0.16) | 0.67 (0.16) | 0.62 (0.16) | 0.64 (0.15) |
| t1/2 (h) | 14.31 (2.39) | 14.43 (2.72) | 14.22 (2.63) | 14.69 (2.28) | 14.53 (2.55) |
| tmax (h)c | 2.00 (0.50, 5.00) | 1.00 (0.50, 4.00) | 1.00 (0.25, 2.50) | 1.50 (0.50, 2.52) | 1.50 (0.25, 2.50) |
AUC0‐∞, area under the plasma concentration‐time curve from time of dose extrapolated to infinity; AUC0‐τ, area under the plasma concentration‐time curve from time of dose administration to time of last quantifiable postdose sample; C24, observed concentration at 24 hours postdose; CL/F, apparent oral clearance; Cmax, maximum observed concentration; DTG, dolutegravir; HMC, high mineral content; LMC, low mineral content; PK, pharmacokinetic; SD, standard deviation; t1/2, terminal elimination phase half‐life; tmax, time of occurrence of Cmax.
DTG 20‐mg(4 5‐mg tablets) treatments were reconstituted in purified, LMC, or HMC water and consumed either immediately (0 minutes) or after a 30‐minute delay.
Data presented as mean (SD) unless otherwise indicated.
Median (range).
Plasma exposure of the DTG dispersible tablet formulation was first compared with that of the pediatric granule formulation. Oral administration of DTG 20 mg (4 5‐mg dispersible tablets) immediately after dispersal in LMC water gave equivalent exposures to the pediatric granule formulation with GLS mean ratios (95%CIs) for AUC0‐∞ and Cmax of 1.07 (1.00, 1.14) and 1.13 (1.05, 1.21), respectively (Table 4).
Table 4.
Statistical Comparison of Plasma DTG PK Parameters
| Geometric Least‐Squares Mean Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Comparisona | AUC0‐∞ | Cmax | CL/F |
| B vs A | 1.07 (1.00, 1.14) | 1.13 (1.05, 1.21) | 0.94 (0.88, 1.00) |
| C vs B | 0.94 (0.88, 1.01) | 0.92 (0.85, 0.99) | 1.06 (0.99, 1.13) |
| D vs B | 1.03 (0.96, 1.10) | 0.99 (0.92, 1.06) | 0.97 (0.91, 1.04) |
| E vs C | 1.05 (0.98, 1.12) | 1.05 (0.97, 1.13) | 0.96 (0.90, 1.02) |
AUC0‐∞, area under the plasma concentration‐time curve from time of dose extrapolated to infinity; CL/F, apparent oral clearance; Cmax, maximum observed concentration; DTG, dolutegravir; HMC, high mineral content; LMC, low mineral content; PK, pharmacokinetic.
Treatment A, DTG 20‐mg pediatric granule in purified water and immediately consumed; treatment B, DTG 20‐mg as 4 5‐mg dispersible tablets dispersed in LMC water and immediately consumed; treatment C, DTG 20‐mg dispersible tablet dispersed in HMC water and immediately consumed; treatment D, DTG 20‐mg dispersible tablet dispersed in LMC water with a 30‐minute delay before consumption; treatment E, DTG 20‐mg dispersible tablet dispersed in HMC water with a 30‐minute delay before consumption.
When the dispersible tablet formulation was administered immediately after dispersion, comparable DTG plasma exposures were observed for HMC and LMC water with GLS mean ratios (95%CIs; HMC vs LMC) of 0.94 (0.88, 1.01) and 0.92 (0.85, 0.99) for AUC0‐∞ and Cmax, respectively.
To evaluate if a 30‐minute delay before consumption affects DTG plasma exposure, the PK parameters of subjects consuming the DTG dispersible tablet in both HMC water and LMC water after 30 minutes were compared with those of subjects immediately consuming the same suspension. The GLS mean ratios (95%CIs) for AUC0‐∞ and Cmax with the 30 minute delay of the dispersible tablet compared with the immediate administration of the same suspension were equivalent: 1.03 (0.96, 1.10) and 0.99 (0.92, 1.06), respectively, for LMC water, and 1.05 (0.98, 1.12) and 1.05 (0.97, 1.13), respectively, for HMC water.
Absorption was rapid for all treatments with no lag time. Terminal elimination phase half‐life, CL/F, and C24 were also comparable across all groups, although the median tmax was earlier for the dispersible tablets (1 hour) administered immediately after dispersion in either LMC or HMC water than for the pediatric granule (2 hours).
Tolerability
All 15 participants completed the trial. AEs were reported in 9 subjects (60%). No SAEs, AEs leading to withdrawal, or deaths from the study occurred. Two reports of nausea were the only drug‐related AEs (n = 1); these were mild in intensity and resolved on the same day. No grade ≥2 laboratory toxicities were reported, and no clinically significant changes in laboratory values, vital signs, or electrocardiogram results occurred during the study.
