Abstract
Benzoyl peroxide (BPO) has been well established as a common medication for acne vulgaris in many countries (e.g. in Europe and the USA), where clinical data have been accumulated over a long time. In Japan, the use of BPO for acne treatment was approved in 2014, and the results of clinical trials in Japanese patients have recently been reported. This review compares clinical study results between Japanese and Western patients. Clinical studies that had been performed in Western countries were searched on the basis of the criteria, double‐blind studies of BPO monotherapy and comparison with a vehicle group. Two reports of Japanese studies were also selected by using the same criteria. Efficacy was assessed by comparing the mean difference between the BPO and the vehicle groups for reduction rate in the number of lesions from baseline, and there were no differences between Japanese and Western patients. Safety assessment also showed that the incidence of adverse events was higher in Japanese patients than in Western patients, but the characteristics of the adverse events were not different. Therefore, we conclude that there are no significant differences in the efficacy and safety of BPO between these patient populations. The efficacy and safety of long‐term use in Japanese patients are also expected to be applicable to those in Western patients.
Keywords: acne vulgaris, benzoyl peroxide, clinical efficacy, ethnicity, safety
Introduction
Acne vulgaris is a chronic inflammatory disease that often affects the hair follicle sebaceous glands on the face, chest and back. It is commonly observed in adolescent males and females, and more than 90% of Japanese develop acne vulgaris during their life.1 As increased value is put on appearance, adolescents with acne may have a low quality of life with regard to their emotional state.2 Therefore, there is a high demand for an efficacious treatment for acne patients. The pathogenesis of acne vulgaris is a complex process involving abnormal lipid metabolism, dyskeratosis and bacterial growth. Antimicrobials form one of the main treatment options, but there are concerns that their long‐term use may cause the development and spread of antibiotic‐resistant bacteria. In fact, it has been reported that isolation of drug‐resistant Propionibacterium acnes from acne patients has increased in Japan.3 Topical benzoyl peroxide (BPO) has been used as a treatment for acne vulgaris in many countries, including those in Europe and the USA, where the corresponding medical guidelines recommend it as the standard treatment for acne vulgaris.4, 5 The mechanism of action of BPO is considered to be its antiseptic activity and keratolytic effects,6, 7 and there is not a concern for the development of drug‐resistant bacteria to BPO. The Japanese medical guideline for acne vulgaris was revised in 2016, and BPO is recommended as a drug for the treatment of inflammatory and non‐inflammatory acne vulgaris lesions and to maintain remission after inflammation has resolved.1 Western countries have been using BPO as acne treatment since the 1960s, and have accumulated much clinical data. Recently, in fact, Lamel et al.8 reviewed and investigated the data from clinical studies conducted in Western countries in patients with acne vulgaris using BPO. In the meantime, in Japan, BPO was approved in 2014 for the treatment of acne vulgaris and has been on the market since 2015, and therefore clinical data of Japanese patients has only recently become available.9, 10, 11 This article reviews the efficacy and safety profiles of BPO, and compares the results of clinical studies between Japanese and Western patients.
Selection of Studies
We selected randomized, double‐blind studies that compared a monotherapy with a BPO gel with its vehicle as a placebo control to evaluate the efficacy and safety of BPO for acne vulgaris. Reports were searched in PubMed and Embase (cut‐off date of 14 July 2016) using “benzoyl peroxide”, “acne vulgaris”, “placebo” or “vehicle”, and “controlled” or “randomized” as key words. Reports hit by the search were further screened on the basis of their titles and abstracts. As a result, 10 studies including one Japanese study by Kawashima et al., 9 were selected for our review. A more recently reported study by Kawashima et al., 10 was also used as another clinical study in Japanese patients (Table 1). These two Japanese studies were independent and separate clinical studies and the results were generated from different subject groups. Clinical studies outside Japan were performed in North America and Europe, which means as a result that this review compared Japanese studies in Japanese patients with Western studies in Western patients (mainly Caucasians). In the following comparison, references were limited to studies with gel formulations of BPO as the Japanese studies used. For comparison of efficacy, references that reported the mean reduction rate in number of lesions from baseline were selected, and four studies in Western patients and two studies in Japanese patients were used for the comparison. To investigate the safety profile, we focused on the reports that included incidences of adverse events (AE), and eight studies in Western patients and two studies in Japanese patients were selected. Table 1 shows the outlines and subject backgrounds of the selected studies.
