Abstract
Cancer Biotherapy and Radiopharmaceuticals (CBR) is officially retracting the published article by Mei Yang, Xueyao Wang, Jiaoyuan Jia, Hongwen Gao, Peng Chen, Xianliang Sha, and Shan Wu entitled, “Tumor Protein D52-Like 2 Contributes to Proliferation of Breast Cancer Cells,” Cancer Biother Radiopharm February 2015, 30(1): 1–7. doi:10.1089/cbr.2014.1723.
The editors of CBR received a letter from an investigator raising concerns regarding this published paper. Upon examining the 5′-GCGG… shRNA sequence described in the article, the investigator discovered that the lentiviral sequence cited to target TPD52L2 actually does not correspond to any TPD52L2 sequence, and does not show homology to any sequence in the non-redundant nucleotide database (as per a Blastn search conducted by the investigator). The investigator claimed that the article reported using the 5′-GCGG… shRNA as a non-targeting control, but the alternative “TPD52L2-targeting” 5′-CCGG… shRNA does not target the TPD52L2 gene as described, or any other sequence in the non-redundant nucleotide database.
The investigator's concern was such that since the article did not employ a lentiviral construct targeting the TPD52L2 gene, the experimental results reported must be invalid. The editors of the CBR contacted the authors of the published paper who confirmed that the sequence was wrong which may have occurred due to a “copy paste error,” and agreed that the report could be misleading and agreed to retract the paper from the literature.
Key words: : breast cancer, knockdown, lentivirus, TPD52L2
Introduction
Breast cancer is the most common cancer of women both in developed and developing regions.1 It is caused by various risk factors, but usually due to somatic genetic alteration, including the oncogenes of HER-2/neu, ERBB2, PIK3CA, and TPD52L2, etc. The traditional therapies of breast cancer patients focus on instances of surgery, radiotherapy, and chemotherapy and significantly prolong survival and effective palliation. However, breast cancer has high motility, invasion, and metastasis, which has become the secondary leading cause of cancer-related deaths for patients.1,2 The molecular mechanisms responsible for breast cancer have previously been understood partially.3 Therefore, providing genetic therapeutic targets for the treatment of breast cancer is urgently needed.
The tumor protein D52-like family, which comprises tumor protein D52 (TPD52), tumor protein D52-like 1 (TPD52L1), tumor protein D52-like 2 (TPD52L2), and tumor protein D52-like 3 (TPD52L3), is characterized by an N-terminal coiled-coil motif. TPD52 was originally identified through its elevated expression level in human breast carcinoma.4 Likewise, TPD52L1 was found to be a cell cycle-regulated protein maximally expressed in the G2/M transition, and thus mediated breast cancer cell growth.5 Altered expression of TPD52 regulated apoptosis and migration of prostate cancer cells.6 In testicular germ cell tumors, TPD52 was exclusively expressed in seminomas and embryonal carcinomas, while it was absent in normal germ cells and most intratubular germ cell neoplasias.7 TPD52L3 was also observed to interact with other TPD52 family members and involved in testis development.8 A previous study revealed that TPD52 and TPD52L2 transcripts were frequently coexpressed in acute lymphoblastic leukemia and acute myeloid leukemia.9 Recently, Zhou et al. demonstrated that TPD52L2 could interact with hABCF3 and enhance liver cancer cell proliferation, while disruption of this interaction significantly decreased cell growth in vitro.10 Thus, it could be concluded that tumor protein D52-like family members are widely involved in human cancers. Nevertheless, the biological role of TPD52L2 in breast cancer cell proliferation has not yet been demonstrated.
In this study, the expression levels of TPD52L2 were first detected in various human breast cancer cell lines. The targeted depletion of TPD52L2 in ZR-75-30 cells was carried out by using lentivirus-mediated RNA interference technology that was used to suppress gene expression in mammalian cells.11 Then, the effects of TPD52L2 knockdown on cell proliferation and cell cycle regulation were evaluated.
