Abstract
Biomarkers are central to the translational medicine strategic focus, though strict criteria need to be applied to their designation and utility. They are one of the most promising areas of medical research, but the “biomarker life-cycle” must be understood to avoid false-positive and false-negative results. Molecular biomarkers will revolutionize the treatment of neurological diseases, but the rate of progress depends on a bold, visionary stance by neurologists, as well as scientists, biotech and pharmaceutical industries, funding agencies, and regulators. One important tool in studying cell-specific biomarkers is multi-parameter flow cytometry. CSF immunophenotyping, or immune phenotypic subsets, captures the biology of intrathecal inflammatory processes, and has the potential to guide personalized immunotherapeutic selection and monitor treatment efficacy. Though data exist for some disorders, they are surprising lacking in many others, identifying a serious deficit to be overcome. Flow cytometric immunophenotyping provides a valuable, available, and feasible “window” into both adoptive and innate components of neuroinflammation that is currently underutilized.
Keywords: CSF immunophenotype, CSF immunobiomarkers, neuroinflammation, pediatric neuroimmune disorders, surrogate markers
Introduction
Biomarker has been defined as a “characteristic that is objectively measured and evaluated as an indication of normal biologic processes, pathogenic processes, or pharmacological responses to a therapeutic intervention”.1 Use of the term dates back to 1980.2 In an era called the “biomarker revolution,” medical biomarker studies are extremely timely and widely recognized as important.3 Now there is a multiplicity of biomarker types and applications (Table 1).4,5 From a US regulatory perspective, integration of biomarkers in drug development would help alleviate stagnation and foster innovation in the development of new medical products, leading to more translational and personalized medicine.6 Biomarker-guided decision making would have a competitive clinical advantage over the existing empirical approach.7
Table 1.
Multiplicity of Biomarker Types and Applications
| Type | Definition/Application |
|---|---|
| Combinatorial | Panel- or pattern-based |
| Diagnostic | Disease-specific |
| Differentiation | Efficacy or safety of drugs within same class |
| Efficacy | Reflects positive outcome of a treatment |
| Exploratory | Delimited, may be driven by discovery, not hypothesis |
| Pharmacodynamic | Contrasting pharmacodynamic patterns may offer utility for treatment (optimal dose?) |
| Prognostic | Predict course of disease |
| Predictive | Provides information on obtaining response to treatment (optimal drug?) |
| Qualified | The data support its use for stated purpose |
| Risk | Can be used for risk stratification |
| Toxicity | Avoid/monitor potential toxic effects |
| Screening | Early disease detection |
| Staging | Differentiates different stages, activity, and subtypes of disease |
| Stand-alone | A single biomarker sufficient for purpose by itself |
| Stratification | Select best treatment for given patient |
| Surrogate | Intended as substitute for clinical endpoint |
| Translational | Can be used in pre-clinical and clinical studies |
The first part of this article discusses the unique need for biomarkers in neurological diseases, the importance of cerebrospinal fluid as the best source, and the life-cycle of the biomarker. It vets the biomarker process and provides tangible steps needed to improve the interpretability of biomarker data for neurological disorders. In the second part, putative CSF cellular immune markers, as revealed by flow cytometric immunophenotyping, are evaluated as candidate biomarkers of neuroinflammation.8 Recent advances in flow cytometry have presented greater capacity to identify and refine immune cell phenotypes.9 Although most immunophenotyping studies have been of peripheral blood, only CSF studies are reviewed here.
Biomarkers
Why are molecular biomarkers uniquely needed for neurological diseases?
Biomarker-guided personalized medicine is not a novel concept, but one applied in most areas of clinical medicine, including some neurological disorders, for years. For example, a stroke specialist will investigate and treat all cardiovascular risks factors s/he can identify, such as hypercoagulable state, sources of embolisms, hyperlipidemia, diabetes and hypertension. Consequently, a stroke patient will receive personalized, rational combination of drugs that target simultaneously all risk factors that contribute to a phenotypical expression of his/her disease. Furthermore, treating physician will not wait for the second stroke to make necessary therapeutic adjustments; rather s/he will use normalization of biomarker measurements as a guiding principle. Of course, this strategy required clinical trials that have proven surrogacy of these biomarkers to the clinically-relevant outcomes, such as mortality from cardiovascular diseases.
