Abstract
Intrathymic signals induce the differentiation of immature CD4+CD8+ double positive (DP) thymocytes into mature CD4+ or CD8+ single positive (SP) T cells. The transcriptional mechanism by which CD8 lineage is determined is not fully understood. The best evidence, which favors the kinetic signaling/co-receptor reversal model, indicates that signaled DP thymocytes terminate CD8 transcription prior to their subsequent re-initiation of CD8 transcription and ultimate differentiation into CD8SP T cells. We and others have shown that CD8 lineage commitment is severely perturbed in mice in which expression of the transcription factor SATB1 is either conventionally knocked out or T cell-specifically knocked down. Here, we demonstrate that, as with normal thymocytes, cultured SATB1-deficient DP thymocytes inactivate CD8 coreceptor transcription following receipt of signals (PMA plus ionomycin) that mimic TCR-mediated positive selection. However, this terminated CD8 transcription is not re-initiated by signals (IL-7) conducive to CD8 differentiation in SATB1-deficient DP. We show that SATB1 specifically binds to a cis-regulatory element within the CD8 enhancer (E8III) known to be required for coreceptor reversal. A requirement in CD8 coreceptor reversal identifies SATB1 as an essential trans-regulator of CD8 lineage fate, whose action may be mediated via recruitment to the E8III DP enhancer.
Keywords: Coreceptor reversal, SATB1, CD8 enhancer
1. Introduction
Most thymus-derived CD8 or CD4 single-positive (SP) T cells develop in the thymus from common CD4+CD8+ double-positive (DP) progenitor cells, expressing both CD4 and CD8, through a process known as positive selection. The mechanism by which uncommitted DP thymocytes differentiate into SP T cells that express a TCR and coreceptor molecules with matching MHC specificities has been a matter of considerable debate and the subject of intense investigation. The initial instructive and stochastic/selective models of T cell lineage determination (Singer, 2002) have been further refined in two related models. The “strength of signal model” proposes that the strength or duration of the TCR signal determines the fate of DP thymocytes; i.e., stronger and/or more prolonged signals drive DP to CD4SP and weaker/less sustained signals direct them to CD8SP. This model contends that lineage commitment occurs in DP thymocytes and results in transcriptional termination of one or the other coreceptor molecules. Alternatively, the “kinetic signaling model” proposes that SP choice of lineage is influenced by differential regulation of coreceptor gene expression. All DP thymocytes are preprogrammed to respond to intrathymic TCR coreceptor signals by transiently terminating CD8 transcription to become CD4+8low intermediate thymocytes. Cells destined to become CD8SP possess the ability to re-initiate CD8 gene transcription, a process termed “coreceptor reversal”, and to differentiate into CD8SP (Brugnera et al., 2000). Strongly supportive of the kinetic signaling model, Sarafova et al (Sarafova et al., 2005) demonstrated that positive selection signals, mediated through the TCR, inactivate the CD8 enhancer region E8III, which has been shown to be essential for CD8 expression in DP thymocytes (Ellmeier et al., 1998).
Several transcription factors are known to bind within CD8 gene regulatory regions to control CD8 transcription. These include GATA3, Ikaros, Runx, MAZR, CDP/Cux, and SATB1 (Banan et al., 1997; Bilic et al., 2006; Harker et al., 2002; Landry et al., 1993; Sato et al., 2005). Expressed predominantly in thymocytes, Special AT-rich Binding protein 1, SATB1 binds to and thereby regulates the function of nuclear matrix attachment sites (MARs) within the CD8α E8III enhancer and other antigen receptor and coreceptor genes, including IgH, Igκ, and TCRα (Cai et al., 2003; Li et al., 1999). Similar to actions ascribed to Runx 3 (Sato et al., 2005), SATB1 has been reported to function by remodeling chromatin structure (Cai et al., 2003; de Belle et al., 1998; Yamaguchi et al., 2006; Yasui et al., 2002; Han et al., 2008). SATB1-null mice lack both CD8SP and CD4SP T cells (Alvarez et al., 2000). SATB1-reduced mice, which express ~20% wild-type levels of SATB1 in their thymocytes but normal levels elsewhere, also display significant reduction in the percentages of their CD8SP T cells (Nie et al., 2005).