Materials and Methods
Cell culture
Human breast cancer cell lines, ZR-75-30, MDA-MB-231, MCF-7, T-47D, BT-474, and human embryonic kidney cell line, HEK293T, were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). MDA-MB-231, T-47D, and HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS; Hyclone). MCF-7 cells were cultured in modified Eagle's medium (Hyclone) supplemented with 10% FBS. BT-474 cells were cultured in RPMI 1640 (Flow Laboratories, Irvine, United Kingdom) supplemented with 10% FBS. ZR-75-30 cells were cultured in Eagle's minimum essential medium (Hyclone) containing 10% FBS, 1 mM sodium pyruvate, and 1% nonessential amino acids. All cells were maintained at 37°C in a 5% CO2 incubator.
TPD52L2 shRNA construction and infection
The cDNA sequence of TPD52L2 was obtained from GenBank (NM_003288.2). To knock down the TPD52L2 gene, the short hairpin RNA (shRNA) sequences, 5′-CCGG GACCATAAAGTCTAAGGTTGTCTCGAGACAACCTTA GACTTTATGGTCTTTTTG-3′ and 5′-GCGGAGGGTTT GAAAGAATATCTCGAGATATTCTTTCAAACCCTCCG CTTTTTT-3′, were used as the TPD52L2 silencing (Lv-shTPD52L2) and control nonsilencing (Lv-shCon) nucleotide sequences, respectively. The shRNA sequences were cloned into the pFH-L vector (Shanghai Hollybio, Shanghai, China) containing the green fluorescent protein (GFP) reporter gene driven by the cauliflower mosaic virus 35S promoter. Lv-shTPD52L2 and Lv-shCon recombinant lentiviruses were constructed by cotransfecting ZR-75-30 cells with pVSVG-I and pCMVΔR8.92 (Shanghai Hollybio, China) used by Lipofectamine 2000 (Invitrogen, Waltham, MA) according to the manufacturer's instructions. To verify the specificity of RNAi, a rescue experiment was performed. A 5′-CCATCAAATCCAAAGTCGT-3′ sequence, which was based on the synonymous codon replacement principle, was used to replace the interference sequence targeting TPD52L2 in the pFH-L vector so that this rescue expression vector could make TPD52L2 mRNA escape degradation.
ZR-75-30 cells were plated at a density of 50,000 cells per well in a six-well plate and then infected with the recombinant lentivirus at a multiplicity of infection of 35 for 72 h. The efficiency of knockdown was detected by quantitative real-time polymerase chain reaction (PCR) (qRT-PCR) and western blot analysis.
Quantitative real-time PCR
To identify the expression levels of TPD52L2 in ZR-75-30, MDA-MB-231, MCF-7, T-47D, BT-474, and HEK293T cells, qRT-PCR analysis was performed. It was also used to detect the knockdown efficiency of TPD52L2 in ZR-75-30 cells infected with Lv-shTPD52L2, Lv-shCon, and control (Con) for 5 days. Total RNA was extracted from cultured cells using Trizol reagent (Invitrogen). The primers used were as follows: TPD52L2, 5′-ATGCAGTGTGGTGCACATTT-3′ (forward) and 5′-AACCCAAACACTTTGGC AAG-3′ (reverse); and actin, 5′-TTCACAGGCAGGACAG AAGA-3′ (forward) and 5′-TTGAAGGTCGCAGAGTT CCT-3′ (reverse). The comparative Ct (ΔΔCt) method was used for data analysis.
Western blot analysis
After lentivirus infection for 5 days, ZR-75-30 cells were washed with ice-cold phosphate-buffered saline (PBS), scraped, centrifuged, and lysed in the RIPA buffer with PMSF. The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Then, the membranes were blocked overnight with 5% milk and probed with primary antibodies against TPD52L2 (1:500, #SAB2501053; Sigma, St. Louis, MO) and GAPDH (1:60,000, #10494-1-AP; Proteintech Group, Inc., Chicago, IL). After incubation with horseradish peroxidase-conjugated secondary antibodies, the expression levels of the target protein were visualized using enhanced chemiluminescence detection reagent (Amersham, Bucks, United Kingdom).