Taste Questionnaire
The taste of the dispersible formulation was rated as neutral/acceptable or very good by 91.7%, with a majority describing the flavor as chalky (75.0%). One subject described the aftertaste of the granule formulation as unpleasant or unacceptable. Only 1 dispersible formulation (treatment B; dispersible tablet in LMC) received unacceptable ratings in taste, mouth feel, aroma, and aftertaste (n = 1 for each).
Discussion
Although several antiretroviral agents are approved for use in pediatric subjects with HIV‐1, alternative formulations are needed to improve dosing and administration. The current commercial formulation tablets have been approved in the United States for use in patients weighing at least 30 kg. The dispersible DTG tablet formulation is currently under development with the aim of improving treatment administration for infants and young children with HIV‐1. A recent relative bioavailability study conducted in healthy adults showed that DTG plasma exposure following the direct administration of the granule formulation was equivalent to that of the granule formulation with water.7 However, DTG exposure following granule administration exceeded that of the adult 50‐mg tablet formulation by 55% to 83%, suggesting that dosage reductions would be required.7 The current analyses indicate that doses of 20 mg for the pediatric dispersible tablet (4 5‐mg tablets) and granule formulations yielded equivalent DTG exposures. Results also demonstrate that dispersing the tablet in HMC water resulted in DTG plasma exposure similar to that of tablet dispersal in LMC water. Comparable DTG exposures were also observed if dispersion of the tablet formulation was withheld for 30 minutes before consumption or consumed immediately. Collectively, these observations suggest that the dispersible tablet can be dispersed using water with a range of mineral content such as that studied here.
DTG is mostly metabolized by uridine diphosphate glucuronosyltransferase 1A1, with a minor component metabolized by cytochrome P450 (CYP) 3A4.14 Prior studies have shown that coadministration of DTG and strong CYP3A inducers, such as carbamazepine and nevirapine, results in clinically relevant reductions in DTG plasma exposure.15, 16 In contrast, CYP3A inhibitors, such as ritonavir, do not cause a clinically meaningful change in DTG exposure.17 DTG causes inhibition of the OCT2/MATE renal transporter, which results in increased exposure to drugs that are OCT2 substrates, such as metformin.18
The influence of cation‐containing agents on DTG bioavailability has been widely investigated, as chelation interactions with metal cation‐containing agents reduce absorption and plasma exposure of INSTIs. Like all integrase inhibitors, DTG binds to magnesium within the active site of the HIV integrase enzyme.19 Thus, high concentrations of divalent and trivalent metal cations can chelate integrase inhibitors, thereby reducing plasma exposures. Previously, calcium and iron supplements were shown to reduce DTG plasma exposure by 39% and 54%, respectively, under fasting conditions; however, administration of a mineral supplement and DTG with a moderate‐fat meal (which had previously been shown to increase DTG exposure) resulted in bioavailability similar to that of DTG administered alone.9, 12 Additionally, concomitant administration of DTG and a magnesium‐ and aluminum‐containing antacid was shown to reduce DTG exposure by 70%.12 These results were consistent with other INSTIs, as the combined administration of metal cation‐containing antacids and raltegravir led to a 67% decrease in raltegravir concentration at 12 hours after administration.10 However, the concentration of divalent and trivalent metal cations in antacids (400 mg/5 mL each of Mg[OH]2 and AL[OH]3) is more than 1000 times greater than that in HMC water (468 mg/L of calcium and 74 mg/L of magnesium).12 These differences in mineral content may in part explain why HMC water produced no significant change in DTG bioavailability when compared with the pediatric granule formulation prepared with purified water. Accordingly, DTG bioavailability in HMC water cannot be extrapolated to exposure when coadministered with antacids because of the substantial difference in divalent and trivalent metal cation concentrations. The DTG dispersible tablet may offer a practical way to administer DTG to infants and young children, although additional analyses are needed to investigate potential differences in PK in children.
The tolerability of the dispersible tablet is similar to that of the pediatric granule formulation. Both formulations of DTG evaluated in this study were well tolerated, with no SAEs, AEs leading to treatment discontinuation, or deaths occurring in study participants. Nausea of mild intensity was the only drug‐related AE and was resolved the same day. These results are consistent with a previous study of the safety profile of the pediatric granule formulation with few AEs and no SAEs.7
There are several limitations to this study. The information provided by the palatability questionnaires is limited. However, most participants described the taste and mouth feel of the dispersible tablet as acceptable, which suggests that taste or flavor will not prohibit development of this formulation. In addition, this study was conducted in adults, and further investigation may be needed to assess the palatability preferences in young volunteers. Future studies will also need to evaluate PK and safety in young children.
Overall, these results indicate that the dispersible tablet formulation of DTG is well tolerated and exhibits a comparable bioavailability to the pediatric granule formulation whether dispersed in LMC or HMC water and whether held for 30 minutes or consumed immediately. These data support the further evaluation of the DTG dispersible tablet formulation in infants and young children with HIV‐1.