These observations prompted us to assess the role of SATB1 in CD8 lineage commitment. We show that signaled DP thymocytes isolated from SATB1-deficient mice initially terminate CD8 transcription to become CD4+8low thymocytes, but unlike wild-type, never re-initiate CD8 transcription. Specific binding of SATB1 to the E8III enhancer suggests that SATB1 may serve as the conduit between this cis-regulator and CD8 lineage commitment.
2. Materials and methods
2.1. Animals
Generation of the SATB1 knockout and the SATB1 reduced mouse strains (KD) have been previously described (Alvarez et al., 2000; Nie et al., 2005). In the knockout model, the targeted allele contains a deletion of the translation start site and the first 5 exons of SATB1 (Alvarez et al., 2000). SATB1 reduced mice (KD) are homozygous for a T cell-specific (CD2 promoter-mediated) SATB1-antisense transgene and heterozygous for the SATB1 null allele. Expression of the antisense RNA was limited to thymocytes, thereby avoiding complications arising from the lack of an essential SATB1 function in other tissues. Thymic SATB1 mRNA and protein expression in KD mice were reduced to ~20% that of WT (Nie et al., 2005). All animal experimentation outlined in this paper was reviewed and approved by the University of Texas at Austin IACUC.
2.2. Cell isolation and coreceptor re-expression assay
DP thymocytes were magnetically separated by sequential positive selections of anti-CD4 and anti-CD8 microbeads using MACS MultiSort Kits according to the manufacturer’s instructions (Miltenyi Biotec Inc. Auburn, CA). Isolated DP thymocytes were used for coreceptor re-expression assays as previously described (Brugnera et al., 2000). Briefly, isolated DP thymocytes (5×106/ml) were stimulated for 18–20 hrs with a combination of phorbal-12-myristate-13-acetate (PMA) (0.2ng/ml) and ionomycin (0.2ug/ml). Cells were washed and subsequently incubated in medium containing recombinant mouse IL-7 (3ng/ml).
2.3. Flow cytometric analysis
Where indicated, thymocytes were harvested and stained with fluorochrome-conjugated antibodies. Flow cytometric analysis was carried out with monoclonal antibodies (BD Pharmingen) specific for the following cell surface molecules: TCRβ (H57-597); CD4 (RM4-5), CD8α (53-6.7), CD25 (7D4), CD5 (53-7.3), and CD69 (H1.2F3). FACS analysis was performed in the FACS core facility of the M. D. Anderson Cancer Center, Science Park-Research Division, Smithville TX.
2.4. PCRs
The method for genotyping the SATB1 KO mice was described previously (Nie et al., 2005). For RT-PCR, total RNA was isolated with TRI REAGENT (MRC, Inc. Cincinnati, OH) and reversed transcribed to cDNA by using a poly(dT) oligonucleotide (SUPERSCRIPTTM II, GIBCO–BRL). We used 58°C, 62°C and 55°C annealing temperatures for the CD8, CD4 and GAPDH primer pairs, respectively. PCR products were analyzed on 1.2% agarose gels.
2.5. EMSA
Nuclear extracts (2–5µg) from Jurkat cells were mixed with poly-(dI-dC) (2µg) in binding buffer (Banan et al., 1997) for 5 min, and end-labeled probe was added. After 20 min incubation, samples were electrophoresed on a 4% polyacrylamide gel, following by autoradiography for 4hr. For antibody inhibition assays, various dilutions of antibodies were added to the reaction before the addition of probes. Anti-SATB1 and anti-CDP polyclonal sera were prepared in rabbits as described previously (Banan et al., 1997).