Indeed, molecular biomarkers (i.e., clinical laboratory tests) have been assessing functions of different cellular components of the endocrine, hematological, gastrointestinal and immune systems, or cardiomyocytes and renal epithelium for decades. In stark contrast, neurologists lack molecular biomarkers that measure physiological functions (or dysfunctions) of the cellular components of the central nervous system (CNS).
Instead, neurology practice and drug development rely on imaging modalities, especially magnetic resonance imaging (MRI), which provides structural information about CNS tissue. The undisputable utility of MRI in neurology practice makes us often forget that structural imaging does not provide molecular or even cellular information. For example, although brain atrophy reflects loss of CNS tissue, it may be masked for a long-time by replacement of one cellular component (e.g., neurons) by another (e.g., microglia, astroglia or immune cells) or by alternative processes such as edema or expansion of extracellular matrix. Furthermore, even in the instances when pathological correlations showed link between certain cellular processes and MRI features, such as perivascular inflammation underlying contrast-enhancing lesions (CELs) in multiple sclerosis (MS), assuming that all CELs are inflammatory causes misdiagnosis of ischemic and malignant lesions,10 while assuming that CELs capture all inflammatory activity underestimates the amount of inflammation e.g. in progressive MS.11 It is rather common radiology practice to call T2/FLAIR white matter lesions of certain size and location “demyelinating”, even though this MRI contrast captures differences in the relaxations of hydrogen protons and therefore cannot possibly differentiate one type of tissue integrity change (e.g. edema) from another (e.g. demyelination or astrogliosis).12
On the other hand, although clinical deficit correctly reflects loss of cellular functions, it provides limited insight about its reversibility or causes. Additionally, clinical deficit lacks sensitivity: i.e., it becomes obvious only after substantial damage to the underlying CNS tissue has accumulated; this is true for virtually any neurological condition where clinico-pathological correlations exists, including Parkinson’s disease, primary-progressive multiple sclerosis (MS) or mild cognitive impairment.
It is reasonable to conclude that this lack of molecular information about CNS tissue is one of the main reasons for the slow therapeutic progress in neurology. Inability to detect earliest stages of CNS diseases prevents initiating treatments at the time when their efficacy is highest. On the other hand, once the clinical defects become apparent, the physiological compensatory processes are exhausted and pathological processes are well-established and wide-spread. To stop the disease progression at this stage requires targeted, rational (i.e. personalized) combination treatments.
However, it is extremely difficult, if not impossible, to develop such effective treatment modalities without molecular biomarkers. Pathology studies revealed presence of different processes (e.g. Ca2+-mediated excitotoxicity, oxidative and nitrosative damage,13–16 endoplasmic reticulum [ER] stress,17–19 mitochondrial dysfunction, 20–22 hypoxia23 and inflammation) common to many established polygenic neurological diseases, with substantial variability between individual patients. Let’s imagine a perfect drug, targeting only one of these processes, being developed and tested: without ability to preselect patients in whom the targeted process is dominant, this drug will have limited efficacy in small, Phase II trials. Furthermore, development of a single, partially effective treatment makes development of further treatments for the same indication more difficult: instead of proving efficacy of future drugs against placebo, we must now prove efficacy against an active comparator, which is much harder. An alternative is to prolong recruitment into clinical trials by focusing on patients who cannot, for whatever reason, benefit from already approved therapy. Using biomarkers specific for different pathogenic processes effectively abolishes these problems.
Thus, development of biomarkers that measure physiological functions of cellular components of CNS tissue, and those that reflect varied intrathecal pathophysiological processes is a prerequisite to accelerate development of neurological therapeutics. Incorporating sample collection for biomarker measurements in contemporary clinical trials for neurological diseases is essential to validate biomarker surrogacy to clinical outcomes, as was achieved in other (e.g. cardiovascular) fields.