Table 1.
Selected references
| Reference | Study region | Regimen | Treatment group | Subject number | Sex, male % | Age, mean | Ethnicity/race | Baseline lesion counts, mean (median), or range | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | NIL | IL | ||||||||
| Eichenfield et al. (2011)17, †, ‡ | USA, Canada, Belize | q.d. × 12 w | BPO 3% | 328 | 38 | 20.6 | White, 77% | 71.6 | 44.6 | 27.0 |
| Vehicle | 332 | 37 | 20.7 | White, 78% | 70.5 | 44.2 | 26.3 | |||
| Gold et al. (2009)15, ‡ | USA, Puerto Rico, Canada | q.d. × 12 w | BPO 2.5% | 415 | 50.1 | 18.4 | White, 62.2% | (76) | (46) | (27) |
| Vehicle | 418 | 46.9 | 18.0 | White, 64.6% | (76) | (46) | (27) | |||
| Gollnick et al. (2009)16, ‡ | USA, Canada, Europe | q.d. × 12 w | BPO 2.5% | 415 | 44.6 | 18.9 | Caucasian, 79.3% | (74) | (45) | (26) |
| Vehicle | 418 | 41.6 | 19.2 | Caucasian, 79.2% | (76) | (46) | (26) | |||
| Thiboutot et al. (2008)18, †, ‡ | USA | q.d. × 12 w | BPO 2.5% | 809 | 43.8 | 19.1 | White, 73.9% | 72.6 | 46.8 | 25.8 |
| Vehicle | 395 | 51.4 | 19.4 | White, 77.2% | 70.2 | 44.0 | 26.1 | |||
| Thiboutot et al. (2007)19, ‡ | USA | q.d. × 12 w | BPO 2.5% | 149 | 64.4 | 16.5 | Caucasian, 76.5% | (74) | (43) | (28) |
| Vehicle | 71 | 56.3 | 16.6 | Caucasian, 73.2% | (78) | (46) | (29) | |||
| Leyden et al. (2001)20, ‡ | USA | b.i.d. × 10 w | BPO 5% | 120 | 48 | 19 | NA | 49 | NA | 19 |
| Vehicle | 120 | 40 | 19 | NA | 47 | NA | 19 | |||
| Tschen et al. (2001)21, ‡ | USA | b.i.d. × 10 w | BPO 5% | 95 | 46‐54 | 19 | NA | NA | NA | 23‐27 |
| Vehicle | 48 | 19 | NA | NA | NA | |||||
| Lookingbill et al. (1997)22, †, ‡ | USA | q.d. × 11 w | BPO 5% | 92 | 49 | 18.5 | NA | 89.9 | 59.2 | 30.7 |
| Vehicle | 58 | NA | 101.6 | 70.1 | 31.5 | |||||
| Mills Jr et al. (1986)13, † | USA | b.i.d. × 8 w | BPO 2.5% | 25 | 48.4 | 20 | NA | NA | NA | 13.6 |
| Vehicle | 25 | NA | NA | NA | 13.7 | |||||
| Kawashima et al. (2014)9, †, ‡ | Japan | q.d. × 12 w | BPO 3% | 178 | 34 | 21.3 | Japanese | 72.1 | 44.2 | 28.0 |
| Vehicle | 182 | 36 | 22.4 | Japanese | 70.3 | 42.7 | 27.5 | |||
| Kawashima et al. (2017)10, †, ‡ | Japan | q.d. × 12 w | BPO 2.5% | 203 | 41.4 | 19.5 | Japanese | 54.9 (50) | 34.7 (29) | 20.1 (18) |
| BPO 5% | 203 | 38.9 | 20.0 | Japanese | 55.9 (51) | 35.8 (30) | 20.1 (18) | |||
| Vehicle | 201 | 45.3 | 19.2 | Japanese | 56.5 (51) | 36.4 (30) | 20.1 (18) | |||
‡Included in comparison of safety. †Included in comparison of efficacy. BPO, benzoyl peroxide; IL, inflammatory lesion; NA, not‐available; NIL, non‐inflammatory lesion; w, weeks.
