Abstract
Cannabis is used widely in the United States, both recreationally and for medical purposes. Current methods for analysis of cannabinoids in human biological specimens rely on complex extraction process and lengthy analysis time. We established a rapid and simple assay for quantification of Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), 11-hydroxy Δ9-tetrahydrocannabinol (11-OH THC) and 11-nor-9-carboxy-Δ9-tetrahydrocannbinol (THC-COOH) in human plasma by U-HPLC-MS/MS using Δ9-tetrahydrocannabinol-D3 as the internal standard. Chromatographic separation was achieved on an Acquity BEH C18 column using a gradient comprising of water (0.1% formic acid) and methanol (0.1% formic acid) over a 6 min run-time. Analytes from 200 µL plasma were extracted using acetonitrile (containing 1% formic acid and THC-D3). Mass spectrometry was performed in positive ionization mode, and total ion chromatogram was used for quantification of analytes. The assay was validated according to guidelines set forth by Food and Drug Administration of United States. An eight-point calibration curve was fitted with quadratic regression (r2>0.99) from 1.56 to 100 ng mL−1 and a lower limit of quantification (LLOQ) of 1.56 ng mL−1 was achieved. Accuracy and precision calculated from six calibration curves was between 85 to 115% while the mean extraction recovery was >90% for all the analytes. Several plasma phospholipids eluted after the analytes thus did not interfere with the assay. Bench-top, freeze-thaw, auto-sampler and short-term stability ranged from 92.7 to 106.8% of nominal values. Application of the method was evaluated by quantification of analytes in human plasma from six subjects.
Keywords: Cannabinoids, THC, CBD, plasma, U-HPLC-MS/MS, Protein precipitation
1. Introduction
Cannabis sativa L., commonly known as marijuana, is one of the most controversial and abused recreational natural product in the world [1]. In a recently published survey by US National Highway Safety Administration, cannabis is the most common illicit drug detected in drivers [2]. The psychoactive properties of marijuana are attributed to a group of compounds known as cannabinoids. Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the two most abundant cannabinoids in marijuana, THC being a strong psychoactive agent [3]. A number of preclinical and clinical trials are currently underway to study the efficacy of marijuana in different disease conditions including HIV, cancer, and pain [4].
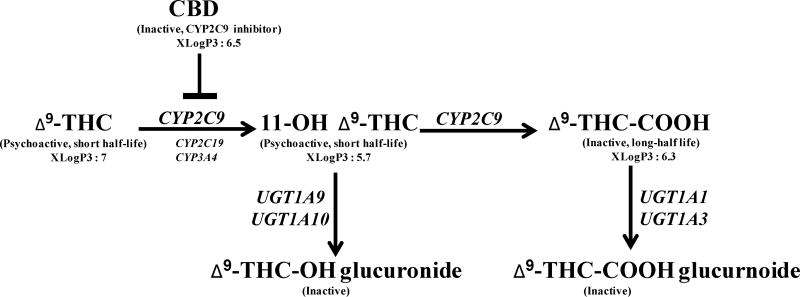
In vitro studies using human liver microsomes have shown that THC is primarily metabolized by cytochrome P450 (CYP)2C9 to a short-lived hydroxylated active metabolite, 11-hydroxy Δ9-tetrahydrocannabinol (11-OH THC) [5][5] (Fig. 1). CYP2C19 and CYP3A4 also oxidize THC but with a very low catalytic activity as compared to CYP2C9 [5]. The primary metabolite is further oxidized by CYP2C9 (major enzyme) and CYP2C19, CYP3A4 (minor enzymes) to generate an inactive metabolite, 11-nor-9-carboxy-Δ9-tetrahydrocannbinol (THC-COOH) [5, 6]. Recently, THC-COOH has emerged as a biomarker for detection of cannabis use in clinical, workplace and forensic fields [7]. Phase II metabolism of THC and its metabolites is complex. 11-OH THC is metabolized primarily by UGT1A9 and UGT1A10 while THC-COOH is metabolized by UGT1A1 and UGT1A3 isoforms, resulting in more hydrophilic metabolites that are renally cleared [8]. Plasma concentrations of THC decrease rapidly due to metabolism and distribution in the tissues. The majority of THC dose is excreted via the feces (30–65%) while hepatic and renal clearance is responsible for the elimination of about 20% of THC in the form of conjugated glucuronic acids and free THC hydroxylated metabolites [9].
Fig. 1.
A schematic diagram of Δ9-tetrahydrocannabinol (THC) metabolism in humans.
CYP: Cytochrome P450; UGT: uridine diphosphate-glucuronosyltransferase, values of xLogP3 were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/)
Concomitant administration of THC with CBD enhances the psychoactive effect of THC as CBD inhibits the drug metabolism enzyme (CYP2C9 and CYP2C19) responsible for clearance of THC [5, 10]. Considering this, US FDA has approved dronabinol and nabilone for therapeutic use but both contain only THC, and no CBD [11]. In contrast, European Medicines Agency (EMA) has approved the use of Sativex (nabiximol), a mouth spray containing THC and CBD for patients with multiple sclerosis [11].
Different screening methods and biological matrices are utilized to detect cannabis use for employment verification or forensic purposes [12]. Preliminary testing of cannabinoids is frequently based on immunoassays, but advanced chromatographic techniques are employed for confirmation and quantification. Quantification is often performed in various human matrices including blood, plasma, serum, saliva and urine using techniques such as HPLC[13], GC-MS [14], and HPLC-MS [15]. However, these traditional methods involve elaborate sample preparation, complex derivatization and lengthy analysis time.
The objective of this study was to develop and validate a simple but rapid analytical method to quantify THC, CBD, 11-OH THC and THC-COOH in human plasma. We used Δ9-tetrahydrocannabinol-D3 (THC-D3) as internal standard (IS) for plasma extraction with acetonitrile (containing 1% formic acid and 10 µg mL−1 IS) followed by drying, reconstitution and subsequent analysis of samples on an ultra-performance liquid chromatography-mass spectrometer (U-HPLC-MS).
2. Materials and methods
2.1. Chemicals and reagents
Certified reference material for THC, THC-D3, CBD, 11-OH THC and THC-COOH were procured from Ceriliant Corporation (Round Rock, Texas). Mass spectrometry grade formic acid, methanol, and acetonitrile (methyl cyanide) were procured from Fisher Scientific, Waltham, MA. Acquity U-HPLC BEH C18 analytical and VanGuard pre-column for chromatography were from Waters Corp., Waltham, MA. Blank human plasma was obtained from BioreclamationIVT, Westbury, NY.