Cerebrospinal fluid (CSF) as the best source of molecular biomarkers for CNS diseases
What are the possible sources of molecular biomarkers for CNS diseases? Two related imaging modalities, positron emission tomography (PET) and single photon emission computed tomography (SPECT) are gaining lot of interest.24 Because these methods have been reviewed elsewhere, we will only highlight their comparison to the source of molecular biomarkers this article focuses on: CSF. Both PET and SPECT imaging use radioactive tracers that bind with high specificity to a single molecule, providing quantitative information about its spatial distribution in CNS tissue. The inclusion of spatial information represents strength of these modalities. The limitations are use of radioactivity, necessity of arterial delivery for some tracers, limited repeatability and focus on a single molecule. Especially the last one: focus on a single molecule limits broad clinical use. A single biomarker is unlikely to capture the dysfunctional pathway in its entirety. Multiplicity of pathogenic mechanisms in fully evolved CNS disease represents analogous challenges to a single biomarker as to an afore-mentioned single therapy. For example, although there is a fair correlation between deposition of extracellular amyloid and loss of neurons in Alzheimer’s disease (AD),25 the mechanisms by which amyloid beta may contribute to neuronal loss are complex and indirect. Furthermore, the direct mechanisms of synaptic and neuronal loss in AD patients are likely diverse, explaining why the correlations between amyloid PET and neurological disability are only fair.26
Instead, molecular tests of high clinical utility should measure multiple (ideally all) contributing processes, captures each pathway by multiple complementary biomarkers and measures loss of physiological functions and gains of pathological functions simultaneously with (at least some) cellular specificity. This is exemplified by liver functions tests, that measure physiological functions of hepatocytes (e.g., albumin, bilirubin) together with intracellular enzymes that are released during hepatocellular damage (e.g. AST, ALT). These requirements can be fulfilled today only by soluble biomarkers, amenable to quantification by multiplex technologies.
Should we strive to identify such clinically-useful soluble biomarkers of CNS diseases in the blood or CSF? The obvious advantages of blood are its accessibility and wide-spread acceptance of serial blood draws. However, blood cannot be used as a reliable source of biomarkers of neuroinflammation. CNS is comparably smaller and more sterile organ than gastrointestinal tract, skin, respiratory system or genitourinary system, all of which represent entry-ports for infectious agents and toxins. Depending on the type, dose and pathogenicity of the invading agent and the presence/absence of immunological memory, the ensuing immune reaction to the pathogen is systemic and overt or subclinical. Yet, both systemic and subclinical immune responses can have undistinguishable serum/plasma biomarker profiles.27 Thus, differentiating systemic immune activation related to neuroinflammation from immune activations related to subclinical infections is not possible. Furthermore, we have reported that significant correlations between inflammatory biomarkers present in the blood and CSF exist paradoxically only in subjects without CNS inflammation.28 This is because under physiological conditions, activated immune cells cross blood brain barrier (BBB) to perform immunosurveillance functions to clear pathogens that may have gained access to the CNS during systemic infection. However, once the CNS inflammation is established, the correlations between blood and CSF disappear28,29 because of selective recruitment of cells from the blood to CNS, followed by selective expansion and preferential retention of specific cells in the intrathecal compartment.
Although soluble biomarkers released exclusively by the cells of the nervous system (e.g. axonal marker neurofilament-light chain [NF-L]) can be successfully measured in the blood using highly-sensitive assays,30 serum levels of these CNS-originating biomarkers explain between 38–60% of variance of the CSF levels.31,32 This 40–62% “noise” is likely due to intra-individual differences in CSF clearance, affected by cardiac output,33 sleep,34 physical activity and aging,35–36 as well as systemic clearance and hepatic metabolism of soluble biomarkers. While this level of accuracy may be sufficient for some indications, such as providing prognostic value of recovery from CNS injury,37 introducing 40–62% noise in pharmacodynamic outcomes in drug development or precision medicine applications may not be acceptable. At any rate, development of sensitive serum/plasma assays for CNS-specific biomarkers is welcomed, but the clinical utility of these assays needs to be evaluated against CSF assays, so that the sensitivity, specificity and correlations of these measurements to clinical outcomes are well understood.