Comparison of Efficacy between Western and Japanese Studies
Efficacy of BPO for acne was assessed on the basis of the percentage reduction in numbers of lesions (inflammatory, non‐inflammatory and total) at the end of the treatment period from baseline. The mean or median percentage reduction of each study is summarized by lesion type (Fig. 1 for inflammatory lesions, Fig. 2 for non‐inflammatory lesions and Fig. 3 for total lesions) and the differences between the BPO and the vehicle groups are also indicated in the figures when the mean values of both groups were available. For the study by Kawashima et al.,9 additional data that were not available in their publication were obtained from the GSK Clinical Study Register,12 because the detailed individual data of this study have been presented on the website, not published in print. Likewise, concerning the study of Kawashima et al.,10 the mean percentage reduction and standard deviation were obtained from the original study report. The BPO concentration of the study drugs ranged 2.5–5% in the Western studies and 2.5–3% in the Japanese studies. It has been reported that there is no difference in the efficacy of BPO within the range of a concentration of 2.5–10%,13 and thus, we did not take the difference in the BPO concentration into account to compare efficacy. In addition, duration of treatment was 8–12 weeks in the Western studies and 12 weeks in the Japanese studies.
Figure 1.
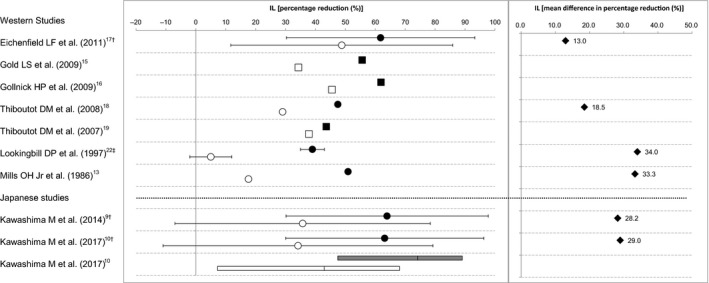
Percentage reduction and mean difference in percentage reduction of inflammatory lesion (IL) counts from baseline between the benzoyl peroxide (BPO) and the vehicle groups. Data indicate the mean (●, BPO; ○, vehicle), the standard deviation (†), the standard error (‡), the median (■, BPO; □, vehicle), the median and interquartile range (gray box, BPO; white box, vehicle), or the mean difference (♦, BPO minus vehicle).
Figure 2.
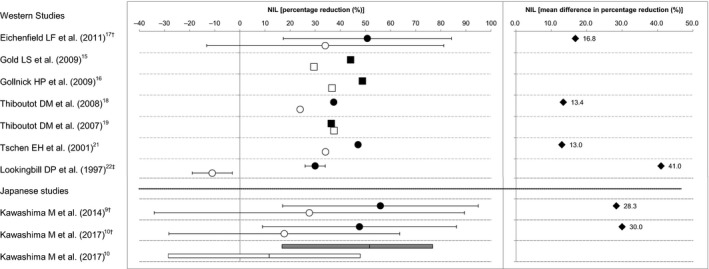
Percentage reduction and mean difference in percentage reduction of non‐inflammatory lesion (NIL) counts from baseline between the benzoyl peroxide (BPO) and the vehicle groups. Data indicate the mean (●, BPO; ○, vehicle), the standard deviation (†), the standard error (‡), the median (■, BPO; □, vehicle), the median and interquartile range (gray box, BPO; white box, vehicle), or the mean difference (♦, BPO minus vehicle).