3. Results
3.1. SATB1-null DP thymocytes fail to re-initiate CD8 gene expression in response to IL-7
Previous studies demonstrated that SATB1-null animals, which typically die around 3 weeks of age, exhibit pronounced reduction in thymocyte numbers and size (Alvarez et al., 2000). CD8SP thymocytes were virtually eliminated in SATB1 KO mice, conceivably because thymocyte development was arrested at the DP stage (Alvarez et al., 2000). To investigate the mechanism underlying the CD8 reduction, DP thymocytes were magnetically sorted from wild type C57BL/6 (B/6) mice and SATB1-null mice that had been backcrossed to B/6 for 7 generations. DP from SATB1 KO and B/6 mice were isolated sequentially, using anti-CD8-FITC, anti-FITC multisort microbeads and anti-CD4 microbeads.
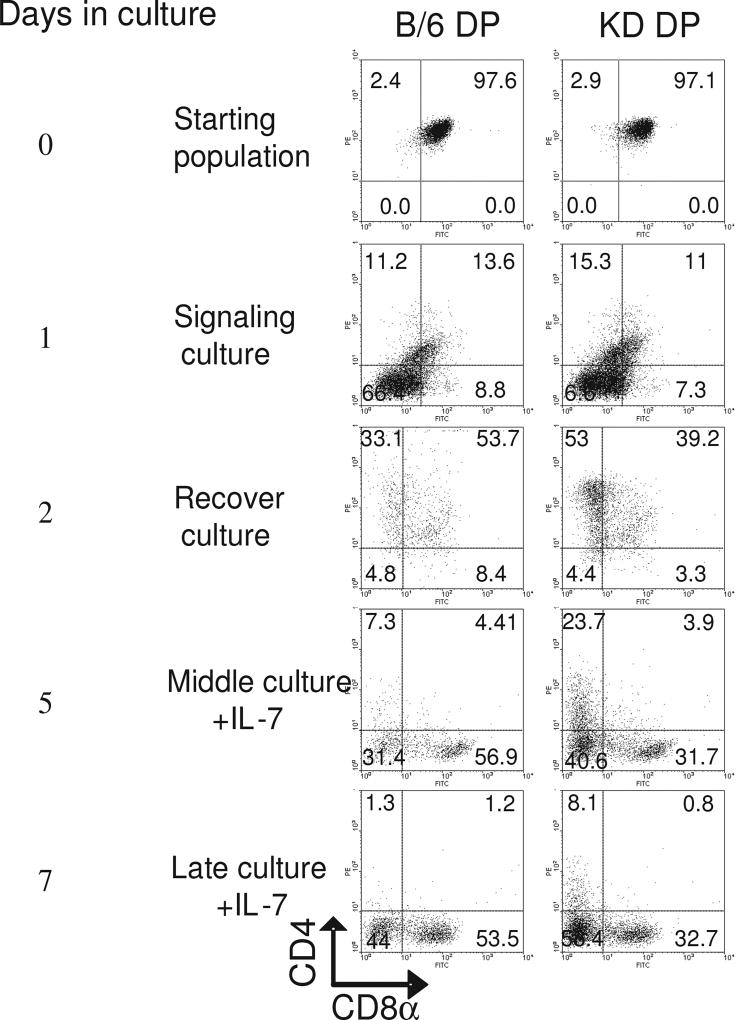
5×106/ml magnetically sorted DP thymocytes from null and wild-type were “signaled” with PMA and ionomycin to mimic positive selection in vitro. Following overnight culture with PMA plus ionomycin, DP from both SATB1-null and B/6 mice converted to CD4lowCD8+ in the signaling culture, simply due to selective internalization of surface CD4 protein by PMA stimulation (Kaldjian et al., 1988). The cells, when placed in nonstimulatory recovery culture, became CD4+CD8low (Figure 1A), because CD8 transcription was actually terminated (Figure 1B). The removal of PMA and ionomycin and the addition of IL-7 gave rise to re-expression of CD8 coreceptor molecules in B/6 cultures on day 3. Although FACS detected CD4+8−, CD4−8−, CD4−8+ and CD4+8+ populations, only CD4−8+ and CD4−8− cells remained after preexisting surface coreceptor molecules were stripped off by pronase treatment (data not shown). Importantly and consistent with the kinetic signaling model, only CD8 coreceptor molecules were actively transcribed at that point in wild-type cultures. However, in SATB1-null cultures, extinguished CD8 expression was never re-initiated (Figure 1B). The numbers of surviving cells determined by Trypan blue staining on day7 were 2.3×106/ml and 2.0×106/ml in B/6 and KO DP cultures, respectively.