2.2. Instrumentation and data processing
An Acquity U-HPLC system equipped with binary pumps, autosampler, inbuilt degasser and column heater coupled with Xevo TQ MS detector (Waters Corp, Milford, MA, USA) was used. A 10 µL sample loop in partial-loop with needle overfill injection mode was used to inject samples. The chromatographic system was controlled with MassLynx Software (V 4.1), and data was processed using the TargetLynx (V 4.1). Samples were centrifuged using an Eppendorf 5810R system (Eppendorf North America, Hauppauge, NY) and extracted samples were dried in Savant SPD1010 SpeedVac system (Thermo Scientific, Holbrook, NY). Calibration curves and graphs were plotted using Prism 6.01 (GraphPad Software Inc., La Jolla, CA).
2.3. LC conditions
Analytes were separated on an Aquity U-HPLC BEH C18 analytical column (2.1 × 50 mm, 1.7 µm particle size, 130Ǻ pore size) preceded by an Acquity U-HPLC BEH C18 VanGuard pre-column (2.1 × 5 mm, 130Ǻ). The flow rate was kept at 0.4 mL min−1, and five µL of sample was injected onto the column. Autosampler was maintained at 10°C throughout the analysis, and the analytical column was maintained at 45°C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). The separation of analytes was achieved by a linear gradient over a run time of 6 min. The gradient conditions were as followed: 75% B to 95% B in 3.5 min, held at 95% B from 3.5 to 4.5 min, 95% B to 75% B at 5.5 min and maintained at 75%B to re-equilibrate the column until the end of run time at 6 min.
2.4. Mass spectrometry conditions
Electrospray ionization (ESI) in positive mode was used for multiple reaction monitoring and quantification of analytes. Major analyte specific mass spectrometer settings used during analysis are given in Table 1. Protonated precursors (M+H)+ were selected based on the intensity, and all analytes were further subjected to fragmentation. Total ion chromatogram (TIC) of all the productions was used for quantification of the analytes. Other parameters used for validation were: capillary voltage 1.30 kV, extractor voltage 3 V, desolvation temperature 500 °C, source temperature 150 °C, desolvation gas flow 1000 L h−1, and collision gas flow 0.15 mL min−1.
Table 1.
Analyte specific mass spectrometry parameters.
| Analyte | Precursor ion (m/z) |
Product ion (m/z) |
Dwell time (s) |
Cone voltage (V) |
Collision voltage (V) |
Retention time (min) |
|---|---|---|---|---|---|---|
| Δ9-THC | 315 | 193, 123, 259 | 0.025 | 26 | 25, 30, 15 | 2.4 |
| CBD | 315 | 193, 123, 259 | 0.025 | 26 | 20, 30, 15 | 1.6 |
| Δ9-THC-D3 | 318 | 196, 262, 126 | 0.025 | 26 | 30, 15, 25 | 2.4 |
| 11-OH Δ9-THC | 331 | 313, 201, 193 | 0.036 | 26 | 15, 25, 30 | 1.4 |
| Δ9-THC-COOH | 345 | 327, 299 | 0.025 | 26 | 15, 20 | 1.6 |
Δ9-THC: Δ9-tetrahydrocannabinol; 11-OH Δ9-THC: 11-hydroxy Δ9-tetrahydrocannabinol; CBD: cannabidiol; Δ9-THC-COOH: 11-nor-9-carboxy-Δ9-tetrahydrocannbinol; Δ9-THC-D3: Δ9-tetrahydrocannabinol-D3. Total ion chromatogram (TIC) of product ions was used for quantification.
2.5. Plasma preparation for analysis
Cannabinoids from spiked human plasma were extracted by a simple protein precipitation method. Calibrators, control blank, double blank and quality control (QC) samples were thawed at 4°C and vortexed thoroughly for 10 s. Subsequently, one mL of acetonitrile (ACN) (containing 1% formic acid (FA) and 10 µg mL−1 THC-D3) was added to 200 µL plasma in a 1.5 mL clear polypropylene tube and vortexed for 10 sec. Double blank was extracted with ACN (containing 1% FA) without any internal standard. After sonicating the mixture for 3 min, samples were vortexed for 10 s and subsequently centrifuged at 10,000 rpm for 5 min. All the extraction steps except drying were carried out at room temperature. After centrifugation, one mL of the supernatant was transferred to a clean glass tube, and the solvent was dried using SpeedVac™ at 60°C. Dried samples were then reconstituted in 200 µL mobile phase (75% A and 25% B) followed by vortex and sonication for 10 s and 3 min, respectively. The solution was transferred to a centrifuge tube and was spun again at 10,000 rpm for 5 min. The resulting clean supernatant was collected and injected into the chromatographic system.
2.6. Validation of the bioanalytical method
Method validation was carried out according to the general recommendation guidelines for bioanalytical methods by the US Food and Drug Administration (FDA) published in 2013 [16]. Various assay validation parameters including selectivity, sensitivity, accuracy, precision, recovery, and stability were determined.
2.6.1. Standard and quality control samples
Pre-prepared reference solutions of THC and CBD (1 mg mL−1 in methanol), and THC-D3, 11-OH THC and THC-COOH (0.1 mg mL−1 in methanol) were procured from Ceriliant. A calibrator stock solution cocktail containing 10 µg mL−1 each of THC, CBD, 11-OH-THC, and THC-COOH was prepared in methanol. The cocktail solution was used to spike blank human plasma to generate calibrators and quality control (QC) samples. The final concentration of solvent in spiked plasma was <5% and all the spiked samples and stocks were stored at −20°C.
2.6.2. Acceptance criteria
The lower limit of quantification (LLOQ) was the lowest concentration of an analyte on a calibration curve and limit of detection (LOD) was the lowest concentration distinguishable from background noise in the blank matrix (S/N>3). LLOQ was selected as the concentration at which bias and coefficient of variation (CV%) were ≤20% of nominal value and a signal to noise ratio (S/N) ≥ 10. Acceptance criterion for QCs (LQC, MQC, and HQC) was bias and CV≤15%. Highest calibrator, defined as Upper Limit of Quantification (ULOQ) of the assay was 100 ng ml−1 for all analytes. HQC response for different anticoagulant within ±20% potassium-EDTA response was considered acceptable.
