Abstract
Adults with clinical depression exhibit systematic errors in their recognition and interpretation of affective stimuli. This study investigated the extent to which depression and phases of pregnancy and postpartum influence affective processing of positive and negative information, and the extent to which affective information processing in pregnancy predicts depressive symptoms in postpartum. Data were collected from 80 unmedicated women, diagnosed with major depressive disorder (MDD) or with no psychiatric disorder and between ages 18 and 44 years, during 32–36 weeks of pregnancy and during 6–8 weeks postpartum. All completed a Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) Axis I review, symptom reports, and a computer task measuring affective information processing. Significant group differences were found in which postpartum women with major depression were less responsive to negative and reactions to negative pictorial stimuli, compared with postpartum healthy women. Also, lower ratings of the intensity and reactions to negative stimuli during pregnancy among depressed women predicted postpartum depression severity, even after controlling for depressive severity and affect ratings in pregnancy. Blunted affective reactivity to negative stimuli is a characteristic of depression that was observed among depressed women during pregnancy and postpartum in our study.
Keywords: Major depression, Pregnancy, Postpartum, Affective disorders, Emotion, Processing
1. Introduction
Major depression during pregnancy and postpartum is a major public health concern invoking increased maternal morbidity and mortality (Lindahl et al., 2005; Grace et al., 2003). Even when pregnancy and postpartum progress normatively, the perinatal phase are emotional experiences for many women. Functionally, women are continually prompted to interpret and react to the environment with responses that ensure the well-being of themselves and their infants. Systematic biases in emotional reactions reflected by greater or less responsiveness to emotional information are likely to have important effects on maternal affective experience. Additionally, systematic biases in emotional reactions impact mother–infant interactions, quality of attachment, and child(rens) own affective experiences (Tronick and Beeghly, 2011).
Data suggest that major depression alters an individual’s ability to accurately recognize the valence, described as positivity or negativity, and affective arousal to emotional scenarios. Research indicates systematic biases in evaluative judgments of emotional information with nonpregnant adults who view discrete emotional facial expressions. Adults with major depression show more errors with visual search tasks (Hammar et al., 2003), exhibit reduced accuracy in identifying positive emotions (Surguladze et al., 2004), and need a higher intensity of positive valence in faces to detect happiness (Joormann et al., 2006) compared with healthy adults. Also, depressed adults show an increased likelihood of interpreting neutral faces as sad relative to non-depressed controls (Csukly et al., 2009; Gollan et al., 2008; Leppänen et al., 2004). Finally, depressed adults perceive heightened sadness in sad faces (Bouhuys et al., 1999; Hale et al., 1997), exhibit a preference for mood-congruent stimuli (sad faces) (Sterzer et al., 2011), and show deficits in emotion recognition (Surguladze et al., 2004). Collectively, numerous studies show that (see Demenescu et al., 2010 for review) adults with major depression exhibit difficulties in cognitive processing of emotional information conveyed by heightened reactivity to negative facial expressions and relatively lower reactivity to positive facial expressions. Characterizing the extent to which perinatal depression is associated with systematic biases in responses to affective stimuli has yet to generate the same level of research attention.
Research shows that depressed women show altered cognitive processing in early pregnancy (Pearson et al., 2010). Yet, to the authors’ knowledge, there are no studies that have examined affective reactivity among depressed females in later stages of pregnancy and also during the first few weeks of postpartum using emotional stimuli that represent social and nonsocial contexts.
Data indicate that women in pregnancy and postpartum have difficulty identifying emotional experience. Healthy pregnant women show increased affective arousal ratings in response to negative stimuli compared with nonpregnant healthy women (Siefritz et al., 2003). Data from brain scans show that healthy pregnant women in their second trimester have an increased activation to fear-related stimuli in the prefrontal cortex relative to other trimesters (Roos et al., 2011), though harm avoidance moderates increased activation, namely, in the left amygdala activation when viewing negative visual pictures of infants faces (Baeken et al., 2009). Likewise, healthy mothers in the early postpartum stage exhibit changes in their capacity to identify visual sensory stimuli from their surrounding environment (Strathearn et al., 2008). And, first time mothers appear to be less primed to respond to positive unfamiliar stimuli as they exhibited greater activation of dopaminergic brain regions, specifically the reward system, to their own happy infant faces versus unfamiliar positive infants compared with controls (Strathearn et al., 2008). Though these data suggest that women in pregnancy and in postpartum have altered reactions to positive and negative stimuli, most of the research has relied on facial expressions, rather than social and nonsocial scenarios. Several studies have used tasks that consist of stimuli unique to motherhood experiences, particularly pictorial stimuli of infant faces. Using a validated paradigm permits cross-comparison with other studies on affective arousal in response to the International Affective Pictures System Task (IAPS) stimuli (Lang et al., 1993).
Also, the field has yet to characterize the extent to which depression exerts systematic differences in affective information processing across the perinatal phases. Participants with postpartum depression and nonpartum depression showed worse recognition of happiness and fear expressions, and participants with postpartum depression (PPD) showed worse recognition of disgust and anger faces compared with depressed nonpartum women (Flanagan et al., 2011). Also, postpartum depressed mothers evaluated neutral baby faces as less neutral (Gil et al., 2010), and showed higher amygdala activity when viewing negative emotional faces (Moses-Kolko et al., 2010). Studies have also shown improved abilities to encode emotional faces in later than earlier stages of pregnancy and that symptoms of anxiety were greatly associated with these encoding abilities (Pearson et al., 2010). In our current study, we examined affective reactivity from later stages of pregnancy until 6 weeks postpartum.