Fig. 1.
Blockade of coreceptor reversal in SATB1-null thymocytes. A. Analysis of DP populations undergoing coreceptor reversal. Magnetically isolated DP thymocytes from C57BL/6 (B/6) wild type or from SATB1-null (KO) mice responded to PMA+ ionomycin (P+I) stimulation (signaling culture) first by terminating CD8 expression and then by converting to CD4+8−. After culture with IL-7, the CD8+ population reappeared in wild type thymocytes, but not in SATB1-null thymocytes. By day 7, signaled DP wild type thymocytes converted to CD4−8+, whereas those from SATB1-null mice remained CD4+8−. B. Analysis of CD4 and CD8 transcription during coreceptor reversal. RT-PCR was performed on RNA isolated from day 2, 3, and 7 cultures. Both wild type and SATB1-null thymocytes selectively extinguished CD8 transcription, but only cells from wild type reinitiated transcription. C. Analysis of the indicated T cell identity and maturation markers in day 7 cultures. The FACS experiments shown in (A) and (B) were representative of 4 independent experiments. lwayshappy
Ablation of SATB1 is known to have broad transcriptional consequences (Alvarez et al., 2000). Thus, we measured expression of genes other than the TCR coreceptors that might be relevant to the flawed attempt at co-receptor reversal observed in SATB1-deficient thymocytes. As shown in Figure 1C, CD3, CD5 and TCRαβ were down-regulated at day 7 in IL-7-supplemented cultures of SATB1-nulls, whereas expression of CD69 and CD25 appeared similar to B/6 controls. It is generally accepted that expression of CD3, TCRαβ and CD5 are up-regulated upon thymocytes maturation. We assume the down-regulations of these surface markers in SATB1-null cultures are consistent with the blockage of CD8 differentiation maturation. In response to treatment with PMA and ionomycin alone (without IL-7), CD4SP rather than CD8SP cells were generated in both B/6 and SATB1-null cultures (data not shown). This confirmed previous findings that cytokine receptor signals transduced by IL-7R are required for differentiation of signaled DP into CD8SP cells (Brugnera et al., 2000; Chong et al., 2003).
3.2. Thymocyte-specific reduction of SATB1 significantly impairs CD8 re-initiation during coreceptor reversal
SATB1-null mice die at ~2–3 wk of age, apparently due to the lack of essential functions of SATB1 in non-thymic tissues, including the brain (Alvarez et al., 2000). As such, the abnormal thymic phenotype in these null mice might be attributed to non-T cell autonomous causes. We generated mice (KD mice) in which the expression of a SATB1-antisense transcripts is targeted specifically to T lymphocytes and have bred these with mice heterozygous for a SATB1-null allele (Nie et al., 2005). Significant reduction in the percentage of CD8SP T cells present in the thymus and in the periphery were observed (Nie et al., 2005).