CSF is collected through lumbar puncture (LP), an invasive procedure with low and manageable side effects. The most frequent side effect is post-LP headache, which can be minimized by use of atraumatic needles of small caliper.38 In vast majority of subjects, the procedure can be performed repeatedly, under local anesthesia and with minimal discomfort. The ability to safely collect 20–25cc of CSF in adult subjects provides sufficient volume for multiple simultaneous applications, such as detailed assessment of cellular compositions,28 analysis of extracellular vesicles,39 multiplex proteomic analysis,40 lipidomics41 and metabolomics.42 The possibility to combine different assays leads to creative applications, such as recently-developed method for quantifying inflammation in human CNS tissue without a need for CNS tissue biopsy.11
Reasons for slow progress in the development of CNS biomarkers
Notwithstanding our enthusiasm for, and stated advantages of CSF biomarkers, the reality is that surprisingly few reached drug development43–46 or clinical practice in the past decades. There are two major reasons for this unsatisfactory outcome: First relate to the quality of biomarker studies. Because biological changes (induced by disease state or therapy) are usually mild to moderate, the technology applied must be highly sensitive and reproducible. To achieve this, all sources of non-biological variance must be identified, quantified and actively suppressed. Pre-analytical variables may contribute to >60% of variance, which often exceeds biological variance caused by diseases or administration of therapies.
Sources of pre-analytical variance are related to sample acquisition (e.g., acquisition method, time of the day, preparation of patient: treatment, diet, pre-medication, container to which the sample is acquired) and sample processing (e.g., placement and transport on ice versus room temperature, delay in processing, centrifugation, ultracentrifugation, fractionation, aliquotting, freezing/thawing). Studies that combined patient cohorts collected by one group of investigators with controls collected by different investigators using non-standardized procedures, can mistakenly interpret inter-group differences caused by pre-analytical variances as features of a disease or therapeutic intervention. Any assay that requires extensive sample preparation (e.g., depletion of most abundant protein species, fractionation) will increase pre-analytical variance and needs to be done blinded, with patient and control samples run side-by-side and intermingled.
Sources of analytical variance relate to introduction of a biases when operators are unblinded, especially when using highly operator-dependent assays such as cloning of antigen-specific T cells or semi-quantitative 2D gels. To limit analytical variance, the investigators should examine and report intra and inter-assay variability, use written, optimized standard operating procedures (SOPs), highly trained personnel working on coded samples, or greater automation. To minimize batch effects and inter-assay variability, reference calibrators should standardize relative or absolute values between the assays.
Finally, sources of post-analytical variance are represented by selective elimination of “outliers” after (rather than before) unblinding and flexible sample sizes leading to multiple “interim analyses” without appropriate disclosure and adjustments of statistical assumptions. Design flaws such as underpowered studies combined with bias to preferentially publish positive results lead to unacceptably high false discovery rates. Biomarker studies routinely fail to adjust statistical analyses for multiple comparisons, using justification that these studies are “discovery” studies that will “need future validation”. However, validation rarely follows, perhaps due to the afore-mentioned publication bias, which keeps negative results preferentially unpublished.
The biomarker field has now matured to a stage when flawed study designs are no longer acceptable;47–49 where standardized collection and processing of samples from large numbers of patients and appropriate controls are performed by same investigators (or consortium that adheres to detailed SOPs), in a blinded fashion and using technologies with adequate and properly disclosed intra- and inter-assay variabilities. Validation of observed findings in an independently collected and processed validation cohort, with pre-determined (and sufficient power) should be part of any new biomarker study performed.
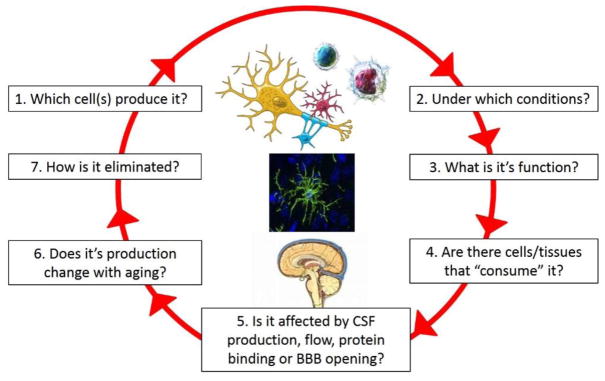
The second problem relates to interpretation of biomarker studies. Ideally, we should understand all aspects of what we call “a biomarker life-cycle” (Figure 1), before we can correctly interpret measured values.
Figure 1.