Quantifying affective reactivity relies on two dimensions: valence, which specifies the dimensions of positivity or negativity evoked by affective stimuli; and, intensity, which characterizes the dimension of affective arousal in response to the stimuli (Lang et al., 1993; Yik et al., 1999). Valence and arousal ratings convey the extent to which individuals experience affective reactivity and may be motivated to approach or withdraw, as is consistent with models of emotion that associate valence with an activated motivational system and arousal with the intensity of the activation (Cacioppo and Gardner, 1999; Davidson and Irwin, 1999; Lang et al., 1993). Higher activation of arousal and valence when viewing negative pictorial stimuli is likely to prompt the motivation to withdraw or avoid the scenario. A mother who is less responsive to negative scenarios may delay avoidance behaviors, inadvertently increasing exposure her to negative scenarios.
This study enrolled two groups (Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) depressed versus no psychiatric disorder) in a prospective, longitudinal design with the first visit during 32–36 weeks of pregnancy and the second visit between 6 and 8 weeks postpartum. At each visit, we collected clinical data and evaluations of affective stimuli using a validated and standardized set of affectively-laden pictorial stimuli. To test the relative differences in affective evaluations, we controlled for maternal self-reported affective state, given data showing that valence ratings may have varying intensity depending on the negative affect state of the individual (Joormann et al., 2010; van Beek and Dubas, 2008). Then, we compared maternal ratings of valence and arousal of positive, neutral, and negative stimuli. This approach sought to: (a) measure affective information processing within two well-characterized groups of unmedicated adults, (b) identify group differences in affective information processing across dimensions of positive and negative affective stimuli in pregnant and postpartum women with and without DSM-IV defined major depression, and (c) evaluate the extent to which affective information processing in pregnancy, after controlling for affective state and depression in pregnancy, would uniquely predict severity of depressive symptoms at the postpartum. We hypothesized that (a) pregnant women with major depression would exhibit attenuated responses to affective stimuli compared to pregnant healthy women; and, (b) postpartum women with major depression would exhibit attenuated responses to affective stimuli compared to postpartum healthy women.
2. Methods
This study uses data collected from one study conducted between 2008 and 2010 at an academic medical center, that was designed to test and compare affective information processing among women with and without major depression in pregnancy and postpartum.
2.1. Participants and procedures
Eighty pregnant women, right handed, unmedicated and between ages 18 and 65 years, were self-referred responding to advertisements placed in prenatal clinics, community groups, and the Internet. All subjects enrolled between 32 and 36 weeks of pregnancy. To ensure reliable identification of major depression, participants were screened with DSM-IV structured clinical interview screen/modules and depression symptom rating scales, along with specific inclusion and exclusion criteria. To ensure reliable identification of depression, one group met Diagnostic and Statistical Manual of Mental Disorders criteria for Major Depressive Disorder (DSM-IV, 4th edition; American Psychiatric Association, 1994) as well as a score ≥ 10 on the Patient Health Questionnaire-9 (PHQ-9, Kroenke et al., 2001) and on the Quick Inventory of Depressive Symptoms Self-Report (QIDS-SR, Rush et al., 1996). The healthy controls did not meet DSM-IV criteria for Major Depressive Disorder and had scores ≤ 9 each on the PHQ-9 and QIDS-SR.
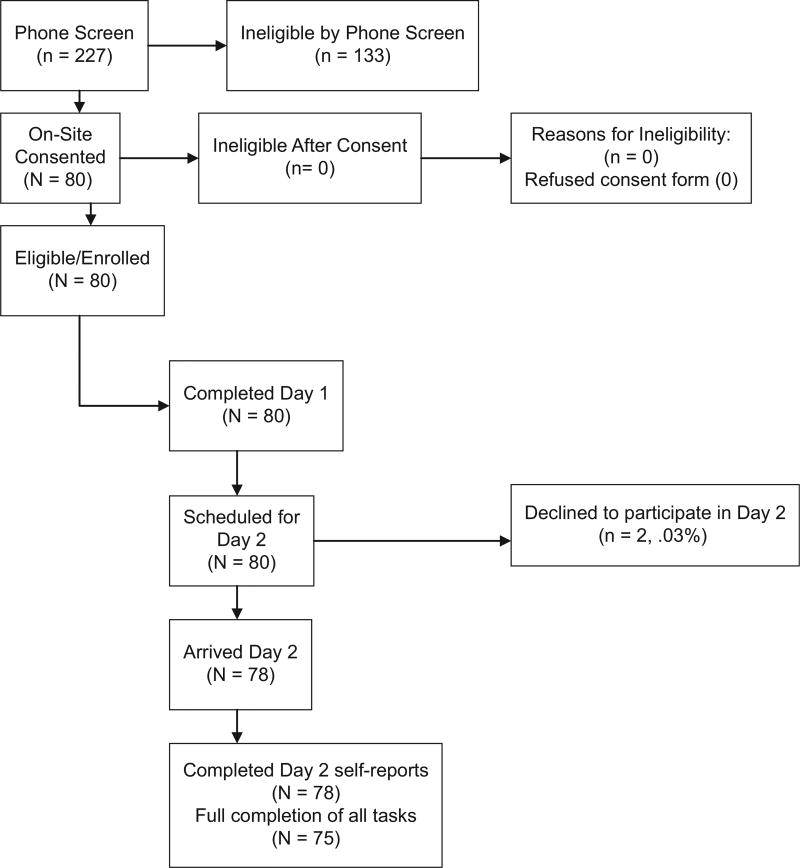
Exclusion criteria were designed to ensure that comorbid illness substances did not influence measurement of valence and arousal. These included (1) comorbid medical or psychiatric illness (i.e., bipolar I and II (lifetime, current), schizophrenia, delusional disorder, organic brain disorder, obsessive-compulsive disorder, specific phobia or philia of animals, Axis II borderline, schizotypal, antisocial; substance use or dependence); (2) imminent risk of suicide or homicide; (3) use of psychotropic medications in the last 2 weeks that might affect valence and arousal ratings; (4) exhibited insufficient understanding of the research procedures to voluntarily participate. In total, 80 participants completed the first visit in pregnancy, 78 completed the second visit postpartum follow-up, of which 75 completed the valence and arousal task. See the CONSORT chart (Fig. 1).