MTT assay
To determine the proliferation of ZR-75-30 cells infected by TPD52L2 shRNA, the MTT assay was performed. The infected and uninfected cells were seeded into a 96-well plate with inoculation density of 2000 cells per well and incubated at 37°C. Twenty microliters of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added into each well to continue incubation at 37°C after daily intervals of exposure to infection. After 3 h, the media were removed and replaced with 100 μL acidic isopropanol containing 10% SDS, 5% isopropanol, and 0.01 M HCl. The absorbance at 595 nm was measured by a microplate reader (Bio-Rad, Hercules, CA) for colorimetric analysis.
Colony formation assay
The infected and uninfected ZR-75-30 cells were trypsinized, centrifuged, and resuspended in the medium and then seeded into six-well plates at 400 cells per well. After culture for 5 days, the natural colonies were formed. Plates were washed with PBS and stained by crystal violet staining according to the instructions. The culture plates were photographed under a fluorescence microscope. Colonies that contained more than 50 cells were counted and analyzed.
Cell cycle analysis
First, the infected and uninfected cells were seeded in six-well plates with a density of 200,000 cells per dish. After incubation for 40 h, the cells were collected and washed with cold PBS. The washed cells were fixed in 75% cold ethanol with incubation for 1 h at 4°C. To stain the cells, 100 μL of prodium iodide (PI) solution with DNase-free RNase of 10 μg/mL was added. A Flow cytometer (Cell Lab Quanta; Beckman Coulter, Pasadena, CA) was used to analyze the samples that filtered through a 50-μm nylon mesh.
Intracellular signaling assay
A PathScan® intracellular signaling array kit (#7323; Cell Signaling Technology, Danvers, MA) was utilized to detect alteration of signaling molecules. ZR-75-30 cell lysate was prepared as mentioned previously. The detection procedure was performed according to the manufacturer's instructions.
Statistical analysis
The SPSS 16.0 software employing Student's t-test was used to analyze all data, which are expressed as mean±standard deviation of three independent experiments. p-Values <0.05 were considered as significantly different.
Results
TPD52L2 was expressed at a high level in ZR-75-30 cells
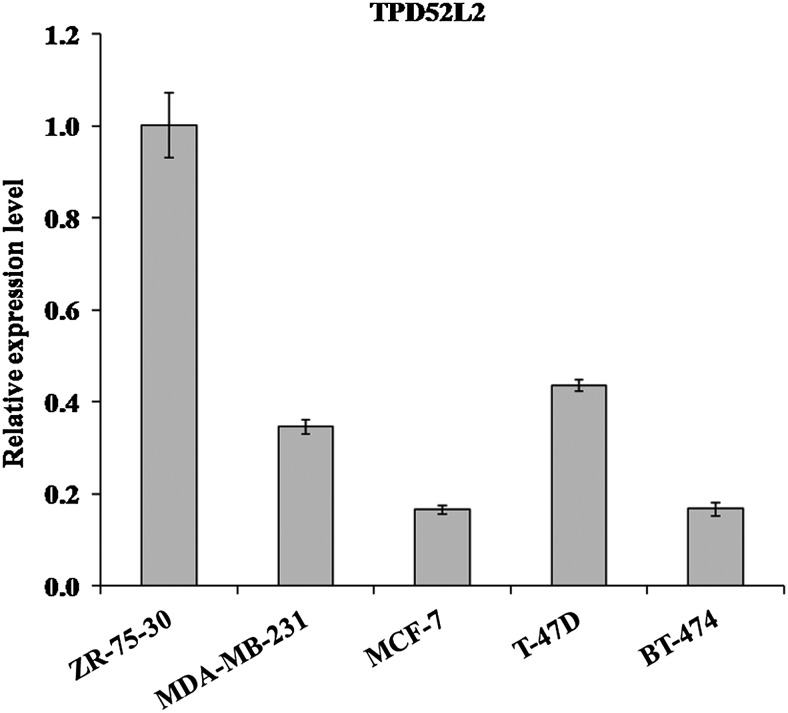
First, qRT-PCR was carried out to measure the expression levels of TPD52L2 in five human breast cancer cell lines ZR-75-30, MDA-MB-231, MCF-7, T-47D, and BT-474. As shown in Figure 1, a higher TPD52L2 expression was observed in ZR-75-30 cells compared with other cell lines. Thus, the authors chose the breast cancer cell line ZR-75-30 for further functional analysis.
FIG. 1.