Author Contributions
All authors contributed equally to this manuscript. A.B., M.H., I.C., M.D., M.C., and B.W. made significant contributions to the conception and design and/or acquisition of data and/or analysis and interpretation of data. Each author also actively participated in the drafting and approval of this manuscript.
Declaration of Conflicting Interests and Financial Disclosure
This study was funded by ViiV Healthcare. A.B. and B.W. are employed by ViiV Healthcare and may receive company stock as part of their incentive packages. M.H., I.C., and M.D. are employed by GlaxoSmithKline and may receive company stock as part of their incentive packages. M.C. is an employee of PAREXEL and has no competing interest. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Acknowledgments
This study was funded by ViiV Healthcare. The authors would like to acknowledge Julie Borland, Ivy Song, Steve Weller, and Fred Jerva for their contributions to the study and Kimberly Adkison for her contribution to the manuscript. Editorial assistance was provided under the direction of the authors by Kristi Porter and Meredith MacPherson and was supported by ViiV Healthcare.
References
- 1. World Health Organization . Global summary of the AIDS epidemic 2014. http://www.who.int/hiv/data/epi_core_july2015.png?ua=1. Accessed September 22, 2016.
- 2. Brichard B, Van der Linden D. Clinical practice treatment of HIV infection in children. Eur J Pediatr. 2009;168(4):387–392. [DOI] [PubMed] [Google Scholar]
- 3. Panel of Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed September 22, 2016.
- 4. Min S, Song I, Borland J, et al. Pharmacokinetics and safety of S/GSK1349572, a next‐generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viani RM, Alvero C, Fenton T, et al. Safety, pharmacokinetics and efficacy of dolutegravir in treatment‐experienced HIV‐1 infected adolescents: forty‐eight‐week results from IMPAACT P1093. Pediatr Infect Dis J. 2015;34(11):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hazra R, Viani R, Acosta E, et al. Pharmacokinetics, safety and efficacy of dolutegravir (DTG; S/GSH1349572) in HIV‐1 positive adolescents: preliminary analysis from IMPAACT P1093. Poster presented at: 19th International AIDS Conference; July 22‐27, 2012; Washington, DC.
- 7. Patel P, Song I, Borland J, et al. Relative bioavailability of a paediatric granule formulation of the HIV integrase inhibitor dolutegravir in healthy adult subjects. Antivir Ther. 2014;19(3):229–233. [DOI] [PubMed] [Google Scholar]
- 8. Hazuda D, Iwamoto M, Wenning L. Emerging pharmacology: inhibitors of human immunodeficiency virus integration. Annu Rev Pharmacol Toxicol. 2009;49:377–394. [DOI] [PubMed] [Google Scholar]
- 9. Song I, Borland J, Arya N, Wynne B, Piscitelli S. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol. 2015;55(5):490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiser JJ, Bumpass JB, Meditz AL, et al. Effect of antacids on the pharmacokinetics of raltegravir in human immunodeficiency virus‐seronegative volunteers. Antimicrob Agents Chemother. 2010;54(12):4999–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramanathan S, Mathias A, Wei X, et al. Pharmacokinetics of once‐daily boosted elvitegravir when administered in combination with acid‐reducing agents. J Acquir Immune Defic Syndr. 2013;64(1):45–50. [DOI] [PubMed] [Google Scholar]
- 12. Patel P, Song I, Borland J, et al. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co‐administered with acid‐reducing agents and multivitamins in healthy volunteers. J Antimicrob Chemother. 2011;66(7):1567–1572. [DOI] [PubMed] [Google Scholar]
- 13. Song IH, Borland J, Savina PM, et al. Pharmacokinetics of single‐dose dolutegravir in HIV‐seronegative subjects with moderate hepatic impairment compared to healthy matched controls. Clin Pharmacol Drug Dev. 2013;2(4):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reese MJ, Savina PM, Generaux GT, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–361. [DOI] [PubMed] [Google Scholar]
- 15. Song I, Weller S, Patel J, et al. Effect of carbamazepine on dolutegravir pharmacokinetics and dosing recommendation. Eur J Clin Pharmacol. 2016;72(6):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dailly E, Allavena C, Gregoire M, et al. Influence of nevirapine administration on the pharmacokinetics of dolutegravir in patients infected with HIV‐1. J Antimicrob Chemother. 2015;70(12):3307–3310. [DOI] [PubMed] [Google Scholar]
- 17. Khatri A, Trinh R, Zhao W, et al. Drug‐drug interaction between the direct‐acting antiviral regimen of ombitasvir/paritaprevir/ritonavir plus dasabuvir and the HIV antiretroviral agents dolutegravir or abacavir plus lamivudine. Antimicrob Agents Chemother. 2016;60(10):6244–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song IH, Zong J, Borland J, et al. The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Acquir Immune Defic Syndr. 2016;72(4):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hare S, Smith SJ, Metifiot M, et al. Structural and functional analyses of the second‐generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol. 2011;80(4):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]