Fig. 1.
Participant flow (CONSORT chart).
2.2. Procedure
Using IRB-approved methods, verbal consent was obtained for the phone interview, written consent upon arrival for the on-site evaluation. On-site participants completed a urine toxicology screen, questionnaires, and the computer task. Participants repeated the same assessment in their postpartum visit within 6–8 weeks.
2.3. Measures
2.3.1. Psychiatric Diagnostic Screening Questionnaire
The Psychiatric Diagnostic Screening Questionnaire (PDSQ, Zimmerman and Mattia, 2001) is a self-report scale with 13 subscales of Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) Axis I disorders encountered in outpatient mental health settings. Data indicate good to excellent levels of internal consistency, test-retest reliability, and discriminant, convergent, and concurrent validity (Zimmerman and Mattia, 2001).
2.3.2. Patient Health Questionnaire
The Patient Health Questionnaire (PHQ-9, Kroenke et al., 2001), a 9-item measure of DSM-IV criteria of Major Depressive Disorder in the past week. Scores 1–4 reflect minimal depression, 5–9 indicate mild severity, 10–14 indicate moderate severity, 15–19 moderately-severe, and 20–27 suggest severe depression. PHQ-9 scores ≥ 10 have high sensitivity (88%), specificity (88%) for major depression, and high internal reliability with a Cronbach alpha of 0.89 (Kroenke et al., 2001). Our Cronbach alphas were 0.90 for the pregnancy visit and 0.89 for the postpartum visit.
2.3.3. Quick Inventory of Depressive Symptoms-Self Report
The Quick Inventory of Depressive Symptoms, Self report (QIDS-SR16; Rush et al., 1996) is a 16-item self-report scale quantifying the frequency, duration, intensity, and severity of depressive symptoms which we used to generate a metric of depressive symptoms in the past week. The QIDS-SR16 total scores range from 0 to 27. The total score is obtained by adding the scores for each of the nine symptom domains of the DSM-IV MDD criteria, reflecting increasing severity (i.e., mild depression = scores 6–10, moderate severity = 11–15, severe = 16–20, very severe = 21–27). The QIDS-SR has good internal consistency for depressed patients, Cronbach alpha 0.81. Our Cronbach alphas were 0.83 for pregnancy visit and 0.86 for postpartum visit.
2.3.4. Positive Affect and Negative Affect Scale
The Positive Affect and Negative Affect Scale (PANAS; Watson et al., 1988) is a 20-item measure of positive and negative dimensions of affect, which we used to control for the effect of affective experience on valence and arousal ratings. Patients rate the extent to which they experienced emotions portrayed by a list of adjectives in the past week. Ratings are made on a five-point scale ranging from “not at all” to “extremely”. The positive affect subscale (10 items) and the negative affect subscale (10 items) have strong internal consistency (α = 0.88; α = 0.85, respectively, Watson et al., 1988).
2.3.5. International Affective Pictures System Task
The International Affective Pictures System Task (IAPS, Lang et al., 2008) is a validated method in which a computer presents emotionally laden scenes (color pictures) to induce affective states, in which participants provide an emotional assessment of valence (pleasant to unpleasant) and arousal (calm to excited). These pictures were selected based on z-score transformed normative ratings of valence (negative to positive) and arousal (high to low) using the full collection. Each stimulus type was divided into four categories based on their normative valence ratings: Positive, Neutral, Negative and Threat. Thirty stimuli were selected from each of the four categories, yielding 120 stimuli.1 We used positive, neutral and threat categories.
Participants were informed that they would see pictures that differed in emotional content, and that they should attend to each picture for the entire time that was presented. Each trial consisted of a 0.5 s baseline period, 4 s stimulus presentation period, and a self-paced rating period. A fixation point appeared at the center of the screen during the baseline period, which was replaced by the stimulus centered on the screen during the stimulus presentation period. For ratings of intensity, participants made two ratings of each picture: First, the participants issued ratings to the picture using an affect matrix, represented as a 5 square (positive: zero to maximum) by 5 square (negative: zero to maximum) matrix (Larsen et al., 2009), with positive affect reflected on the horizontal axis and negative affect on the vertical axis (Norris et al., 2004). Participants were instructed to move their mouse to one of the 25 cells in the 5 × 5 matrix to indicate their positive and negative feelings (intensity). Then, we asked for arousal ratings, asking participants to rate their reaction to the affective stimuli on a 9-point scale (1–9). Histograms of valence and arousal ratings of stimuli in pregnancy were normally distributed in the depressed group and in the healthy group.