Relative expression levels of TPD52L2 in human breast cancer cell lines. TPD52L2 expression was evaluated in five human breast cancer cell lines, ZR-75-30, MDA-MB-231, MCF-7, T-47D, and BT-474. Data are represented as mean±standard deviation (SD) of three independent experiments.
TPD52L2 knockdown in ZR-75-30 cells mediated by lentivirus-based RNAi
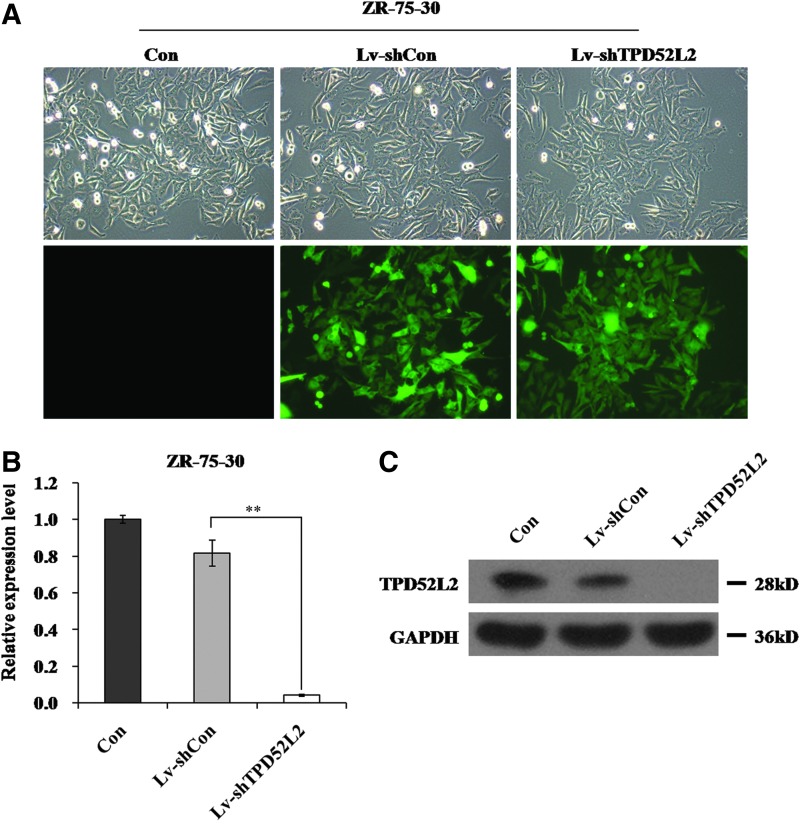
To elucidate the function of TPD52L2 in breast cancer, the TPD52L2 gene expression was inhibited using lentivirus-mediated RNA silencing in ZR-75-30 cells. The authors observed that more than 90% of ZR-75-30 cells showed GFP fluorescence, indicating a successful infection (Fig. 2A). qRT-PCR and western blot analysis were performed to confirm whether Lv-shTPD52L2 can decrease TPD52L2 expression in ZR-75-30 cells. The mRNA level of TPD52L2 was significantly reduced in the Lv-shTPD52L2 group by ∼95% compared with the control group (Fig. 2B). The protein level of TPD52L2 was also obviously downregulated in the Lv-shTPD52L2 group compared with controls (Fig. 2C). All these data showed that Lv-shTPD52L2 could specifically and strongly reduce TPD52L2 expression in ZR-75-30 cells.
FIG. 2.