2.6.3. Selectivity and specificity
U-HPLC-MS/MS methods are highly specific for an analyte; however, endogenous matrix components can interfere with the analysis of samples. Selectivity of the assay in blank plasma was assessed visually for any presence of endogenous matrix components at the analyte specific retention times. Further, we studied the selectivity in plasma from seven different donors at LLOQ. The sensitivity of the method was the lowest analyte concentration measured with acceptable accuracy and precision (LLOQ).
2.6.4. Accuracy and precision
An eight-point calibration curve with concentrations ranging from 1.56–100 ng mL−1 was prepared, and QC samples were at 6.25, 25, and 75 ng mL−1 for low quality control (LQC), middle quality control (MQC), and high quality control (HQC), respectively. Inter-run precision and accuracy of the assay were calculated from six different calibration curves.
2.6.5. Stability and recovery
The recovery was conducted at three QC levels (LQC, MQC, HQC) in triplicates. A set of QCs was prepared in extracted blank plasma, and another was prepared in mobile phase (75% A, 25% B). The assay recovery was calculated by comparing the mean peak areas of QC in blank plasma and mobile phase (representing 100% recovery).
The stability of all the analytes was investigated at LQC and HQC in duplicates. Three cycles of freeze and thaw, bench top (6 h), auto-sampler (10°C for 24 h) and short-term (1-week) stability studies were conducted for all analytes.
2.6.6. Phospholipids elution and matrix effect
We studied the co-elution of analytes and major phospholipids reported previously in the literature by monitoring the precursor ion (Q1) for m/z 496, 522, 524, 758 and 782 and product ion (Q3) with m/z184 [17, 18]. Chromatographic conditions were optimized to separate the elution region of phospholipids and analytes of interest. A post-column divert valve was used to guide unwanted portion of chromatographic runs, mainly containing phospholipids.
Matrix effect was studied using post-column infusion method as described elsewhere [17, 19]. The region of ion suppression was identified by continuous infusion of a solution containing 50 ng ml−1 of all the analytes and simultaneous injection of extracted blank plasma solution or mobile phase.
2.6.7. Anticoagulant specificity
Drug-free plasma was spiked in triplicates at HQC in plasma isolated using three separate anticoagulants i.e. sodium heparin, potassium-EDTA, and sodium fluoride-potassium oxalate. Analyte/internal standard ratio for all the analytes was compared to find percent variability of recovered concentration as compared to potassium EDTA.
2.7. Application of the proposed method
The proposed method was developed in collaboration with co-authors at Brown University for estimation of analytes in human plasma from self-reported marijuana users. The clinical study had received approval from Institutional Review Board at Merriam Hospital, Providence, Rhode Island. In this manuscript, we report the application of the method by estimating cannabinoids in plasma from six subjects.
3. Results
3.1. LC-MS/MS assay
THC and CBD have a molecular weight of 314.45 g mol−1 and show a similar precursor to product ion transitions (314➔123, 193, and 259). We evaluated several mobile phases and C18 analytical columns and found that the current approach provided adequate separation of the two major constituents (THC and CBD) of marijuana. The retention time (RT) for THC-D3, 11-OH THC, and THC-COOH was 2.4, 1.4, and 1.6 min, respectively. THC eluted at 2.4 and CBD at 1.6 min. The mean deviation in RT over the six validation runs for all the analytes was less than 0.5%. Carryover inspected by two successive double blank injections after HQC sample was not significant (<0.05%). Limit of detection for the method was found to be ~0.78 ng ml−1. The precursor and product ions used in the assay (Table 1) were found to be in agreement with the fragmentation proposed previously [20].
3.1.1. Specificity and sensitivity
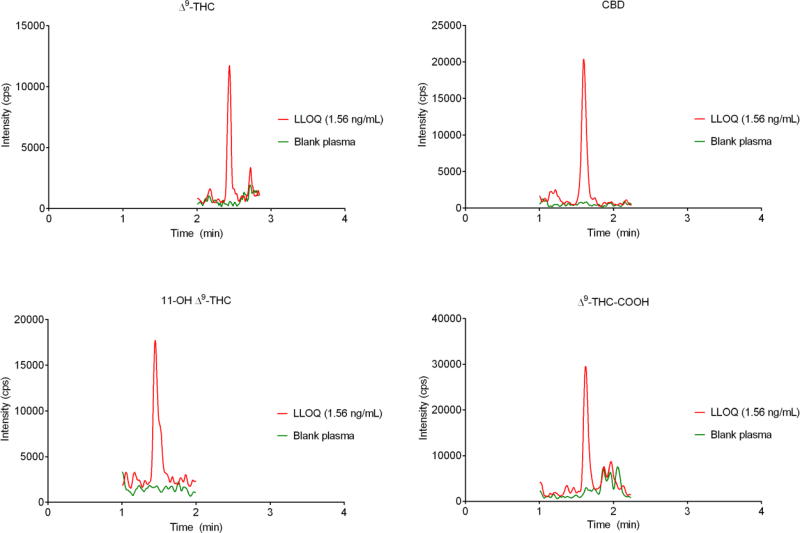
No interference was visually observed at the retention time of analytes in blank plasma extracted from seven different donors. An LLOQ of 1.56 ng mL−1 was achieved for all analytes, and a chromatogram of an extracted LLOQ is shown in Fig. 2.
Fig. 2.
Chromatograms for blank plasma (green) and spiked plasma sample at the lower limit of quantification (red).
3.1.2. Precision and accuracy
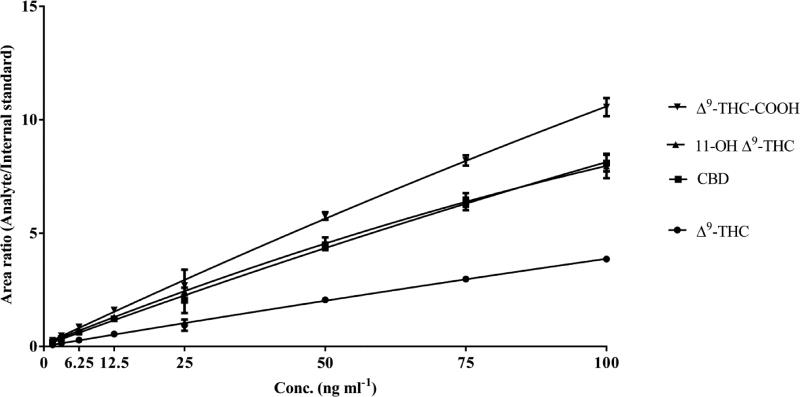
An eight-point calibration curve (Fig. 3) from a range of 1.56 to 100 ng mL−1 was fitted with quadratic regression and correlation coefficient (r2) was ≥0.99 for all the analytes while using a 1/x2 weighting factor. Deviations in calibrators and QC samples were less than 10% of nominal concentrations for all of the compounds. The accuracy of the assay for different analytes was between 85.94 to 113.01% of their nominal values for calibrators (Table 2) and between 93.48 to 103.6% for inter-run QCs (Table 3).