2.4. Statistical analyses
Tests of baseline differences in demographic and clinical characteristics were investigated using one sample t-tests or Analysis of Variance (ANOVA) for continuous variables (depression severity, valence and arousal ratings of emotional stimuli) and Chi-square tests of independence for categorical variables (gender, ethnicity, marital status, employment, and educational background). We conducted one sample t-tests to evaluate the mean score of the groups against a hypothesized population mean of zero. Then, we tested for group differences using a GLM ANOVA (emotion category: positive, neutral, negative) to examine group differences (depressed versus healthy) on valence and arousal ratings collected at pregnancy and postpartum. Analyses were two-tailed at the 0.05 level of significance. Significant interactions were probed using simple effects analyses as specified by Tabachnik and Fidell (2001). Finally, we used hierarchical regression to examine the contribution of valence and arousal ratings in pregnancy to depressive symptom severity in postpartum. In each regression, demographic variables (education and marital status) and depression in pregnancy (QIDS-SR total score) were entered in the first step. Positive and negative affect (PANAS) were entered to control for the correlation between depression and affect in the second step. Arousal ratings of positive, negative and neutral stimuli were entered to permit direct comparison of coefficients in the third step. The valence ratings of positive, neutral and negative were added in the fourth step to examine contribution, and the valence by arousal interaction of each of the three stimuli sets were entered in the fifth step to examine the interactive effect above and beyond that of the separate valence and arousal ratings. We conducted two separate regression analyses to determine the extent to which demographic, clinical, and affective reactivity variables (in pregnancy) predicted depressive symptoms in postpartum in depressed and healthy controls.
3. Results
3.1. Sample characteristics
Baseline means, standard deviations of demographic and clinical variables by group are shown in Table 1. Twenty-seven participants were depressed (MQIDS-SR = 14.30, SD = 4.3) and 53 were healthy (MQIDS-SR = 6.34, S.D. = 2.6). Pregnant women with major depression were younger by an average of 3 years than healthy women, t(78) = − 2.21, p < 0.05. Pregnant women with major depression were less likely to have a college education, and be married, .
Table 1.
Sociodemographic characteristics by group in pregnancy (N = 80).
| Depressed (n = 27) |
Nondepressed (n = 53) |
|
|---|---|---|
|
|
|
|
| n (%) | n (%) | |
| Ethnicity | ||
| Caucasian | 16 (17.6) | 33 (43) |
| African American | 8 (8) | 8 (14) |
| Asian | 0 (1) | 5 (4) |
| Hispanic | 3 (4) | 7 (8) |
| Educationa | ||
| Partial high school | 3 (3.8) | 1 (1.2) |
| High school graduate | 1 (1.2) | 1 (1.2) |
| Partial college training | 10 (12.5) | 9 (11.2) |
| College graduate | 11 (13.8) | 26 (32.5) |
| Completed graduate training | 2 (2.5) | 16 (20.0) |
| Employment | ||
| Unemployed | 4 (5.0) | 5 (6.2) |
| Employed | 14 (17.5) | 35 (43.8) |
| Full-time Student | 3 (3.8) | 4 (5.0) |
| Not working to care for child, no public aid | 6 (7.5) | 9 (11.2) |
| Maritala | ||
| Never married | 14 (17.5) | 12 (15) |
| Married | 11 (13.8) | 39 (48.8) |
| Separated | 1 (1.2) | 2 (2.5) |
| Divorced | 1 (1.2) | 0 (0) |
| Age (mean, S.D.) | 27.5 (6.8) | 30 (4.9) |
Significant difference between two groups at p < 0.05.
Group differences in severity of depression in pregnancy were significant, FQIDS-SR (1, 80) = 94.95, p < 0.001; FPHQ-9 (1, 80) = 76.42, p < 0.001, as well as in postpartum, FQIDS-SR (1, 77) = 14.66, p < 0.001; FPHQ-9 (1, 77) = 27.11, p < 0.001. Also, group differences were significantly different for positive affect in pregnancy, FPANAS-PA (1, 79) = 40.39, p < 0.001, and for negative affect in pregnancy, FPANAS-NA (1, 79) = 89.15, p < 0.001), as well as for positive affect in postpartum, FPANAS-PA (1, 77) = 15.21, p < 0.001, and for negative affect in postpartum, FPANAS-NA (1, 77) = 20.65, p < 0.001). Pregnancy QIDS-SR score was positively correlated with pregnancy PANAS Negative Affect in the depressed group, r(27) = 0.65, p < 0.001, as well as with the healthy group, r(53) = 0.54, p < 0.001. Likewise, QIDS-SR was inversely correlated with PANAS PA in the depressed group, r(27) = −0.64, p < 0.00, as well as the healthy group, r(53) = −0.40, p < 0.01. These correlations provide rationale for including PANAS subscales as covariates in regressions.
Means and standard deviations of clinical variables are shown in Table 2.2
Table 2.