Magnetically purified DP populations from wild type B/6 mice and SATB1-KD mice were induced to undergo co-receptor reversal by the method described above. As shown in Figure 2, day 2 recovery cultures of wild type B/6 contained substantially more CD8+CD4low and CD8+CD4− cells than did SATB1-KD cultures (53.7% and 8.4% versus 39.2% and 3.3%, respectively). On day 5 in media containing IL-7, there were more CD8+CD4− cells (56.9% vs 31.7%) and fewer CD4+CD8− cells (7.3% vs 23.7%) in B/6 cells relative to SATB1-KD cells. The cells displaying CD4+CD8− on day 5 may result from cells failing to correctly undergo the process of co-receptor reversal (consequently underwent apoptosis), thereby transitioning into the CD4−CD8− population on day 7 (Figure 2). These data indicate that SATB1 acts in a T cell autonomous manner, and its T cell-specific reduction significantly impairs CD8 transcriptional re-initiation at the DP stage.
Fig. 2.
Impairment of coreceptor reversal in mice in which SATB1 was specifically reduced in thymocytes. DP thymocytes were purified from C57BL/6 (B/6) wild type mice and from SATB1-reduced mice (KD mice) homozygous for a T cell-specific SATB1-antisense transgene and heterozygous for the SATB1 null allele. Analyses were carried out as described in the legend to Figure 1A. Percentage of cells within each quadrant is indicated.
3.3. The CD8 DP-specific E8III enhancer is a target of SATB1
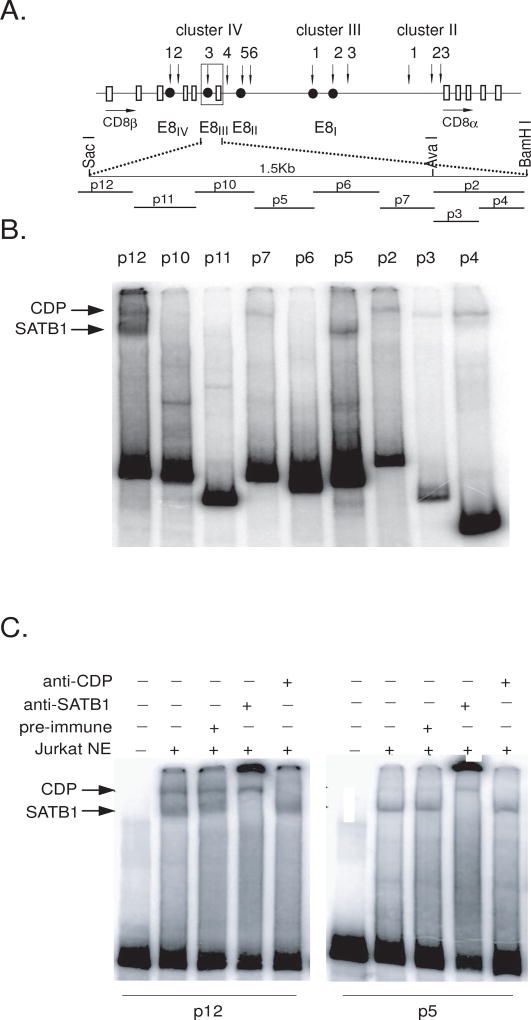
Transgenic studies identified E8III (an ~4 kb region residing ~8 kb downstream of the CD8ß gene; Figure 3A) as an enhancer of CD8 expression whose activity was restricted to DP thymocytes (Ellmeier et al., 1998). Further studies indicated that an ~1.5 kb sub-region of E8III was sufficient to promote coreceptor reversal during positive selection of DP thymocytes (Sarafova et al., 2005). Therefore, we reasoned that SATB-1 might reinitiate CD8 DP transcription (Figure 2B) through E8III.
Fig. 3.