Fig. 2.
Calibration curves for different cannabionoids; data represent mean ± SD of six validation runs.
Table 2.
Summary of assay parameters for calibrators.
| Nominal Conc. (ng mL−1) |
1.56 (LLOQ) |
3.12 | 6.25 | 12.50 | 25.00 | 50.00 | 75.00 | 100.00 (ULOQ) |
r2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Δ9-THC | Mean | 1.48 | 3.08 | 6.16 | 12.60 | 25.26 | 50.28 | 74.31 | 100.32 | 0.9947 |
| SD | 0.19 | 0.15 | 0.24 | 0.31 | 0.37 | 0.47 | 0.76 | 0.33 | ||
| %CV | 13.06 | 5.02 | 3.91 | 2.47 | 1.47 | 0.94 | 1.03 | 0.33 | ||
| %Bias | −4.89 | −1.75 | −1.38 | 0.79 | 1.05 | 0.55 | −0.92 | 0.32 | ||
|
| ||||||||||
| CBD | Mean | 1.38 | 3.11 | 6.27 | 12.74 | 25.18 | 50.14 | 74.46 | 100.27 | 0.9919 |
| SD | 0.11 | 0.11 | 0.17 | 0.32 | 0.41 | 0.41 | 0.94 | 0.44 | ||
| %CV | 8.19 | 3.55 | 2.69 | 2.51 | 1.61 | 0.82 | 1.27 | 0.44 | ||
| % Bias | −11.59 | −0.71 | 0.28 | 1.92 | 0.73 | 0.29 | −0.72 | 0.27 | ||
|
| ||||||||||
| Δ9-THC-COOH | Mean | 1.36 | 3.05 | 6.26 | 12.72 | 25.42 | 50.37 | 73.93 | 100.50 | 0.9932 |
| SD | 0.26 | 0.21 | 0.23 | 0.39 | 0.50 | 0.51 | 0.68 | 0.33 | ||
| %CV | 19.40 | 6.80 | 3.74 | 3.04 | 1.96 | 1.02 | 0.91 | 0.33 | ||
| % Bias | −13.01 | −2.60 | 0.12 | 1.74 | 1.67 | 0.73 | −1.43 | 0.50 | ||
|
| ||||||||||
| 11-OH Δ9-THC | Mean | 1.78 | 3.27 | 6.19 | 12.60 | 24.72 | 49.79 | 75.82 | 99.50 | 0.9929 |
| SD | 0.24 | 0.24 | 0.33 | 0.33 | 1.03 | 0.89 | 2.56 | 1.31 | ||
| %CV | 13.30 | 7.41 | 5.27 | 2.64 | 4.17 | 1.79 | 3.38 | 1.32 | ||
| % Bias | 14.06 | 4.38 | −0.90 | 0.82 | −1.11 | −0.43 | 1.10 | −0.50 | ||
Data from 6 validation runs, n=6 for each concentration (two replicates for concentration per validation run), %CV calculated as (SD/mean) ×100, %bias calculated as 100×(mean-nominal)/nominal
Table 3.
Summary of inter-run assay parameters for quality control (QC) samples.
| QC sample Nominal (ng mL−1) |
Conc. | LLOQ* 1.56 |
LQC# 6.25 |
MQC# 25 |
HQC# 75 |
ULOQ* 100 |
|
|---|---|---|---|---|---|---|---|
| Δ9-THC | Mean | 1.57 | 6.66 | 25.07 | 75.22 | 101.03 | |
| SD | 0.02 | 0.17 | 0.18 | 1.06 | 0.68 | ||
| %CV | 1.52 | 2.50 | 0.72 | 1.06 | 0.68 | ||
| % Bias | 0.94 | 6.52 | 0.26 | 0.29 | 1.03 | ||
|
| |||||||
| CBD | Mean | 1.53 | 6.43 | 24.10 | 76.85 | 102.61 | |
| SD | 0.01 | 0.20 | 0.09 | 1.05 | 1.14 | ||
| %CV | 0.17 | 3.04 | 0.38 | 1.37 | 1.11 | ||
| % Bias | −2.08 | 2.85 | −3.60 | 2.47 | 2.61 | ||
|
| |||||||
| 11-OH Δ9-THC | Mean | 1.53 | 6.43 | 23.98 | 80.95 | 100.65 | |
| SD | 0.09 | 0.17 | 0.07 | 1.69 | .30 | ||
| %CV | 5.61 | 97.18 | 0.27 | 2.09 | 1.29 | ||
| % Bias | −1.78 | 2.70 | −4.07 | 7.93 | 0.65 | ||
|
| |||||||
| Δ9-THC-COOH | Mean | 1.52 | 6.46 | 24.55 | 76.92 | 102.77 | |
| SD | 0.04 | 0.07 | 0.13 | 1.00 | 0.44 | ||
| %CV | 2.35 | 1.16 | 0.54 | 1.30 | 0.43 | ||
| % Bias | −2.74 | 3.43 | −1.80 | 2.56 | 2.77 | ||
Data from 6 validation runs n=18 for each concentration (three replicates for concentration per validation)
n=12 for each concentration (two replicates for concentration per validation), %CV calculated as (SD/mean)×100, %bias calculated as 100×(mean-nominal)/nominal
3.1.3. Recovery and stability
The extraction recovery of the method for all analytes in the assay ranged from 92.24–99.90% (Table 4). Stability of the method was assessed at LQC and HQC for all the analytes (n=3). Bias for auto-sampler, freeze-thaw, bench-top and short-term stability ranged from −4.40 to 13.72% (Table 5).
Table 4.