Understanding a biomarker life-cycle
For example, abnormally high levels of a biomarker can be caused by its increased secretion, which is the most commonly entertained interpretation. However, we should not forget that decreased consumption of a biomarker will have the same effect. For example, consumption of CSF B cell activating factor (BAFF) by expanded number of intrathecal B cells keeps CSF BAFF levels in MS patients in the physiological (i.e., healthy volunteer; HV) range. Administering B cell-depleting therapy rituximab into CSF partially depletes intrathecal B cells and unmasks BAFF elevations.43 Understanding biomarker lifecycle and combining measurements of the biomarker with the measurements of its cellular producers and consumers will allow mathematical normalizations of the measured biomarker levels for the observed differences in cellular components and thus reveal its true, (e.g., pathological) levels. The cellular components may be measured directly (e.g. by flow cytometry)28 or indirectly, by another cell-specific soluble analyte. Thus, understanding lifecycle of biomarkers in the multiplex assay(s) will eventually allow system-level analysis, either based on hypothesis-driven conceptual models, or using machine (statistical) learning.50
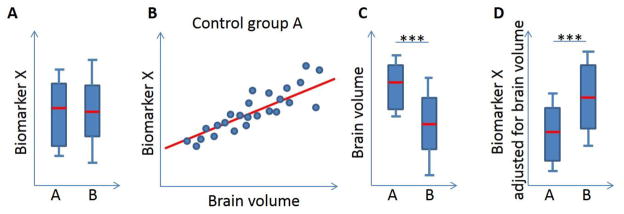
We’ll illustrate this concept on a simplified example of the Biomarker X (Figure 2) that is expressed only in axons and released under two conditions: Small amounts of this biomarker are released physiologically, during axonal maintenance; but larger quantities of the Biomarker X are released during acute axonal damage. Let’s now imagine that we measured identical CSF levels of the Biomarker X in healthy volunteers (HV; Fig 2A, group A) as in patients (Fig 2A, group B). We will interpret these findings as an evidence that our patient cohort does not have acute axonal damage.
Figure 2.
Adjustment of biomarker levels based on mechanistic understanding of its lifecycle
However, what if we observed a significant correlation between CSF levels of biomarker X and brain fractional volume in HVs (Fig 2B) and significantly lower brain fractional volumes in the patient cohort in comparison to HVs (Fig 2C)? Those observations should change our interpretation! The correlation between brain volume and Biomarker X in the HVs provides an opportunity to assess the physiological release of Biomarker X as a function of measured brain volume. Because patients have lower brain volume, their physiological secretion of Biomarker X should be significantly lower than what we have measured. In other words, if we mathematically adjust CSF levels of Biomarker X to the measured brain volume, we will conclude that patients have significantly higher levels than they should have, which means that in addition to physiological release, patients have superimposed pathological release of Biomarker X due to acute axonal transections. The NF-L is a prime candidate biomarker for such assessment of its relationship to MRI-derived brain volumetric data.
In conclusion, we are only at the beginning of exploring full potential of CSF biomarkers. Future biomarker studies need to collect high quality clinical (i.e. scales of neurological and cognitive disability) and imaging data (i.e., especially volumetric data and/or composite measures such as Combinatorial MRI Scale of CNS tissue destruction COMRIS-CTD),51 to facilitate systems-wide analysis of relationships between biomarkers, neurological functions and structural integrity of CNS tissues. In parallel, in-vitro and in-vivo models need to generate knowledge-base for understanding biomarker-life cycles and relationships between biomarkers measured by multiplex assays. Finally, biomarker studies need to be incorporated to clinical trials to generate surrogacy data. Although we have little doubt that molecular biomarkers will revolutionize treatment of neurological diseases, how fast we’ll achieve desired progress depends on the willingness of the involved parties (i.e., patients, providers, scientists, pharmaceutical and biotech industry, funding agencies and regulators) to take bold, visionary stance.52
Potential Biomarkers Revealed by CSF Immunophenotyping