Knockdown efficiency of TPD52L2 in ZR-75-30 cells. (A) Green fluorescent protein (GFP) expression indicates that ZR-75-30 cells were successfully infected with Lv-shCon and Lv-shTPD52L2. (B) Knockdown efficiency of TPD52L2 was analyzed by quantitative real-time PCR. Actin was used as an internal gene. (C) TPD52L2 protein levels in ZR-75-30 cells were detected by western blot. GAPDH was used as an internal control. Data are represented as mean±SD of three independent experiments. **Represents p<0.01. Color images available online at www.liebertpub.com/cbr
TPD52L2 knockdown suppressed proliferation and colony formation in ZR-75-30 cells
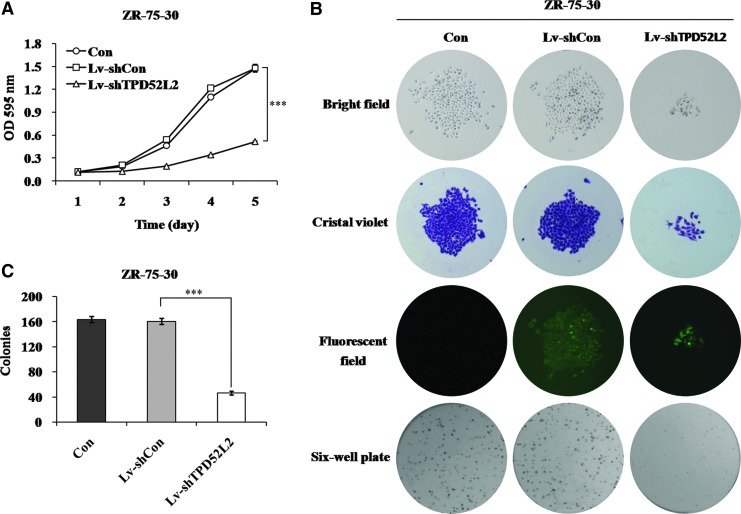
The authors further elucidate whether TPD52L2 knockdown affects proliferation of ZR-75-30 cells by using MTT assay. The proliferation ability of ZR-75-30 cells was significantly suppressed after TPD52L2 depletion (Fig. 3A). Downregulation of TPD52L2 resulted in decrease of cell proliferation by 72% and 65% compared with Lv-shCon infected cells on day 4 and 5 after infection. As shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/cbr), the phenotype of cell proliferation inhibition by Lv-shTPD52L2 could be rescued by re-expression of TPD52L2, suggesting that the TPD52L2 RNAi was specific. The difference between the rescue group and Lv-shCon group might result from the cytotoxicity produced during transduction.
FIG. 3.
TPD52L2 knockdown suppresses ZR-75-30 cell proliferation and colony formation. (A) ZR-75-30 cell proliferation was measured by MTT assay. (B) Representative images of single colony (the upper three panels) and colonies with crystal violet staining (the lowest panels). (C) The number of colonies was significantly reduced after Lv-shTPD52L2 infection. GFP expression indicates that ZR-75-30 cells were successfully infected with Lv-shCon and Lv-shTPD52L2 during the colony formation. Data are represented as mean±SD of three independent experiments. ***Represents p<0.001. Color images available online at www.liebertpub.com/cbr
Moreover, the authors examined the colony morphology and total number of colonies in ZR-75-30 cells by the colony formation assay. It could be seen from Figure 3B that TPD52L2 knockdown resulted in a significant reduction in both colony size and numbers. The total number of colonies was significantly reduced by nearly 71% in the Lv-shTPD52L2 group in contrast to the Lv-shCon group (Fig. 3C). Taken together, loss of the TPD52L2 function could suppress the proliferation and colony formation ability of ZR-75-30 cells.
TPD52L2 knockdown blocked cell cycle progression in ZR-75-30 cells
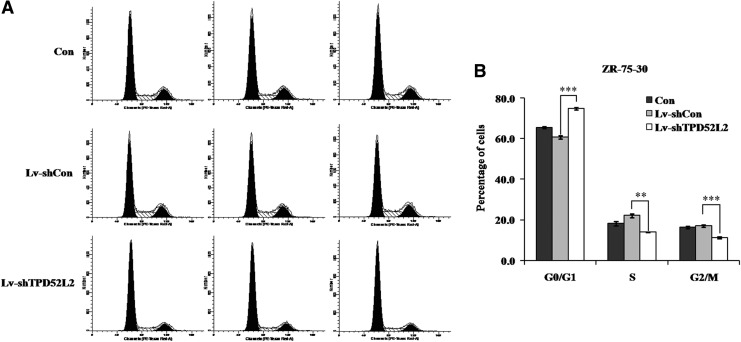
To investigate the mechanisms underlying the impact of TPD52L2 knockdown on cell proliferation and colony formation, flow cytometry analysis was used to detect cell cycle distribution of ZR-75-30 cells (Fig. 4A). As shown in Figure 4B, ∼75% of Lv-shTPD52L2 infected cells were arrested at the G0/G1 phase of the cell cycle, whereas the percentage of control cells in the G0/G1 phase was no more than 65%. Simultaneously, the cell population in Lv-shTPD52L2 groups was reduced by 37% and 34% in the S phase and G2/M phase, respectively, in contrast to Lv-shCon groups. These results showed that TPD52L2 knockdown in ZR-75-30 cells blocked cell cycle progression in the G0/G1 phase, which could contribute to cell growth inhibition.