Extraction recovery of different analytes expressed as a percentage.
| LQC (%) | MQC (%) | HQC (%) | |
|---|---|---|---|
| Δ9-THC | 93.76±5.20 | 99.90±0.24 | 92.24±1.36 |
| CBD | 96.28±3.72 | 99.68±1.92 | 94.81±0.67 |
| 11-OH Δ9-THC | 96.58±3.73 | 99.56±0.76 | 95.70±0.71 |
| Δ9-THC-COOH | 97.45±1.69 | 96.96±2.51 | 92.37±0.69 |
LQC-6.25 ng mL−1, MQC-25 ng mL−1, HQC-75 ng mL−1, n=3
Table 5.
Result of stability studies performed at low quality control (LQC) and high quality control (HQC) levels.
| Auto-sampler stability (24 hours, 10°C) |
Freeze-thaw stability (3 cycles) |
Bench-top (6 hour) |
Short term (1 week) |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| LQC | HQC | LQC | HQC | LQC | HQC | LQC | HQC | ||
| Δ9-THC | Mean | 6.66 | 75.50 | 6.43 | 73.18 | 6.94 | 77.18 | 7.11 | 72.77 |
| % Bias | 6.61 | 0.67 | 2.93 | −2.42 | 11.06 | 2.90 | 13.72 | −2.97 | |
| % CV | 2.44 | 0.55 | 2.53 | 3.91 | 4.78 | 1.85 | - | 3.74 | |
|
| |||||||||
| CBD | Mean | 6.69 | 75.51 | 6.47 | 73.04 | 6.68 | 79.13 | 6.60 | 74.85 |
| % Bias | 7.04 | 0.68 | 3.48 | −2.61 | 6.92 | 5.50 | 5.62 | −0.19 | |
| % CV | 1.18 | 1.93 | 1.22 | 1.72 | 2.32 | 1.85 | 4.82 | 3.26 | |
|
| |||||||||
| 11-OH Δ9-THC | Mean | 6.47 | 77.78 | 6.36 | 71.70 | 6.04 | 78.51 | 6.97 | 82.43 |
| % Bias | 3.47 | 3.70 | 1.69 | −4.40 | −3.34 | 4.69 | 11.54 | 9.91 | |
| % CV | 3.65 | 2.51 | 1.24 | 1.28 | 2.62 | 2.10 | 1.06 | 1.94 | |
|
| |||||||||
| Δ9-THC-COOH | Mean | 6.84 | 74.05 | 6.27 | 72.14 | 6.44 | 76.06 | 6.72 | 75.81 |
| % Bias | 9.41 | −1.27 | 0.33 | −3.81 | 2.96 | 1.42 | 7.50 | 1.08 | |
| % CV | 5.42 | 2.56 | 2.95 | 1.11 | 0.91 | 1.14 | 0.99 | 6.13 | |
%Bias calculated as 100×(mean-nominal)/nominal; CV calculated as 100×SD/mean; n=2 LQC-6.25 ng mL−1, HQC-75 ng mL−1
3.1.4. Phospholipids and matrix effect
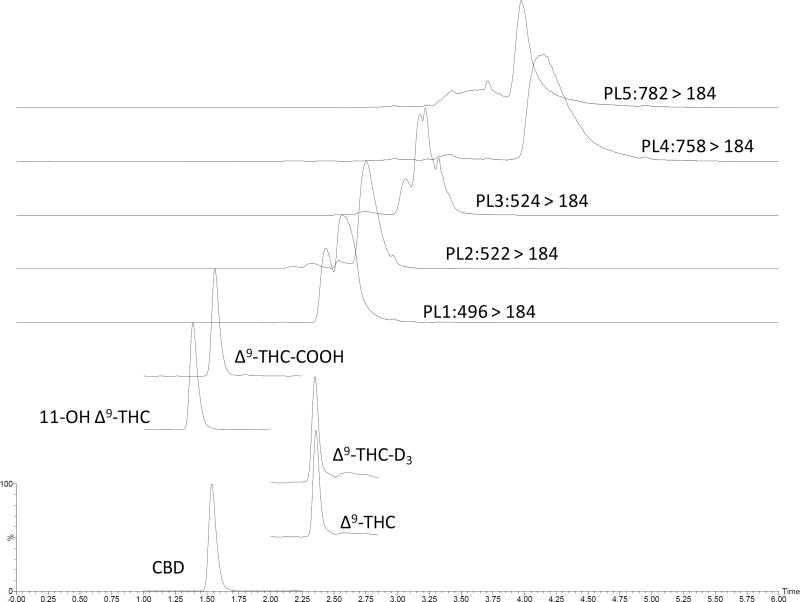
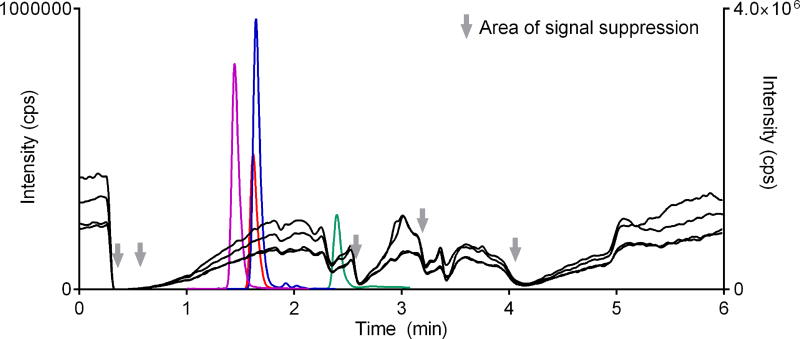
Most of the phospholipids eluted after the analytes. However we noticed some overlap for THC (Fig. 4). Post-column infusion showed that there was some degree of suppression at the RT of THC and THC-D3 (Fig. 5). Though THC and THC-D3 elution may have slightly overlapped with one of the phospholipids (Q1 m/z 496), any suppressive effect was normalized when analyte/IS area was calculated.
Fig. 4.
Elution of analytes and major plasma phospholipids in chromatography. PL-Phospholipids Phospholipids precursor m/z 496, 522, 524, 758 and 782; product m/z 184.
Fig. 5.
The region of signal suppression due to the elution of endogenous matrix components observed by injection of extracted blank plasma and continuous infusion of analytes post-column.