Figure 3.
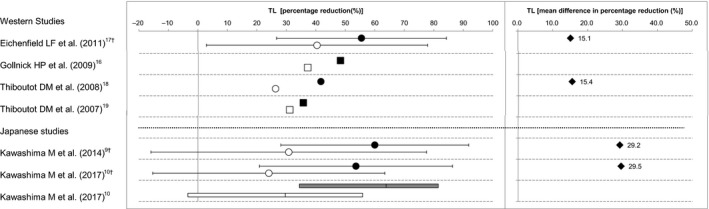
Percentage reduction and mean difference in percentage reduction of total lesion ( TL) counts from baseline between the benzoyl peroxide (BPO) and the vehicle groups. Data indicate the mean (●, BPO; ○, vehicle), the standard deviation (†), the median (■, BPO; □, vehicle), the median and interquartile range (gray box, BPO; white box, vehicle), or the mean difference (♦, BPO minus vehicle).
We consider that there is no significant difference in percentage reduction in inflammatory lesion count between the Western and the Japanese studies for both of the BPO and the vehicle groups. The mean difference in percentage reduction in inflammatory lesion count was 13–34% in the Western studies and 28–29% in the Japanese studies, indicating that the differences between the BPO and the vehicle groups for both Western and Japanese studies were clinically meaningful (Fig. 1). In addition, the mean differences in percentage reduction in inflammatory lesion count in the Japanese studies were within the range observed in the Western studies. Therefore, we conclude that the efficacy profiles of BPO for the inflammatory lesions are similar between the Western and the Japanese studies.
We consider that there is no significant difference in percentage reduction in non‐inflammatory lesion count between the Western and the Japanese studies for both the BPO and vehicle groups. The mean differences in percentage reduction in non‐inflammatory lesion count were 13–41% in the Western studies and 28–30% in the Japanese studies, indicating that the differences between the BPO and the vehicle groups for both Western and Japanese studies (Fig. 2) were clinically meaningful. In addition, the mean differences in percentage reduction in non‐inflammatory lesion count in the Japanese studies were within the range observed in the Western studies. Therefore, we conclude that the efficacy profiles of BPO for the non‐inflammatory lesions are similar between the Western and the Japanese studies.
We consider that there is no significant difference in percentage reduction in total lesion count between the Western and the Japanese studies for both the BPO and vehicle groups. The mean differences in percentage reduction in total lesion count were 15% in the Western studies and 29–30% in the Japanese studies, indicating that the differences between the BPO and the vehicle groups for both Western and Japanese studies (Fig. 3) were clinically meaningful. However, the mean differences in percentage reduction in total lesion count in the Japanese studies were not within the range observed in the Western studies. This was because only two studies in the Western studies calculated the mean percentage reduction in total lesion count. As the efficacy of BPO for the inflammatory lesions and non‐inflammatory lesions is similar between the Western and the Japanese studies, we conclude that the efficacy of BPO for the total lesions is also similar between the Western and the Japanese studies.
Comparison of Safety between Western and Japanese Studies
The incidences of AE were compared between the selected studies (Table 2). In both Western and Japanese studies, the following safety characteristics were commonly reported: most AE were mild in severity and consisted mainly of skin irritation at the application site, no serious treatment‐related AE were observed, and the occurrence of AE was higher during the early than later treatment period. Therefore, we conclude that there are no qualitative differences in the safety profile of BPO between the Western and Japanese patient populations. However, the incidence of AE was higher in Japanese studies than Western studies (Table 2). Most treatment‐related AE in the Japanese studies consisted of skin irritation at the application site, suggesting higher sensitivity to local irritation of BPO in Japanese patients. It has been reported that the skin barrier structure of Asians, including Japanese, is weaker than that of Caucasians,14 which may be a reason for the higher incidence of AE in Japanese studies.
Table 2.