SATB1 binds to sites within the CD8 DP enhancer E8III. A. Structure of the mouse endogenous CD8 gene locus and E8III probes. Vertical arrows indicate DNase hypersensitive sites; solid circles, characterized transcriptional control elements; open boxes, exons; horizontal arrows indicate the direction of transcription. Positions of the probes used for EMSA are shown as dashes below. B. Gel shift assays demonstrate SATB1 binding to E8III. EMSA was performed with Jurkat T cell nuclear extract and radiolabeled E8III probes indicated in (A). Reactions were analyzed on 4% nondenaturing polyacrylamide gels. Retarded bands were detected with probes p12, p7, p5 and p2. Arrows indicate putative SATB1 or CDP/Cux complexes. C. Specificity and confirmation of SATB1 binding by EMSA/antibody supershifts. Jurkat nuclear extracts were preincubated with PBS (lane2), pre-immune serum(Lane3), anti-SATB1 serum (lane4), or anti-CDP serum (lane5) then incubated with probe p12 (lanes 1–5, left panel) or probe p5 (lanes 1–5, right panel). Lanes 1, probe only. The arrows indicate SATB1 or CDP/Cux complexes.
As an initial test of this hypothesis, we determined whether SATB1 binds E8III in vitro. The 1.5 kb region was divided into seven ~200 bp probes (Figure 3A), and EMSA was performed with Jurkat nuclear extract. DNA corresponding to probe P12, located at the 5’ end of the 1.5 kb region, formed two protein complexes (Figure 3B). Their specificity and identity were confirmed by antibody supershifts as SATB1 and CDP/Cux (Figure 3C and data not shown). Another, albeit significantly weaker, SATB1 binding site was identified within probe P5, near the center of the 1.5 kb region (Figure 3B). SATB1, by virtue of its MAR-binding, matrix-targeting and atypical homeodomains, recognizes sequences highly enriched in ATC content, which have high potential for unwinding by base unpairing (Dickinson et al., 1997; Seo et al., 2005). The sequence of P12 contains several ATC-rich regions which may be possible binding sites for SATB1 or CDP/Cux.
4. Discussion
Thymocytes from conventional SATB1 nulls arrest at the DP stage (Alvarez et al., 2000). T cell-specific SATB1 knockdowns show significant reduction of CD8SP thymocytes, unperturbed levels of other major populations (DN, DP and CD4SP), and normal CD8 transcriptional initiation during the DN to DP transition (Nie et al., 2005). Since newly selected DP thymocytes have been shown to downregulate or completely terminate the expression of CD8, regardless of whether they eventually mature into CD4SP or CD8SP (Brugnera et al., 2000), the in vivo data suggested that SATB1 might control the re-initiation of CD8 transcription after positive selection. The results presented here show that SATB1 is indispensable for this critical step. This implicates SATB1 in the specification of CD8SP differentiation and provides strong support for the kinetic signaling model.
Through binding to the E8III enhancer, SATB1 may regulate CD8 transcriptional re-initiation by remodeling the chromatin structure at the CD8 locus. This speculation is supported by three sets of observations. First, unlike most transcription factors which regulate individual genes by binding to their target sequences, SATB1-binding sites are commonly located within MARs associated with transcriptional enhancers (Banan et al., 1997; Dickinson et al., 1992; Seo et al., 2005). SATB1 has been shown to participate in cell type-specific gene regulation at the level of higher order chromatin structure by binding to DNA from the major groove side at the bases of MAR-mediated chromosomal loop domains (de Belle et al., 1998; Yamaguchi et al., 2006). By recruiting ATP-dependent chromatin remodeling complexes to the MAR attachment sites, SATB1 has been shown to mediate region-specific histone modifications that regulate genes over long distances (Cai et al., 2003; Yasui et al., 2002). Thus, the function of SATB1 is clearly associated with chromatin regulation. Second, while the reduction of SATB1 protein in thymocytes regulated the maturation of CD8SP cells, the level of CD8 gene expression was not decreased in any population (Nie et al., 2005). This is consistent with its established role in chromatin remodeling and supports the notion that SATB1 acts to direct DP fate at the level of CD8 locus accessibility. Third, at least five separable enhancers reside within the CD8 gene locus (Ellmeier et al., 1998; Hostert et al., 1997). E8III enhancer-driven reporter gene expression is exclusively detected in DP thymocytes (Ellmeier et al., 1998), suggesting that trans-acting factors, together with E8III, function in a stage-specific manner for activating the CD8 gene. Studies from several groups indicated that expression of CD8 is epigenetically regulated by the alteration of chromatin structure and/or nuclear compartmentalization via interaction with Ikaros and MAZR (Avitahl et al., 1999; Bilic et al., 2006; Harker et al., 2002). Also mutations in the chromatin-remodeling SWI/SNF-like complex resulted in misregulation of CD8SP thymocyte development (Chi et al., 2002).