3.1.5. Effect of different anticoagulants
Although the method was validated with blank plasma collected with potassium-EDTA, as part of method adaptability, the effect of various anticoagulants on the extraction of analytes spiked at HQC was studied. We found that variability of results among sodium heparin and sodium fluoride-potassium oxalate was less than ± 15% as compared to potassium-EDTA (Table 6). The results from sodium heparin were found to be closer to potassium-EDTA than sodium fluoride-potassium oxalate.
Table 6.
Anticoagulant effect on bias and variability studied at HQC level.
| Sodium Heparin | Potassium-EDTA | Sodium fluoride-potassium oxalate |
||
|---|---|---|---|---|
| Δ9-THC | %Bias | 0.02 | −0.02 | −6.65 |
| %CV | 4.77 | 2.29 | 13.28 | |
|
| ||||
| CBD | %Bias | −11.06 | −0.01 | 5.02 |
| %CV | 9.44 | 5.93 | 13.34 | |
|
| ||||
| 11-OH Δ9-THC | %Bias | −13.05 | 0.01 | 3.90 |
| %CV | 9.79 | 1.71 | 10.67 | |
|
| ||||
| Δ9-THC-COOH | %Bias | −4.87 | 0.01 | 9.66 |
| %CV | 6.73 | 6.44 | 12.7 | |
%Bias calculated with respect to potassium-EDTA, n=3
3.2. Application of the assay for quantification of cannabinoids in human plasma
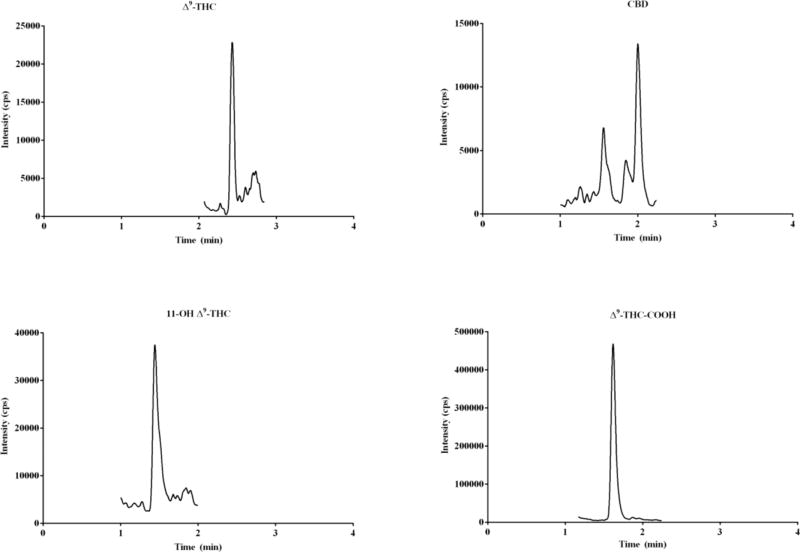
The assay was successfully applied for quantification of cannabinoids in plasma from six subjects (Table 7). Traces of THC-COOH was quantified in all subjects while THC was only detected in the plasma of three subjects. CBD was not detected in any of the samples, and 11-OH THC was found in only two subjects. Chromatogram of cannabinoids that was quantified in plasma from one self-reported cannabis user is shown in Fig. 6.
Table 7.
Concentration of cannabinoids estimated in human plasma from six self-reported cannabis users.
| Δ9-THC | CBD | 11-OH Δ9-THC | Δ9-THC-COOH | |
|---|---|---|---|---|
| Subject 1 | <LLOQ | <LLOQ | <LLOQ | 9.80 |
| Subject 2 | 3.18 | <LLOQ | 3.47 | 35.25 |
| Subject 3 | <LLOQ | <LLOQ | <LLOQ | 8.40 |
| Subject 4 | <LLOQ | <LLOQ | <LLOQ | 6.28 |
| Subject 5 | 2.55 | <LLOQ | 1.69 | 24.07 |
| Subject 6 | 2.34 | <LLOQ | <LLOQ | 44.51 |
<LLOQ represent concentrations below lower limit of quantification, Concentrations in ng mL−1
Fig. 6.
Representative chromatogram of cannabinoids quantified in plasma from one self-reported cannabis user study volunteer.
4. Discussion and conclusion
Previously reported methods for quantification of cannabinoids in human plasma and serum rely on tedious, multi-step liquid-liquid extraction or solid-phase extraction techniques. We have validated a U-HPLC-MS/MS assay utilizing simple protein precipitation for extraction of THC, CBD, 11-OH THC and THC-COOH from human plasma. The novelty of the current assay lies in its simple extraction method, low plasma requirement, and comparatively shorter run-time than published LC-MS/MS-based assays. Moreover, LLOQ of the current assay for all analytes (1.56 ng mL−1) was comparable to published studies that employed more elaborate sample preparation techniques and large plasma volume for analysis [21–23]. Although the extraction solvent diluted the analytes, we dried the samples after extraction and reconstituted to achieve a lower quantification range suitable for clinical analysis. Interestingly, the method was also found to be more sensitive (LOD < 1 ng mL−1) than some of the available methods with complex sample preparation and analysis techniques [15, 24]. We also found that all the analytes in the study ionize well in positive ion mode [M+H]+ which was in contrast to some published reports having used negative ion mode (M−H)− for THC-COOH and 11-OH THC [25, 26]. This approach allowed us to analyze all the analytes in positive ion operation mode.
Anticoagulant used for collection of plasma could influence the analysis and stability of analytes. Scheidweiler and colleagues recently reported a long-term stability (between 6 and 9 months) study for cannabinoids depending on the anticoagulant and storage conditions [27]. These authors concluded that for accurate quantitative analysis of THC and metabolites, blood should be collected with sodium fluoride-potassium oxalate as an anticoagulant and samples can be accurately quantified within 12 weeks from the collection when stored at −20°C.
Phospholipids are responsible for endogenous matrix effects and ion suppression in the analysis of compounds in human plasma and serum [18]. The inclusion of major phospholipids’ transition enabled us to avoid co-elution of phospholipids of analytes and post-column infusion allowed to find the regions of ion suppression. Ion suppression in ACN protein precipitation methods is a common drawback of such assays [19]. However, where good separation between analytes and region of suppression is not achieved, an appropriate internal standard should be included in the assay to account for the suppression of co-eluting analyte.