Incidences of adverse events
| Reference | Regimen | Treatment group | Subject number | Subjects with any AE, % | Subjects with AE related to treatment, % |
|---|---|---|---|---|---|
| Eichenfield et al. (2011)17 | q.d. × 12 w | BPO 3% | 328 | 31 | 2 |
| Vehicle | 332 | 26 | 2 | ||
| Gold et al. (2009)15 | q.d. × 12 w | BPO 2.5% | 415 | NA | NA |
| Vehicle | 418 | NA | NA | ||
| Gollnick et al. (2009)16 | q.d. × 12 w | BPO 2.5% | 415 | 33 | 13 |
| Vehicle | 418 | 28 | 8 | ||
| Thiboutot et al. (2008)18 | q.d. × 12 w | BPO 2.5% | 809 | NA | 5.9 |
| Vehicle | 395 | NA | 6.1 | ||
| Thiboutot et al. (2007)19 | q.d. × 12 w | BPO 2.5% | 149 | 29.5 | 6.7 |
| Vehicle | 71 | 26.8 | 5.6 | ||
| Leyden et al. (2001)20 | b.i.d. × 10 w | BPO 5% | 120 | 29.2 | NA |
| Vehicle | 120 | 20.8 | NA | ||
| Tschen et al. (2001)21 | b.i.d. × 10 w | BPO 5% | 95 | 52 | 27 |
| Vehicle | 48 | 50 | 19 | ||
| Lookingbill et al. (1997)22 | q.d. × 11 w | BPO 5% | 92 | NA | 2.2 |
| Vehicle | 58 | NA | 1.7 | ||
| Kawashima et al. (2014)9 | q.d. × 12 w | BPO 3% | 178 | 58 | 30 |
| Vehicle | 182 | 47 | 5 | ||
| Kawashima et al. (2017)10 | q.d. × 12 w | BPO 2.5% | 204 | 56.4 | 37.3 |
| BPO 5% | 204 | 58.8 | 38.7 | ||
| Vehicle | 201 | 47.3 | 12.9 |
AE, adverse events; BPO; benzoyl peroxide; NA, not available; w, weeks.
For patients enrolled in the Western studies, there was a possibility of developing tolerability to BPO because it was very common for these patients to be using BPO, including over‐the‐counter products, when these studies were conducted. However, it was assumed that most Japanese patients were BPO naive, because no BPO drugs had been approved in Japan at the time. Another possible factor for a higher incidence of AE in Japanese studies was the use of moisturizers. The two Western studies required the concomitant treatment with a moisturizer for subjects who were assessed to have dry skin by the investigator.15, 16 Because the use of moisturizers had to be decided by the investigator and/or the subjects, the effect of moisturizers was unclear.
BPO for Long‐term Treatment
Kawashima et al., 11 was the only study that reported the safety and efficacy of a long‐term treatment with BPO monotherapy. Acne vulgaris frequently relapses when treatment is discontinued after symptoms have improved; thus, remission has to be maintained, even after inflammation has resolved. Because there are no reports of BPO‐resistant bacteria, it is commonly believed that the long‐term maintenance use of BPO would be reasonable. The efficacy and safety for long‐term treatment (up to 52 weeks) with both 2.5% and 5% BPO gel in Japanese patients were reported.10 This report showed that the number of lesions decreased over time from the start of treatment to 12 weeks, and that this status was maintained thereafter. Regarding safety, most treatment‐related AE occurred within the first 12 weeks of treatment, and the incidence rate was low thereafter. Furthermore, the AE that were observed only after the first 12 weeks, and not before that, were dermal symptoms at the application site, and most cases were mild. On the basis of these results, we concluded that no new safety concerns due to prolonged treatment would arise after the early treatment phase.
As previously described, there were no differences in efficacy between Japanese and Western studies up to 12 weeks of treatment. The main mechanism of action of BPO is believed to be oxidative activity at the application site, for which little ethnic difference is expected. Therefore, the efficacy of long‐term use reported in Japanese patients would be similar in Western patients. Safety profiles up to 12 weeks did not indicate any clinically meaningful differences between Japanese and Western patients, and no serious problems occurred after 12 weeks in the Japanese study, suggesting that long‐term use of BPO in Western patients would be as safe as 12‐week treatment.