Flow cytometric immunophenotyping refers to the process of identifying and distinguishing various lymphocyte subsets in a fluid sample by phenotypic characteristics as revealed by fluorochrome-conjugated monoclonal antibody tags that fluoresce in the laser beams of the flow cytometer. Due to methodological advances,53 multi-color (polychromatic) flow cytometry (11 – 12 colors) allows a broader range of immune cells to be phenotyped in a single but sufficient CSF sample. Not without shortcomings,54 multiparameter flow cytometry is “truly coming of age” in the 21st century,55 providing a treasure-trove of other potential biomarkers beyond the scope of this review.9 The 50-fold concentration of the CSF cell pellet obviates the need for higher volume requirements in controls, whose CSF leukocyte counts are only 1–3/cu mm.28 Putting straight CSF from patients with non-inflammatory disorders into the flow cytometer otherwise uniformly results in a false report of no cells found. The irony is that most patients with neuroinflammatory disorders will at some point in their course undergo a lumbar puncture, but with only traditional, low yield testing for neuroinflammation. If a clinical panel of lymphocyte subsets were measured as well for detailed cellular assessment, the informational value of the LP would be enhanced tremendously.52
Analysis of CSF lymphocyte subset distribution in healthy young adults traces back more than two decades.56 The human CSF immunophenotype in healthy individuals and non-inflammatory disorder controls reveals a distinctive distribution of immune cells compared to peripheral blood. The vast majority are T cells, most of which are central memory cells.57 Of those, approximately 75% are CD4+ T helper cells and 25% are CD8+ cytotoxic/suppressor T cells.8,56 The remainder of CSF immune cells constitutes only a fraction of the total and are present in small numbers, but gain in importance in neuroinflammation. These include natural killer-like T cells (NKT), NK cells, γδ T cells, B cells, plasmablasts, plasma cells, and dendritic cells. This mixture of immune cells in CSF comprises cells recruited from circulating blood and those activated intrathecally, exiting the CNS when effectors functions are completed.28 CSF immune cells can be further qualified based on markers of maturation (DR45R isoforms), activation (HLA-DR, CD25), among others.28
Enumeration of CD4+ T cell subsets58 by flow cytometry reveals that T cells are dynamic.59 Differentiated by the hallmark cytokine secreted, there were two helper lineages in the late 1980s: Th1, secreting IFN-γ, and Th2, secreting IL-4. In 2005, a new Th17 lineage producing IL-17A was described, which has been reviewed,60 leading to the current view of a Th17/Th1 paradigm of autoimmune neuroinflammation.61 Th17 cells are involved in some neuroinflammatory disorders, not others. For example, the frequency of CSF IL-17-producing CD4+ cells is increased in cerebral vasculitis caused by secondary angiitis of the CNS and giant cell vasculitis, not stroke.62
The CSF immunophenotype also reports on other T cells that can be measured by flow cytometry. NKT cells (CD3+CD56+) can act as effector or regulatory cells. Gamma/delta T cells (γδ T cells), which express γ and δ chains rather than the usual α and β chains of the T cell receptor, regulate the extent and duration of inflammation.63 Regulatory T cells (Tregs) come in many varieties. CD4+CD25+FoxP3+ Tregs suppress the cytotoxic effects of CD8+ effector T cells, delimiting CNS damage.64 A non-T cell found by immunophenotyping is the natural killer (NK) cell (CD3-CD56+), of which there are multiple subsets,65 including regulatory CD56bright NK cells.
The multiplicity of human B cell subsets has been extensively reviewed.53 Beyond transitional B cells appearing in the course of B cell development, mature B cells may be naïve (IgD+CD27−) or memory (non-switched IgD+CD27+, or switched IgD-CD27+).53 CD5+ B cells are also referred to as “fetal derived CD5+ B1 cells” or self-reactive “innate-like B cells”.66,67 Regulatory B cells (Bregs), such as those that are IL-10-competent, can suppress immune reactions,53 whereas dysregulated B cell signals orchestrate loss of tolerance and autoantibody production.68
Plasmablasts (IgD-CD27+CD38+CD138−), of which there are short-lived and long-lived subgroups, are the main B cell effector subset in MS69 and are migratory IgG-producing cells in NMO.70 Terminally differentiated B cells are plasma cells (IgD-CD27+CD38+CD138+), which are large in size and the most efficient antibody producers. Plasmablasts are usually CD19+, while terminally differentiated plasma cells lack B cell lineage markers. The frequency of plasmablasts is very low/undetectable in non-inflammatory diseases, but may be elevated in various inflammatory diseases.7
Two subsets of dendritic cells can be detected in CSF, but in non-inflammatory neurological disorders, they constitute only up to 1% of CSF mononuclear cells.71 Myeloid DC are identified by flow cytometry as lin-CD11c+HLA-DR+DC123(dim), and plasmacytoid DC as lin-CD11c-HLA-DR+CD123(high).