Clinical characteristics of groups in pregnancy and postpartum (N = 80).
| N | Mean | SD | SE | 95% confidence interval for mean | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Lower bound | Upper bound | ||||||
| Pregnancy | |||||||
| PHQa | MDD | 27 | 12.74 | 4.93 | 0.95 | 10.79 | 14.69 |
| HEA | 53 | 3.79 | 3.99 | 0.55 | 2.69 | 4.89 | |
| QIDSa | MDD | 27 | 14.30 | 4.71 | 0.91 | 12.44 | 16.16 |
| HEA | 53 | 6.34 | 2.60 | 0.36 | 5.64 | 7.07 | |
| PAa | MDD | 27 | 12.44 | 4.01 | 0.77 | 10.86 | 14.03 |
| HEA | 53 | 18.23 | 3.77 | 0.52 | 17.19 | 19.27 | |
| NAa | MDD | 27 | 15.58 | 4.25 | 0.82 | 13.91 | 17.27 |
| HEA | 53 | 8.96 | 2.04 | 0.28 | 8.39 | 9.52 | |
| Postpartum | |||||||
| PHQa | MDD | 26 | 8.88 | 5.72 | 1.12 | 6.58 | 11.19 |
| HEA | 52 | 3.15 | 3.91 | 0.54 | 2.07 | 4.24 | |
| QIDSa | MDD | 26 | 10.42 | 5.81 | 1.14 | 8.08 | 12.77 |
| HEA | 52 | 6.19 | 3.87 | 0.54 | 5.11 | 7.27 | |
| PAa | MDD | 26 | 14.46 | 5.67 | 1.11 | 12.17 | 16.75 |
| HEA | 52 | 18.71 | 3.85 | 0.53 | 17.63 | 19.78 | |
| NAa | MDD | 26 | 12.34 | 4.47 | 0.88 | 10.53 | 14.14 |
| HEA | 52 | 8.89 | 2.23 | 0.31 | 8.28 | 9.52 | |
QIDS-SR = Quick Inventory of Depressive Symptoms-Self Report; PHQ-9 = Patient Health Questionnaire-9 Item; PA = Positive Affect Subscale of Positive Affective Negative Affect Scale; NA = Negative Affect Subscale of Positive Affective Negative Affect Scale.
Significant difference between two groups at p < 0.05.
3.2. Group differences in affective reactivity
Table 3 outlines the means and standard deviations of valence and arousal ratings for group by visit. First, to assess group differences in affective reactivity in pregnancy, we computed the average of the valence ratings and the arousal ratings within the positive, neutral, and negative categories. The averages were subjected to a multivariate GLM ANOVA (valence and arousal ratings across three categories). Study results indicated no significant group differences, F(1, 79) = 1.73, p = 0.13.
Table 3.
Arousal and valence ratings by pregnancy phase.
| Phase | Arousal/valence | Group | n | Mean | SD | SE | Lower bound | Upper bound |
|---|---|---|---|---|---|---|---|---|
| Pregnancy | Arousal negative | MDD | 27 | 8.60 | 3.28 | 0.63 | 7.30 | 9.89 |
| HEA | 53 | 9.74 | 3.05 | 0.42 | 8.90 | 10.58 | ||
| Arousal positive | MDD | 27 | 8.28 | 3.39 | 0.65 | 6.94 | 9.63 | |
| HEA | 53 | 8.73 | 2.84 | 0.39 | 7.95 | 9.51 | ||
| Arousal neutral | MDD | 27 | 4.88 | 2.56 | 0.49 | 3.87 | 5.90 | |
| HEA | 53 | 5.12 | 2.01 | 0.28 | 4.57 | 5.68 | ||
| Valence negative | MDD | 27 | −4.12 | 0.93 | 0.18 | −4.49 | −3.75 | |
| HEA | 53 | −4.34 | 1.21 | 0.17 | −4.67 | −4.00 | ||
| Valence positive | MDD | 27 | 3.63 | 1.53 | 0.29 | 3.03 | 4.24 | |
| HEA | 53 | 4.29 | 1.39 | 0.19 | 3.91 | 4.68 | ||
| Valence neutral | MDD | 27 | 0.86 | 0.94 | 0.18 | 0.49 | 1.24 | |
| HEA | 53 | 0.97 | 0.86 | 0.12 | 0.73 | 1.21 | ||
| Arousal positive | MDD | 27 | 8.28 | 3.39 | 0.65 | 6.94 | 9.63 | |
| HEA | 53 | 8.73 | 2.84 | 0.39 | 7.95 | 9.51 | ||
| Postpartum | Arousal negativea | MDD | 26 | 7.36 | 4.09 | 0.80 | 5.71 | 9.01 |
| HEA | 49 | 10.23 | 2.73 | 0.39 | 9.47 | 11.04 | ||
| Arousal positive | MDD | 26 | 7.79 | 4.22 | 0.83 | 6.09 | 9.49 | |
| HEA | 49 | 8.60 | 2.69 | 0.38 | 7.83 | 9.38 | ||
| Arousal neutral | MDD | 26 | 4.71 | 2.63 | 0.52 | 3.65 | 5.77 | |
| HEA | 49 | 5.12 | 2.12 | 0.30 | 4.51 | 5.73 | ||
| Valence negativea | MDD | 26 | −3.89 | 1.54 | 0.30 | −4.51 | −3.27 | |
| HEA | 49 | −4.55 | 1.10 | 0.16 | −4.87 | −4.23 | ||
| Valence positive | MDD | 26 | 3.55 | 1.92 | 0.38 | 2.78 | 4.33 | |
| HEA | 49 | 4.13 | 1.43 | 0.20 | 3.73 | 4.54 | ||
| Valence neutral | MDD | 26 | 0.76 | 0.89 | 0.18 | 0.41 | 1.13 | |
| HEA | 49 | 0.92 | 0.87 | 0.12 | 0.67 | 1.17 |
MDD = Major Depressive Disorder.