Limitations
In this article, we compared the results of clinical studies performed in Japan with those performed in the USA and Europe. However, the comparison was based on historical data, and we should account for its limitations. Baseline severity of acne symptoms of participants, variability in the evaluation when counting lesions and recognition of AE may be different between studies and therefore affect the evaluation of safety and efficacy. In the study of Kawashima et al.,10 the ratio of participants with relatively mild symptoms was higher than in the other studies, which limits the comparison of efficacy. Furthermore, this study included a change for the worse in local skin tolerability score as an AE, and this could also increase the incidence rate of AE in that study. With regard to the long‐term safety in Western patients, it was an extrapolation from the comparison of the short‐term (12‐week) treatment results between Western and Japanese safety data. No concrete data were available to predict the long‐term safety in Western patients.
Conclusion
This review summarizes clinical trials performed in Japan and those performed in the USA and Europe and compares them for efficacy and safety of BPO. Regarding the efficacy, the results of the Japanese studies are within the range of fluctuations of the studies performed in the USA and Europe, suggesting that there are no differences between these patient populations. Although the safety assessment of the Japanese studies suggested that AE at the application site occurred more often in Japanese patients than Western patients, most of the AE were mild. Except for the difference in the frequency of AE, there were no differences in their respective safety profiles. It is also suggested that the preferable efficacy and safety profiles of the long‐term use of BPO shown in Japanese patients would be applicable to Western patients.
Conflict of Interest
This review was sponsored by Maruho Co. Ltd (Kyoto, Japan). M. K. received consultant fees and honoraria for lectures and publications from Maruho Co. Ltd. T. N. is an employee of Maruho Co. Ltd. M. D. was an employee of Maruho Co. Ltd at the time of the study.
Acknowledgment
Editorial assistance in the preparation of this manuscript was provided by WysiWyg Co. Ltd.
References
- 1. Hayashi N, Akamatsu H, Iwatsuki K et al Guidelines for the treatment of acne vulgaris 2016. Jpn J Dermatol 2016; 26: 1045–1086. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 2. Hayashi N, Higaki Y, Kawamoto K, Kamo T, Shimizu S, Kawashima M. A cross‐sectional analysis of quality of life in Japanese acne patients using the Japanese version of Skindex‐16. J Dermatol 2004; 31: 971–976. [DOI] [PubMed] [Google Scholar]
- 3. Nakase K, Nakaminami H, Noguchi N, Nishijima S, Sasatsu M. First report of high levels of clindamycin‐resistant Propionibacterium acnes carrying erm(X) in Japanese patients with acne vulgaris. J Dermatol 2012; 39: 794–796. [DOI] [PubMed] [Google Scholar]
- 4. Strauss JS, Krowchuk DP, Leyden JJ et al Guidelines of care for acne vulgaris management. J Am Acad Dermatol 2007; 56: 651–663. [DOI] [PubMed] [Google Scholar]
- 5. Nast A, Dréno B, Bettoli V et al European evidence‐based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol 2012; 26(Suppl 1): 1–29. [DOI] [PubMed] [Google Scholar]
- 6. Decker LC, Deuel DM, Sedlock DM. Role of lipids in augmenting the antibacterial activity of benzoyl peroxide against Propionibacterium acnes . Antimicrob Agents Chemother 1989; 33: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waller JM, Dreher F, Behnam S et al ‘Keratolytic’ properties of benzoyl peroxide and retinoic acid resemble salicylic acid in man. Skin Pharmacol Physiol 2006; 19: 283–289. [DOI] [PubMed] [Google Scholar]