We hypothesize that following cessation of CD8 coreceptor transcription in response to TCR signal-mediated inactivation of E8III, SATB1 recruits a chromatin remodeling complex by binding to its p12 site within this enhancer (Figure 3A). This triggers histone modifications (Cai et al., 2003; Yasui et al., 2002) that establish a specific euchromatic code within the CD8 regulatory locus. These events, in cooperation with promoter-enhancer binding of other transcriptional regulators, initiate an accessible chromatin conformation within the locus that favors CD8α gene expression. In the absence of SATB1, such a chromatin state is not achieved, resulting in the blocking of CD8 transcriptional re-initiation and, consequentially, in class I-restricted CD8SP lineage commitment.
SATB1 has the potential to affect many cellular processes because of its interaction with MARs. We cannot rule out the contribution of additional or alterative mechanisms for this failure to reverse CD8 coreceptor transcription. Nonetheless, a more detailed mechanistic analysis of CD8 lineage commitment as affected by SATB1 and the E8III enhancer deserves future attention.
Acknowledgments
We are grateful to Dr. Terumi Kohwi-Shigematsu for providing mouse breeders containing a targeted SATB1 allele, to Kent Claypool for FACS analysis, to June Harriss for technical assistance, and to Mark Brown and Loren Probst for assistance in preparation of the manuscript. These studies were supported by National Institutes of Health Grant AI47209 (to P.W.T.).
Abbreviations
- DN
double negative
- DP
double positive
- MAR
matrix-associated region
- KO
knockout
- KD
knockdown
- SP
single positive
- SATB1
Special AT-rich Binding protein 1
- Runx
Runt-related protein
- MAZR
MAZ-Related.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors concur with the submission and that the material submitted for publication has not been previously reported and is not under consideration for publication elsewhere; the authors have no financial conflict of interest.
References
- Alvarez JD, Yasui DH, Niida H, Joh T, Loh DY, Kohwi-Shigematsu T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000;14:521–35. [PMC free article] [PubMed] [Google Scholar]
- Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–43. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- Banan M, Rojas IC, Lee WH, King HL, Harriss JV, Kobayashi R, Webb CF, Gottlieb PD. Interaction of the nuclear matrix-associated region (MAR)-binding proteins, SATB1 and CDP/Cux, with a MAR element (L2a) in an upstream regulatory region of the mouse CD8a gene. J Biol Chem. 1997;272:18440–52. doi: 10.1074/jbc.272.29.18440. [DOI] [PubMed] [Google Scholar]
- Bilic I, Koesters C, Unger B, Sekimata M, Hertweck A, Maschek R, Wilson CB, Ellmeier W. Negative regulation of CD8 expression via Cd8 enhancer-mediated recruitment of the zinc finger protein MAZR. Nat Immunol. 2006;7:392–400. doi: 10.1038/ni1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E, Bhandoola A, Cibotti R, Yu Q, Guinter TI, Yamashita Y, Sharrow SO, Singer A. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- Cai S, Han HJ, Kohwi-Shigematsu T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat Genet. 2003;34:42–51. doi: 10.1038/ng1146. [DOI] [PubMed] [Google Scholar]
- Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature. 2002;418:195–9. doi: 10.1038/nature00876. [DOI] [PubMed] [Google Scholar]
- Chong MM, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–87. doi: 10.1016/s1074-7613(03)00078-5. [DOI] [PubMed] [Google Scholar]