Additionally, we confirmed that acidic extraction conditions did not interfere with the assay by conversion of CBD into THC. A previous study reported the unsuitability of derivatizing reagents (Trifluoroacetic anhydride, TFAA) for quantification of cannabinoids due to the conversion of CBD to THC under acidic conditions [28]. The suitability of our method was investigated by extraction of plasma spiked with CBD (0, 12.5, 25, 50 and 100 ng mL−1) and subsequent analysis using the assay described above. We found no conversion of CBD into THC at the extraction conditions as evident from the lack of any peak for THC. Authors speculate that low concentration of formic acid (1%) used in our method does not cause conversion of CBD to THC.
The use of cannabis with ethanol is usually reported among fatal motor vehicle accidents, and the detrimental effects appear to be dose-dependent [29]. A clear consensus among different US states on the permissible THC concentration is lacking; however, 5 ng mL−1 is commonly reported as the cutoff limit [30]. Also, estimating the time of last use of cannabis in user is complicated due to polymorphic differences and different metabolism in frequent versus non-frequent users. Higher plasma and urine concentration of THC metabolites were reported in frequent marijuana users without any change in other pharmacokinetic parameters namely, area under the curve, the volume of distribution and elimination half-lives [31]. Sachse-Seeboth et al. found that AUC of THC in CYP2C9 *3/*3 carriers (slow metabolizers) was almost 3-fold higher than *1/*1 carriers (fast metabolizers), suggesting the faster metabolism and clearance in later cases [32]. Huestis’ group at National Institute on Drug Abuse has developed several models for prediction of the last cannabis use from THC and THC/THC-COOH ratio [33, 34]. Therefore, two additional metabolites of THC (11-OH THC and THC-COOH) are frequently quantified along with THC. Similar to THC, the primary metabolite 11-OH-THC, has a short half-life in blood but in contrast, THC-COOH remains in circulation from days to weeks because of its longer half-life [35]. Cut off concentration of THC-COOH metabolite (15 ng mL−1) is well within the range of currently established method [36].
The application of the proposed method for analysis of clinical samples was examined by quantification of cannabinoids in human plasma. We could detect THC in three subjects who had self-reported use of marijuana. Since the blood collection was not part of a controlled study, the authors have no information on the time of last use or the concentration of THC present in marijuana. However, the presence of THC-COOH, which is a long half-life metabolite of THC, supported the frequent use of cannabis by all the subjects [37].
Overall, a simple protein precipitation method for extraction of analytes of interest presents a fast and economical tool for quantitative analysis of cannabinoids. The method was applied successfully for quantification of all the analytes relevant to study THC exposure in plasma and can be easily adapted for similar pharmacokinetic studies in human.
Acknowledgments
This work was supported by National Institute of Health [grant number P30GM110759, U54GM115677, and K24HD080539]; and Brown University [grant number P01AA019072]. Partial support by National Institutes of Health [grant number R15-GM101599, UH3-TR000963] is also greatly appreciated.
Abbreviations
- 11-OH THC
11-hydroxy Δ9-tetrahydrocannabinol
- ACN
acetonitrile
- CBD
cannabidiol
- CV
coefficient of variation
- CYP
cytochrome P450
- ESI
electrospray ionization
- FA
formic acid
- FDA
Food and Drug Administration
- HQC
high quality control
- IS
internal standard
- ISR
incurred sample reanalysis
- LLOQ
lower limit of quantification
- LQC
low quality control
- ME
matrix effect
- MeOH
methanol
- MQC
middle quality control
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- MW
molecular weight
- Q1
precursor ion
- QCs
quality controls
- RT
retention time
- S/N
signal to noise ration
- SD
standard deviation
- TFAA
Trifluoroacetic anhydride
- THC
Δ9-tetrahydrocannabinol
- THC-COOH
11-nor-9-carboxy-Δ9-tetrahydrocannbinol
- THC-D3
Δ9-tetrahydrocannabinol-D3
- UGT
Uridine diphosphate-glucuronosyltransferase
- U-HPLC-MS
Ultra-high performance liquid chromatography mass spectrometer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Authors declare no potential conflict of interest.
References
- 1.Word Drug Report 2015. [accessed 12.09.16];United Nations Office on Drugs and Crime (UNODC) 2015 https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 2.Berning RC, Wochinger K. Results of the 2013–2014 National roadside survey of alcohol and drug use by drivers. (Traffic Safety Facts Research Note. Report No. DOT HS 812 118) Washington, DC: National Highway Traffic Safety Administration; 2015. [accessed 12.09.16]. http://www.nhtsa.gov/Driving-Safety/Research-&-Evaluation/Alcohol-and-Drug-Use-By-Drivers. [Google Scholar]
- 3.Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.National Institute on Drug Abuse; National Institutes of Health. [accessed 12.09.16];U.S. Department of Health and Human Services. https://www.drugabuse.gov/publications/drugfacts/marijuana-medicine,
- 5.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Bland TM, Haining RL, Tracy TS, Callery PS. CYP2C–catalyzed delta9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochem. Pharmacol. 2005;70:1096–1103. doi: 10.1016/j.bcp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Karschner EL, Schwope DM, Schwilke EW, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Predictive model accuracy in estimating last Delta9-tetrahydrocannabinol (THC) intake from plasma whole blood cannabinoid concentrations in chronic, daily cannabis smokers administered subchronic oral THC. Drug. Alcohol. Depend. 2012;125:313–319. doi: 10.1016/j.drugalcdep.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, Finel M, Miller GP, Radominska-Pandya A, Moran JH. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab. Dispos. 2009;37:1496–1504. doi: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin. Pharmacol. Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 10.Yamaori S, Koeda K, Kushihara M, Hada Y, Yamamoto I, Watanabe K. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab. Pharmacokinet. 2012;27:294–300. doi: 10.2133/dmpk.dmpk-11-rg-107. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012;87:172–186. doi: 10.1016/j.mayocp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAMHSA drug testing. [accessed 12.09.16];The Substance Abuse and Mental Health Services Administration. http://www.samhsa.gov/workplace/resources/drug-testing.