- 8. Lamel SA, Sivamani RK, Rahvar M, Maibach HI. Evaluating clinical trial design: systematic review of randomized vehicle‐controlled trials for determining efficacy of benzoyl peroxide topical therapy for acne. Arch Dermatol Res 2015; 307: 757–766. [DOI] [PubMed] [Google Scholar]
- 9. Kawashima M, Hashimoto H, Alio Sáenz AB, Ono M, Yamada M. Is benzoyl peroxide 3% topical gel effective and safe in the treatment of acne vulgaris in Japanese patients? A multicenter, randomized, double‐blind, vehicle‐controlled, parallel‐group study. J Dermatol 2014; 41: 795–801. [DOI] [PubMed] [Google Scholar]
- 10. Kawashima M, Sato S, Furukawa F et al Twelve‐week, multicenter, placebo‐controlled, randomized, double‐blind, parallel‐group, comparative phase II/III study of benzoyl peroxide gel in patients with acne vulgaris: a secondary publication. J Dermatol 2017; 44: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawashima M, Nagare T, Katsuramaki T. Open‐label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris: a secondary publication. J Dermatol 2017; 44: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. GSK Clinical Study Register [homepage on the internet] . Middlesex: GlaxoSmithKline plc.; c2001–17 [Cited 2017 June 8.] Available from URL: http://www.gsk-clinicalstudyregister.com/study/115288#rs
- 13. Mills OH Jr, Kligman AM, Pochi P, Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. Int J Dermatol 1986; 25: 664–667. [DOI] [PubMed] [Google Scholar]
- 14. Muizzuddin N, Hellemans L, Van Overloop L, Corstjens H, Declercq L, Maes D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. Dermatol Sci 2010; 59: 123–128. [DOI] [PubMed] [Google Scholar]
- 15. Gold LS, Tan J, Cruz‐Santana A et al A North American study of adapalene‐benzoyl peroxide combination gel in the treatment of acne. Cutis 2009; 84: 110–116. [PubMed] [Google Scholar]
- 16. Gollnick HP, Draelos Z, Glenn MJ et al Adapalene‐benzoyl peroxide, a unique fixed‐dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double‐blind, controlled study in 1670 patients. Br J Dermatol 2009; 161: 1180–1189. [DOI] [PubMed] [Google Scholar]
- 17. Eichenfield LF, Alió Sáenz AB. Safety and efficacy of clindamycin phosphate 1.2%‐benzoyl peroxide 3% fixed‐dose combination gel for the treatment of acne vulgaris: a phase 3, multicenter, randomized, double‐blind, active‐ and vehicle‐controlled study. J Drugs Dermatol 2011; 10: 1382–1396. [PubMed] [Google Scholar]
- 18. Thiboutot D, Zaenglein A, Weiss J, Webster G, Calvarese B, Chen D. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once‐daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol 2008; 59: 792–800. [DOI] [PubMed] [Google Scholar]
- 19. Thiboutot DM, Weiss J, Bucko A et al Adapalene‐benzoyl peroxide, a fixed‐dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double‐blind, controlled study. J Am Acad Dermatol 2007; 57: 791–799. [DOI] [PubMed] [Google Scholar]
- 20. Leyden JJ, Berger RS, Dunlap FE, Ellis CN, Connolly MA, Levy SF. Comparison of the efficacy and safety of a combination topical gel formulation of benzoyl peroxide and clindamycin with benzoyl peroxide, clindamycin and vehicle gel in the treatments of acne vulgaris. Am J Clin Dermatol 2001; 2: 33–39. [DOI] [PubMed] [Google Scholar]
- 21. Tschen EH, Katz HI, Jones TM et al A combination benzoyl peroxide and clindamycin topical gel compared with benzoyl peroxide, clindamycin phosphate, and vehicle in the treatment of acne vulgaris. Cutis 2001; 67: 165–169. [PubMed] [Google Scholar]
- 22. Lookingbill DP, Chalker DK, Lindholm JS et al Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double‐blind investigations. J Am Acad Dermatol 1997; 37: 590–595. [DOI] [PubMed] [Google Scholar]