- de Belle I, Cai S, Kohwi-Shigematsu T. The genomic sequences bound to special AT-rich sequence-binding protein 1 (SATB1) in vivo in Jurkat T cells are tightly associated with the nuclear matrix at the bases of the chromatin loops. J Cell Biol. 1998;141:335–48. doi: 10.1083/jcb.141.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson LA, Dickinson CD, Kohwi-Shigematsu T. An atypical homeodomain in SATB1 promotes specific recognition of the key structural element in a matrix attachment region. J Biol Chem. 1997;272:11463–70. doi: 10.1074/jbc.272.17.11463. [DOI] [PubMed] [Google Scholar]
- Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992;70:631–45. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- Ellmeier W, Sunshine MJ, Losos K, Littman DR. Multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity. 1998;9:485–96. doi: 10.1016/s1074-7613(00)80632-9. [DOI] [PubMed] [Google Scholar]
- Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–93. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, Kioussis D. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell. 2002;10:1403–15. doi: 10.1016/s1097-2765(02)00711-6. [DOI] [PubMed] [Google Scholar]
- Hostert A, Tolaini M, Festenstein R, McNeill L, Malissen B, Williams O, Zamoyska R, Kioussis D. A CD8 genomic fragment that directs subset-specific expression of CD8 in transgenic mice. J Immunol. 1997;158:4270–81. [PubMed] [Google Scholar]
- Kaldjian E, McCarthy SA, Sharrow SO, Littman DR, Klausner RD, Singer A. Nonequivalent effects of PKC activation by PMA on murine CD4 and CD8 cell-surface expression. Faseb J. 1988;2:2801–6. doi: 10.1096/fasebj.2.12.3261700. [DOI] [PubMed] [Google Scholar]
- Landry DB, Engel JD, Sen R. Functional GATA-3 binding sites within murine CD8 alpha upstream regulatory sequences. J Exp Med. 1993;178:941–9. doi: 10.1084/jem.178.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Moy L, Pittman N, Shue G, Aufiero B, Neufeld EJ, LeLeiko NS, Walsh MJ. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. J Biol Chem. 1999;274:7803–15. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- Nie H, Maika SD, Tucker PW, Gottlieb PD. A role for SATB1, a nuclear matrix association region-binding protein, in the development of CD8SP thymocytes and peripheral T lymphocytes. J Immunol. 2005;174:4745–52. doi: 10.4049/jimmunol.174.8.4745. [DOI] [PubMed] [Google Scholar]
- Sarafova SD, Erman B, Yu Q, Van Laethem F, Guinter T, Sharrow SO, Feigenbaum L, Wildt KF, Ellmeier W, Singer A. Modulation of coreceptor transcription during positive selection dictates lineage fate independently of TCR/coreceptor specificity. Immunity. 2005;23:75–87. doi: 10.1016/j.immuni.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–28. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Seo J, Lozano MM, Dudley JP. Nuclear matrix binding regulates SATB1-mediated transcriptional repression. J Biol Chem. 2005;280:24600–9. doi: 10.1074/jbc.M414076200. [DOI] [PubMed] [Google Scholar]
- Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr Opin Immunol. 2002;14:207–15. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Tateno M, Yamasaki K. Solution structure and DNA-binding mode of the matrix attachment region-binding domain of the transcription factor SATB1 that regulates the T-cell maturation. J Biol Chem. 2006;281:5319–27. doi: 10.1074/jbc.M510933200. [DOI] [PubMed] [Google Scholar]
- Yasui D, Miyano M, Cai S, Varga-Weisz P, Kohwi-Shigematsu T. SATB1 targets chromatin remodelling to regulate genes over long distances. Nature. 2002;419:641–5. doi: 10.1038/nature01084. [DOI] [PubMed] [Google Scholar]