FIG. 4.
TPD52L2 knockdown induces cell cycle arrest in ZR-75-30 cells. (A) The cell cycle distribution of infected and uninfected cells was analyzed by flow cytometry. (B) Cell numbers of the Lv-shTPD52L2 group were increased significantly in the G0/G1 phase of the cell cycle, while they decreased apparently in S and G2/M phases. Data are represented as mean±SD of three independent experiments. **Represents p<0.01 and ***represents p<0.001.
TPD52L2 knockdown promoted GSK3β phosphorylation in ZR-75-30 cells
To explore the molecular basis for TPD52L2-mediated cell proliferation, the PathScan intracellular signaling array kit was utilized. The detected signaling pathways were involved in cell proliferation, growth, cell cycle, survival, or apoptosis. The alterations of modifications that occurred in signaling proteins are shown in Figure 5. As a result, knockdown of TPD52L2 in ZR-75-30 cells resulted in elevated phosphorylation of GSK3β (Ser9). These results suggested that the involvement of TPD52L2 in breast cancer cell growth may be partly through the modulation of related signaling proteins.
FIG. 5.
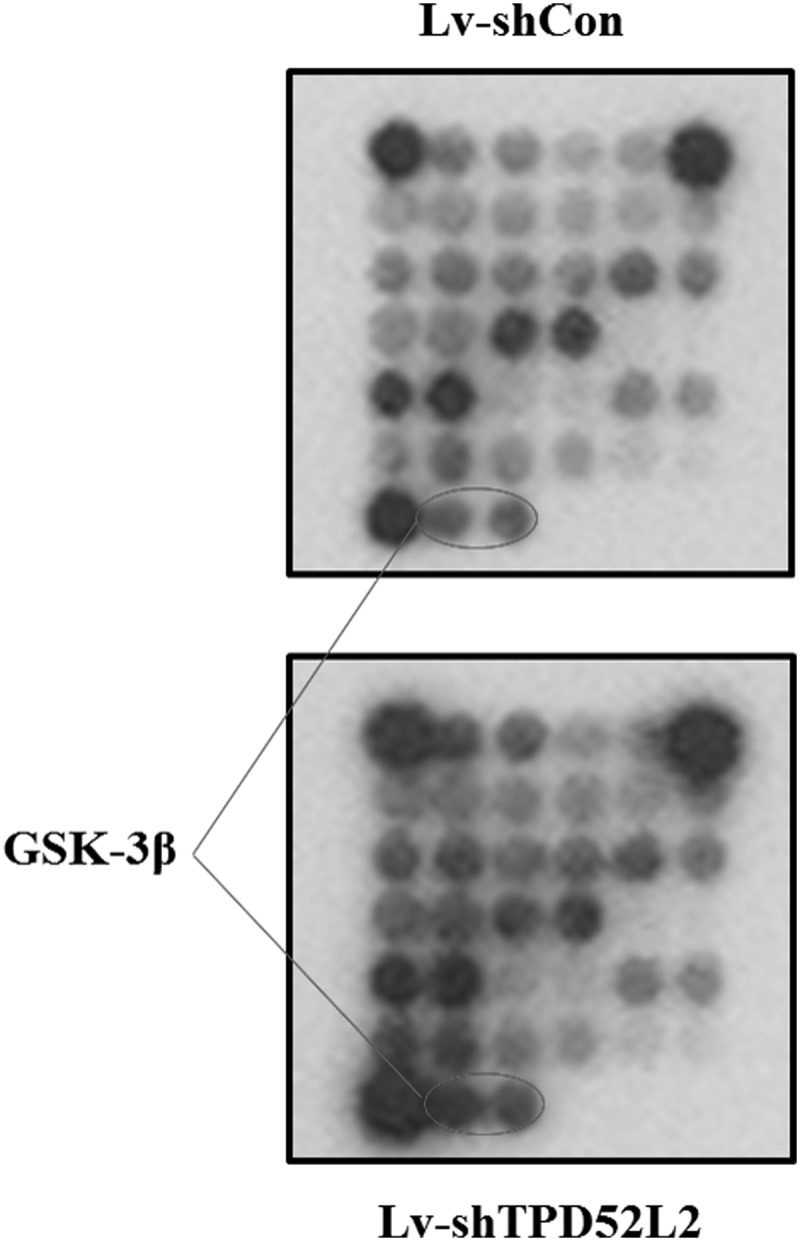
Alterations of protein modification are detected through intracellular signaling assay. Modifications of effector proteins were analyzed in ZR-75-30 cells from Lv-shCon and Lv-shTPD52L2 groups.