HEA = Healthy Controls.
SD = Standard Deviation, SE = Standard Error.
Significant difference between two groups at p < 0.05.
Results of the GLM Analyses of Variance for postpartum ratings of valence and arousal revealed a significant main effect for group, F(1, 74) = 5.25, p < 0.05, in which postpartum women with major depression demonstrated significantly lower arousal ratings for negative stimuli compared with healthy women, F(1, 74) = 13.45, p < 0.001. Also, postpartum women with major depression issued significantly lower valence ratings of negative stimuli, F(1, 74) = 4.65, p = 0.03, compared with healthy women.
3.3. Hierarchical regressions of postpartum depression
Results of the hierarchical regression predicting depressive symptoms at the postpartum visit among depressed subjects at pregnancy are presented in Table 4. In the group of pregnant participants with major depression, the first step examined the extent to which demographic variables and baseline depression score significantly predicted depression symptoms in postpartum. Age, education, and marital status were non-significant predictors of the outcome. Baseline depression (QIDS-SR) and positive and negative affective states (PANAS) were not significant. In the third and fourth regression steps, arousal ratings of negative stimuli in pregnancy uniquely contributed to the prediction of depression severity at the postpartum follow-up [β = −0.439, t(25) = −2.39, SE = 5.33, p = 0.025]. With each point increase on the QIDS-SR at, postpartum, arousal ratings of negative stimuli decreased by 0.775. The remaining ratings of valence and arousal and their interaction terms entered in the third, fourth, and fifth steps were non-significant. The final model accounted for 19% (R2 = 0.19) F(1, 22) = 7.7, p = 0.008) of the variance in depression severity at postpartum.
Table 4.
Hierarchical regression analysis for variables predicting depression at postpartum with depressed participants at pregnancy visit (n = 25).
| Beta | t | p | |
|---|---|---|---|
| Step 1 | |||
| Age | 0.053 | −0.019 | 0.297 |
| Gender | 0.278 | −0.102 | 1.604 |
| Ethnicity | 0.784 | 0.920 | 0.122 |
| Step 2 | |||
| QIDS | 0.208 | 1.070 | 0.296 |
| Step 3 | |||
| PANAS NA | 0.253 | 1.342 | 0.193 |
| PANAS PA | −0.251 | −1.346 | 0.191 |
| Step 4 | |||
| Arousal negativea | −0.439 | −2.39 | 0.025 |
| Arousal positive | 0.160 | 0.717 | 0.481 |
| Arousal neutral | 0.280 | 1.351 | 0.190 |
| Step 5 | |||
| Valence negative | −0.063 | −0.305 | 0.763 |
| Valence positive | 0.165 | 0.817 | 0.422 |
| Valence neutral | 0.168 | 0.896 | 0.380 |
| Step 6 | |||
| Arousal × Val negative | −0.045 | −0.098 | 0.923 |
| Arousal × Val positive | 0.207 | 1.034 | 0.312 |
| Arousal × Val neutral | 0.173 | 0.936 | 0.359 |
QIDS-SR = Quick Inventory of Depressive Symptoms – Self Report; PANAS NA = Negative Affect Subscale of PANAS; PANAS PA = Positive Affect Subscale of PANAS; Val = Valence.
Significant difference between two groups at p < 0.05.
Results of the hierarchical regression among pregnant subjects with no psychiatric disorder are presented in Table 4. The first step examined whether demographic variables, or baseline depression score significantly predicted depression symptoms at postpartum. Education was non-significant and dropped from the final regression analyses. Depression in pregnancy (QIDS-SR) was significant, [β = 0.38, t(51) = 2.80, SE = 3.46, p < 0.01], suggesting that for each point increase on the QIDS-SR in postpartum, the QIDS-SR score in pregnancy increased by half a point (0.57). In the second step, the PANAS negative affect subscale significantly predicted postpartum depressive symptoms [β = 0.39, t(51) = 3.04, SE = 3.36, p < 0.01], as did PANAS positive affect [β = 0.42, t(51) = 3.01, SE = 3.12, p < 0.01]. In the third and fourth regression steps, neither arousal ratings, valence ratings, nor the interaction terms uniquely predicted depression symptoms in postpartum. The final model accounted for 62% (R2 = 0.39, adjusted R2 = 0.35) of the variance in depressive symptom severity at postpartum.
4. Discussion
Our results indicate group differences among depressed and healthy women in early postpartum. Postpartum women with major depression were less responsive to negative stimuli, with lower ratings of intensity and reactions to negative pictorial stimuli, compared with postpartum healthy women. Also, lower ratings of the intensity and reactions to negative stimuli during pregnancy among depressed women predicted postpartum depression severity, even after controlling for depressive severity and self-reported affect ratings in pregnancy. These data indicate that information processing of neutral and positive visual information remains stable among women with depression, given the lack of group differences between healthy and depressed groups in pregnancy and in postpartum. However, women with postpartum depression were significantly less responsive to negative stimuli, with lower ratings of intensity and arousal, compared with postpartum healthy women. It may be that during the later stages of pregnancy, depressed women demonstrate similar patterns in valence and intensity ratings of positive and neutral stimuli similar to their healthy counterparts whereas attenuated responses to negative stimuli are indicative of depressive status.