- 13.De Backer B, Debrus B, Lebrun P, Theunis L, Dubois N, Decock L, Verstraete A, Hubert P, Charlier C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:4115–4124. doi: 10.1016/j.jchromb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F. Simultaneous sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extract. J. Anal. Toxicol. 2005;29:782–789. doi: 10.1093/jat/29.8.782. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira H, Verstraete A, Proenca P, Corte-Real F, Monsanto P, Vieira DN. Validated method for the simultaneous determination of Delta9-THC Delta9-THC-COOH in oral fluid, urine and whole blood using solid-phase extraction and liquid chromatography-mass spectrometry with electrospray ionization. Forensic Sci. Int. 2007;170:148–155. doi: 10.1016/j.forsciint.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Bioanalytical method validation, Guidance for Industry. [accessed 10.21.16];US Food and Drug Administration, 2013. 2013 http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf.
- 17.Macwan JS, Ionita IA, Dostalek M, Akhlaghi F. Development validation of a sensitive, simple, and rapid method for simultaneous quantitation of atorvastatin and its acid and lactone metabolites by liquid chromatography-tandem mass spectrometry (LC-MS/MS) Anal. Bioanal. Chem. 2011;400:423–433. doi: 10.1007/s00216-011-4804-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Wujcik CE. Overcoming ionization effects through chromatography: a case study for the ESI-LC-MS/MS quantitation of a hydrophobic therapeutic agent in human serum using a stable-label internal standard. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:2003–2010. doi: 10.1016/j.jchromb.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Bonfiglio R, King RC, Olah TV, Merkle K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 1999;13:1175–1185. doi: 10.1002/(SICI)1097-0231(19990630)13:12<1175::AID-RCM639>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Maralikova B, Weinmann W. Simultaneous determination of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol and 11-nor-9-carboxy- Delta9-tetrahydrocannabinol in human plasma by high-performance liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004;39:526–531. doi: 10.1002/jms.616. [DOI] [PubMed] [Google Scholar]
- 21.Aizpurua-Olaizola O, Zarandona I, Ortiz L, Navarro P, Etxebarria N, Usobiaga A. Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis. Drug Test. Anal. 2016 doi: 10.1002/dta.1998. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson RA, Moolchan ET, Barnes A, Levine B, Huestis MA. Validated method for the simultaneous determination of Delta 9-tetrahydrocannabinol (THC), 11-hydroxy-THC and 11-nor-9-carboxy-THC in human plasma using solid phase extraction and gas chromatography-mass spectrometry with positive chemical ionization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;798:145–154. doi: 10.1016/j.jchromb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Jagerdeo E, Schaff JE, Montgomery MA, LeBeau MA. A semi-automated solid-phase extraction liquid chromatography/tandem mass spectrometry method for the analysis of tetrahydrocannabinol and metabolites in whole blood. Rapid Commun. Mass Spectrom. 2009;23:2697–2705. doi: 10.1002/rcm.4174. [DOI] [PubMed] [Google Scholar]
- 24.Concheiro M, de Castro A, Quintela O, Cruz A, Lopez-Rivadulla M. Development and validation of a method for the quantitation of Delta9tetrahydrocannabinol in oral fluid by liquid chromatography electrospray-mass-spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;810:319–324. doi: 10.1016/j.jchromb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Tai SS, Welch MJ. Determination of 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in a urine-based standard reference material by isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. J. Anal. Toxicol. 2000;24:385–389. doi: 10.1093/jat/24.6.385. [DOI] [PubMed] [Google Scholar]
- 26.Zanchetti G, Floris I, Piccinotti A, Tameni S, Polettini A. Rapid and robust confirmation and quantification of 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) in urine by column switching LC-MS-MS analysis. J. Mass Spectrom. 2012;47:124–130. doi: 10.1002/jms.2034. [DOI] [PubMed] [Google Scholar]
- 27.Scheidweiler KB, Himes SK, Desrosiers NA, Huestis MA. In vitro stability of free and glucuronidated cannabinoids in blood and plasma collected in plastic gray-top sodium fluoride tubes following controlled smoked cannabis. Forensic Toxicol. 2016;1:179–185. [Google Scholar]
- 28.Andrews R, Paterson S. Production of identical retention times and mass spectra for {delta}9-tetrahydrocannabinol and cannabidiol following derivatization with trifluoracetic anhydride with 1,1,1,3,3,3-hexafluoroisopropanol*. J. Anal. Toxicol. 2012;36:61–65. doi: 10.1093/jat/bkr017. [DOI] [PubMed] [Google Scholar]
- 29.Downey LA, King R, Papafotiou K, Swann P, Ogden E, Boorman M, Stough C. The effects of cannabis and alcohol on simulated driving: Influences of dose and experience. Accid. Anal. Prev. 2013;50:879–886. doi: 10.1016/j.aap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney GR, Huestis MA. Effect of Blood Collection Time on Measured Delta9-Tetrahydrocannabinol Concentrations: Implications for Driving Interpretation and Drug Policy. Clin. Chem. 2016;62:367–377. doi: 10.1373/clinchem.2015.248492. [DOI] [PubMed] [Google Scholar]
- 31.Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J. Anal. Toxicol. 1992;16:228–235. doi: 10.1093/jat/16.4.228. [DOI] [PubMed] [Google Scholar]
- 32.Sachse-Seeboth C, Pfeil J, Sehrt D, Meineke I, Tzvetkov M, Bruns E, Poser W, Vormfelde SV, Brockmoller J. Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin. Pharmacol. Ther. 2009;85:273–276. doi: 10.1038/clpt.2008.213. [DOI] [PubMed] [Google Scholar]
- 33.Huestis MA, Barnes A, Smith ML. Estimating the time of last cannabis use from plasma delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol concentrations. Clin. Chem. 2005;51:2289–2295. doi: 10.1373/clinchem.2005.056838. [DOI] [PubMed] [Google Scholar]
- 34.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) J. Anal. Toxicol. 1992;16:283–290. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- 35.Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther. Drug Monit. 2006;28:155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 36.SAMHSA drug concentration cut off. [accessed 12.09.16];Effective October 2010 The Substance Abuse and Mental Health Services Administration. 2010 http://www.samhsa.gov/sites/default/files/workplace/2010GuidelinesAnalytesCutoffs.pdf.
- 37.Lee D, Bergamaschi MM, Milman G, Barnes AJ, Queiroz RH, Vandrey R, Huestis MA. Plasma Cannabinoid Pharmacokinetics After Controlled Smoking and Ad libitum Cannabis Smoking in Chronic Frequent Users. J. Anal. Toxicol. 2015;39:580–587. doi: 10.1093/jat/bkv082. [DOI] [PMC free article] [PubMed] [Google Scholar]