Discussion
Breast cancer is one of the most commonly diagnosed female cancers around the world. It has been characterized with a distinct metastatic pattern typically involving spread in the lymph nodes, bone marrow, lung, and liver.12 To identify the function of TPD52L2 in breast cancer cells, the lentivirus-mediated RNAi technology was used, which serves as an effective way to knock out or knock down gene expression.13 The expression of TPD52L2 at transcription and translation levels was specifically downregulated by the constructed lentivirus in the breast cancer cell line ZR-75-30. Knockdown of TPD52L2 resulted in inhibited cell proliferation and impaired colony formation. Moreover, in the rescue experiment, the phenotype of cell growth inhibition by RNAi could be rescued by re-expression of TPD52L2, indicating the importance of TPD52L2 in breast cancer cells.
Several genes have been reported to function in the control of cell proliferation by regulating cell cycle progression. For example, GPER1 agonist G-1 attenuates endothelial cell proliferation and SMC1A silencing suppresses glioblastoma cell proliferation mainly due to disorders in G0/G1, S, and G2/M phases.14,15 TPD52L1 is a member of the TPD52 family and its expression is highly upregulated at the G2/M transition in breast cancer.5 It is also pointed out that deregulated expression of cell cycle-regulated protein TPD52L1 can adversely affect mitosis completion.5 In this study, knockdown of TPD52L2 in ZR-75-30 cells increased the cell percentage of the G0/G1 phase and decreased the cell populations in the S phase and G2/M phase, which could contribute to inhibition of cell proliferation. To further explore the underlying signaling pathways mediated by TPD52L2 in breast cancer cells, the authors detected the modifications on a set of cellular proteins using a PathScan intracellular signaling array kit. Knockdown of TPD52L2 in ZR-75-30 cells resulted in elevated phosphorylation of GSK3β. Phosphorylation of the multifunctional kinase GSK-3β at Ser9 inhibits its activity.16,17 Inhibition of GSK-3β enhances reovirus-induced apoptosis in colon cancer cells.18 In addition, the inhibition of GSK-3β in breast cancer cells leads to phenotype changes that are characteristic of more aggressive malignancy, including epithelial to mesenchymal transition (EMT) and cancer stem cell properties.19 It has been shown that ribophorin II promotes breast tumor initiation and metastasis through the functional suppression of GSK-3β.20 Therefore, the authors suggested that knockdown of TPD52L2 might also induce cell apoptosis and EMT, which needs further investigation.
Previous studies showed that TPD52 performed high-level transcription in prostate cancer, while its downregulation was associated with decreased cell proliferation and invasion in multiple cell types.21,22 Its coiled-coil motif is indeed necessary for interactions with TPD52 proteins and these interactions could be stabilized by the C-terminal protein regions.23 TPD52L2, a member of the tumor protein D52-like family, could interact with hABCF3 to enhance liver cancer cell proliferation,10 suggesting that TPD52L2 may interact with the hABCF3-like protein through its coiled-coil structure and further implicate in the development of cancer. The authors thus supposed that TPD52L2 may be involved in the mitosis process through interaction with the hABCF3-like protein to promote breast cancer cell proliferation. Further studies are required to confirm this hypothesis in in vivo experiments.