Though the testing materials differ, these results align with data from a prior study on emotional recognition of negative stimuli, in which women with postpartum depression showed worse recognition of expressions of fear, disgust, and anger (Flanagan et al., 2011). Though recognition studies differ from valence and arousal assignment studies, both suggest that affective information processing is blunted for women with depression. Also, neurocognitive function, specifically, slower speed of information processing in postpartum phase (DeGroot et al., 2006) – depending on depression severity – may influence interpretation of emotional stimuli (Harris et al., 1996). This finding suggests that major depression during postpartum alters information processes used to detect negative stimuli.
To the extent that these women are less responsive to negative scenarios, they may have lower motivation and behavior to navigate away from aversive encounters. Also, lower responsiveness to negative stimuli in women who are depressed in pregnancy, controlling for depression severity and self-reported affect, significantly predicted depressive symptoms in postpartum. These results are consistent with work showing the partial contribution of self-reported affective experience to depression in nonpregnant samples (Bradley et al., 1997; Bouhuys et al., 1999). Our data complement work showing blunted reactivity among depressed mothers in terms of lower activation in brain areas that relate to emotional response. Specifically, non-depressed mothers showed greater neural activation in brain areas that relate to emotional response relative to depressed mother, though given the sample of 11 in each group, this study will require replication (Laurent and Ablow, 2012).
Impaired response to negative scenarios may maintain depressive status across pregnancy into postpartum. In contrast, only ratings of self-reported affect in pregnancy predicted depression symptom severity in postpartum. Women without depression showed stable scores of positive affect across time and of negative affect though the scores were lower than published norms for nonpartum samples (Watson et al., 1988). Our results align with data on the affective structure of postpartum depression in which positive and negative affect were correlated with depressive symptoms, and predictive of depression and anhedonia subscales on the Edinburgh Depression Postpartum Scale (Tuohy and McVey, 2008). One potential explanation is that women with lower negative affect may constrain their defensive responses in aversive scenarios, while also showing poor adaptation with lower positive scores (Fredrickson, 2001). Clinically, the PANAS self-report provides the clinician with a very simple and brief method to evaluate affect, and to the extent that healthy pregnant women endorse lower scores on the affect scales, clinicians may be prompted to track depressive symptoms for the rest of the perinatal phase.
Study results should be interpreted abiding several limitations. First, the IAPS is a well-validated approach to test affective information processing, though maternal or infant content may optimally evoke affective reactivity with peripartal samples. Data indicate that modified versions of pictorial images related to pregnancy, motherhood, infant faces (Gil et al., 2010; Baeken et al., 2009), and infant vocalizations reveal a heightened affective response to negative cues (Siefritz et al., 2003; Landi et al., 2011). Also, our study examined two time points, affective responses shift over time during postpartum. Finally, we did not include women with nonpartum-related depression, and thus, a study that includes this comparison group will permit us to determine the extent to which blunted affective reactivity to negative stimuli is specific to postpartum maternal experience.
Acknowledgments
This research was supported by the Northwestern University’s Feinberg School of Medicine Institute of Women’s Health Research, Chicago, IL. We also thank the Northwestern Women’s Board for their support with this project. We gratefully acknowledge Catherine Norris and John T. Cacioppo for their assistance with the IAPS protocol, and our research team, including Shandra Brown, Justin Birnholz, Kallio Hunnicutt-Ferguson, Bjorn Hanson, Noah Yulish, for interviewing participants.
Footnotes
The picture numbers for stimuli in the International Affective Pictures System Task: 1050, 1051, 1052, 1111, 1113, 1120, 1201, 1205, 1274, 1275, 1300, 1301, 1321, 1390, 1525, 1590, 1726, 1930, 2038, 2191, 2200, 2210, 2215, 2278, 2339, 2345, 2346, 2385, 2397, 2441, 2445, 2499, 2512, 2595, 2691, 2692, 2700, 2704, 2717, 2840, 2850, 3022, 3216, 3220, 3300, 4606, 4610, 4617, 4623, 4624, 4625, 4641, 5270, 5450, 5471, 5520, 5660, 5849, 5973, 6210, 6211, 6213, 6244, 6250, 6311, 6410, 6550, 6555, 6836, 6840, 7006, 7009, 7030, 7037, 7038, 7041, 7050, 7170, 7186, 7235, 7242, 7249, 7250, 7260, 7280, 7289, 7359, 7360, 7361, 7390, 7400, 7430, 7470, 7480, 7500, 7508, 8120, 8371, 8461, 8496, 8540, 9041, 9070, 9090, 9101, 9265, 9280, 9290, 9300, 9301, 9342, 9373, 9390, 9402, 9419, 9424, 9530, 9592, 9630, and 9830.