The cell proliferation in a primary and metastatic tumor is a basic characteristic during breast cancer development.24 In consequence, control of cell proliferation will effectively inhibit tumor growth and metastasis. This study shows that TPD52L2 knockdown inhibits the proliferation of breast cancer ZR-75-30 cells by accumulating cells in the G0/G1 phase. This investigation may provide a potential therapeutic approach for the treatment of breast cancer.
Supplementary Material
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Shadeo A, Lam WL. Comprehensive copy number profiles of breast cancer cell model genomes. Breast Cancer Res 2006;8:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoeli-Lerner M, Yiu GK, Rabinovitz I, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 2005;20:539. [DOI] [PubMed] [Google Scholar]
- 3.Nahta R, Yu D, Hung M-C, et al. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006;3:269. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JA, Nourse CR, Basset P, et al. Identification of homo- and heteromeric interactions between members of the breast carcinoma-associated D52 protein family using the yeast two-hybrid system. Oncogene 1998;16:873. [DOI] [PubMed] [Google Scholar]
- 5.Boutros R, Byrne JA. D53 (TPD52L1) is a cell cycle-regulated protein maximally expressed at the G2-M transition in breast cancer cells. Exp Cell Res 2005;310:152. [DOI] [PubMed] [Google Scholar]
- 6.Ummanni R, Teller S, Junker H, et al. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J 2008;275:5703. [DOI] [PubMed] [Google Scholar]
- 7.Alagaratnam S, Hardy JR, Lothe RA, et al. TPD52, a candidate gene from genomic studies, is overexpressed in testicular germ cell tumours. Mol Cell Endocrinol 2009;306:75. [DOI] [PubMed] [Google Scholar]
- 8.Cao Q, Chen J, Zhu L, et al. A testis-specific and testis developmentally regulated tumor protein D52 (TPD52)-like protein TPD52L3/hD55 interacts with TPD52 family proteins. Biochem Biophys Res Commun 2006;344:798. [DOI] [PubMed] [Google Scholar]
- 9.Barbaric D, Byth K, Dalla-Pozza L, et al. Expression of tumor protein D52-like genes in childhood leukemia at diagnosis: Clinical and sample considerations. Leuk Res 2006;30:1355. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Lin Y, Shi H, et al. hABCF3, a TPD52L2 interacting partner, enhances the proliferation of human liver cancer cell lines in vitro. Mol Biol Rep 2013;40:5759. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Hu Y-H. Lentivirus-mediated RNA Interference. Chin J Cell Biol 2006;4:001 [Google Scholar]
- 12.Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y-L, Yu J-M, Song X-R, et al. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol Ther 2006;5:1320. [DOI] [PubMed] [Google Scholar]
- 14.Holm A, Baldetorp B, Olde B, et al. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J Vasc Res 2011;48:327. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Zhang Z, Wang R, et al. siRNA-mediated knockdown of SMC1A expression suppresses the proliferation of glioblastoma cells. Mol Cell Biochem 2013:381:209. [DOI] [PubMed] [Google Scholar]
- 16.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs KM, Bhave SR, Ferraro DJ, et al. GSK-3beta: A bifunctional role in cell death pathways. Int J Cell Biol 2012;2012:930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min HJ, Koh SS, Cho IR, et al. Inhibition of GSK-3beta enhances reovirus-induced apoptosis in colon cancer cells. Int J Oncol 2009;35:617. [PubMed] [Google Scholar]
- 19.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 2010;16:2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi RU, Takeshita F, Honma K, et al. Ribophorin II regulates breast tumor initiation and metastasis through the functional suppression of GSK3beta. Sci Rep 2013;3:2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Kamili A, Hardy JR, et al. Tumor protein D52 represents a negative regulator of ATM protein levels. Cell Cycle 2013;12:3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin MA, Varambally S, Beroukhim R, et al. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res 2004;64:3814. [DOI] [PubMed] [Google Scholar]
- 23.Sathasivam P, Bailey AM, Crossley M, et al. The role of the coiled-coil motif in interactions mediated by TPD52. Biochem Biophys Res Commun 2001;288:56. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Ho P-C, Lo Y-H, et al. Interaction of proliferation cell nuclear antigen (PCNA) with c-Abl in cell proliferation and response to DNA damages in breast cancer. PLoS One 2012;7:e29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.