Pregnancy QIDS-SR score was positively correlated with pregnancy PANAS Negative Affect in the depressed group, r(27) = 0.65, p < 0.001, as well as with the healthy group, r(53) = 0.54, p < 0.001. Likewise, QIDS-SR was inversely correlated with PANAS PA in the depressed group, r(27) = −0.64, p < 0.00, as well as the healthy group, r(53) = −0.40, p < 0.01. These correlations provide rationale for including PANAS subscales as covariates in regressions.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. APA; Washington, DC.: 1994. [Google Scholar]
- Baeken C, De Raedt R, Ramsey N, Van Schuerbeek P, Hermes D, Bossuyt A, Lehman L, Vanderhasselt M-A, DeMay J, Luypaert R. Amygdala responses to positively and negatively valenced baby faces in healthy female volunteers: influences of individual differences in harm avoidance. Brain Research. 2009;1296:94–103. doi: 10.1016/j.brainres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behavior Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn CM. Depressed patients perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. Journal of Nervous and Mental Disease. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL. Emotion. Annual Review of Psychology. 1999;50:191–214. doi: 10.1146/annurev.psych.50.1.191. [DOI] [PubMed] [Google Scholar]
- Csukly G, Czobor P, Szily E, Takacs B, Simon L. Facial expression recognition in depressed subjects the impact of intensity level and arousal dimension. Journal of Nervous and Mental Disease. 2009;197:98–103. doi: 10.1097/NMD.0b013e3181923f82. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- DeGroot RHM, Vuurman EFPM, Hornstra G, Jolles J. Differences in cognitive performance during pregnancy and early motherhood. Psychological Medicine. 2006;36:1023–1032. doi: 10.1017/S0033291706007380. [DOI] [PubMed] [Google Scholar]
- Demenescu LR, Kortekaas R, den Boer JA, Aleman A. Impaired attribution of emotion to facial expressions in anxiety and major depression. PLoS One. 2010;5(12):e15058. doi: 10.1371/journal.pone.0015058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JT, White H, Carter BG. Differential impairments in emotion face recognition in postpartum and nonpostpartum depressed women. Journal of Affective Disorders. 2011;128:314–318. doi: 10.1016/j.jad.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. American Psychologist. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil S, Teissedre F, Chambres P, Droit-Volet S. The evaluation of emotional facial expressions in early postpartum depression mood: a difference between adult and baby faces? Psychiatry Research. 2010;186:281–286. doi: 10.1016/j.psychres.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Research. 2008;159:18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of PPD on child cognitive development and behavior: a review and critical analyses of the literature. Archives of Women’s Mental Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Hale WW, Jansen JHC, Bouhuys AL, Van Den Hoofdakker RH. The judgment of facial expressions by depressed patients, their partners and controls. Journal of Affective Disorders. 1997;47:63–70. doi: 10.1016/s0165-0327(97)00112-2. [DOI] [PubMed] [Google Scholar]
- Hammar A, Lund A, Hugdahl IK. Long-lasting cognitive impairment in unipolar major depression: a 6-month follow-up study. Psychiatry Research. 2003;118(2):189–196. doi: 10.1016/s0165-1781(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Harris ND, Deary I, Harris MB, Lees MM, Wilson JA. Peripartal cognitive impairment: secondary to depression? British Journal of Health Psychology. 1996;1:127–136. [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Joormann J, Nee DE, Berman MG, Jonides J, Gotlib IH. Interference resolution in major depression. Cognitive. Affective and Behavioral Neuroscience. 2010;10:21–33. doi: 10.3758/CABN.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford JJV, Mencl WE, Woehunsky PD, Potenza MN, Mayes LC. Maternal neural responses to infant cries and faces: relationships with substance use. Frontiers in Psychiatry. 2011;3:1–13. doi: 10.3389/fpsyt.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: a single-item measure of positivity and negativity. Cognition and Emotion. 2009;23:453–480. [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive & Affective Neuroscience. 2012;7(2):125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of neutral faces. Psychiatry Research. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and postpartum. Archives of Women’s Mental Health. 2005;8:77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. American Journal of Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CJ, Chen EE, Zhu DC, Small SL, Cacioppo JT. The interaction of social and emotional processes in the brain. Journal of Cognitive Neuroscience. 2004;16:1818. doi: 10.1162/0898929042947847. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Cooper RM, Penton-Voak IS, Lightman SL, Evans J. Depressive symptoms in early pregnancy disrupt attentional processing of infant emotion. Psychological Medicine. 2010;40:621–631. doi: 10.1017/S0033291709990961. [DOI] [PubMed] [Google Scholar]
- Roos A, Robertson F, Lochner C, Vythilingum B, Stein D. Altered prefrontal cortical function during processing of fear-relevant stimuli in pregnancy. Behavioral Brain Research. 2011;222:200–205. doi: 10.1016/j.bbr.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Siefritz E, Esposito F, Neuhoff JG, Luhti A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Hilgenfeldt T, Freudenberg P, Bermpohi F, Adli M. Access of emotional information to visual awareness in patients with major depressive disorder. Psychological Medicine. 2011;41:1615–1624. doi: 10.1017/S0033291710002540. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a Smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Tabachnik B, Fidell LS. Using Multivariate Statistics. 4. Allyn & Bacon; Needham Heights, MA: 2001. [Google Scholar]
- Tronick E, Beeghly M. Meaning making and infant mental health. American Psychologist. 2011;66(2):107–119. doi: 10.1037/a0021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy A, McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. British Journal of Clinical Psychology. 2008;47:153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- van Beek Y, Dubas J. Decoding basic and non-basic facial expressions and depressive symptoms in late childhood and adolescence. Journal of Nonverbal Behavior. 2008;32:53–64. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yik MS, Russell JA, Feldman Barrett L. Structure of self-reported current affect: integration and beyond. Journal of Personality and Social Psychology. 1999;77:600–619. [Google Scholar]
- Zimmerman M, Mattia J. The psychiatric diagnostic screening questionnaire: development, reliability and validity. Comprehensive Psychiatry. 2001;42:175–189. doi: 10.1053/comp.2001.23126. [DOI] [PubMed] [Google Scholar]