Immune cell ratios may provide additional information. One is the CD4/CD8 T cell ratio, as an indicator of possible T cell dysregulation. The CSF monocyte/ B cell ratio, which is decreased in RR-MS, is another useful index.28 The T cell/monocyte ratio in clinically isolated syndrome identifies patients at risk of rapid disease progression.72 The NK cell CD56bright/CD56dim ratio is increased in MS.65
CSF immunophenotyping for biomarkers of disease activity
Comparison of immunophenotyping results from studies of various neuroinflammatory disorders is provided in Table 2. Frequency data are shown, but some studies also included counts of CSF cells, as noted in the table legend. There was abundant data for MS, a modest amount for paraneoplastic disorders, but surprisingly little or no immunophenotyping data for NMO, AE, ADEM, NPSLE, traumatic brain injury, or stroke.
Table 2.
CSF Cellular Immune Abnormalities in Key Pediatric- and Adult-onset Neuroinflammatory Disorders (Relative Proportion/%)
| Disease/ref | B Cells | PB | PC | T Cells | NK | DC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| N | CD19 | CD5 | CD27 | CD4 | CD8 | NKT | γδ-T | |||||
| RR-MS†28 | 51 | ↑ | ↑ | ↑ | ↑ | ↔ | ↔ | ↔ | ↔/↑ | ↔ | ↔ | |
| PP-MS28 | 61 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||
| SP-MS28 | 30 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||
| *pMS76 | 25 | ↑ | ↑ | |||||||||
|
| ||||||||||||
| NMO82 | X | ? | ↑ | ↑ | ||||||||
| *pNMO77 | 1 | ↑ | ||||||||||
|
| ||||||||||||
| PNS/Hu‡74,75 | 11 | ↑ | ↔ | ↔ | ↓ | ↔ | ||||||
| PCD80 | 1 | ↑ | ||||||||||
| *pOMS81,84 | 36 | ↑ | ↑ | ↑ | (↑) | (↓) | ↔ | ↔ | ↑/↔ | ↔ | ||
|
| ||||||||||||
| ADEM | ? | |||||||||||
|
| ||||||||||||
| AE | ? | |||||||||||
| *pAE78 | 2 | ↑ | ||||||||||
|
| ||||||||||||
| NPSLE | ? | |||||||||||
| *pNPSLE79 | 1 | ↑ | (↓) | ↔ | ↔ | ↑ | ||||||
|
| ||||||||||||
| RE86 | 17 | ? | ↑ | ↑ | ||||||||
Abbreviations: PB = plasmablasts; PC = plasma cells; NKT = natural killer cell-like T cells; γδ T cells = TCR-gamma/delta cells; NK = natural killer cells; RR-MS = relapsing-remitting multiple sclerosis; PP-MS = primary progressive MS; NMO = neuromyelitis optica; PNS = paraneoplastic syndrome; Hu = anti-Hu syndrome or anti-ANNA1; PCD = paraneopastic cerebellar degeneration; OMS = opsoclonus-myoclonus syndrome; ADEM = acute disseminated encephalomyelitis; AE = anti-NMDAR encephalitis; NPSLE = neuropsychiatric lupus; RE = Rasmussen encephalitis
Frequency arrows: ↑ = increased above non-inflammatory disorder controls; ↓ = decreased; ↔ = not different than controls; ? = not reported’
RR MS: absolute counts in RR-MS were elevated for B cells and T cells (CD3+, CD4+, CD8+).28
Absolute CSF counts in anti-Hu were elevated for B cells, memory B cells, CD4+ T cells, and CD8+ T cells Prefatory p = pediatric-onset disorder Anti-Hu: increased absolute counts of CSF DC.75
An increased proportion of CSF B cells was common across diseases.28,73–76 Although there were no data from adults with NMO, AE, or NPSLE in adults, there were case reports of increased CSF B cell percentage for each disorder in pediatric-onset counterparts.77–79 One adult with PCD paraneoplastic syndrome exhibited elevated CSF B cell frequency, though the T cell abnormalities were the focus of the report.80 In untreated pOMS, the percentage of multiple B cell subsets was elevated in CSF.81
Plasmablast increases were reported in pMS, NMO, and pOMS, but they were not routinely measured, so possible abnormalities in other disorders cannot be ruled out. In NMO, activated lymphocytes and/or plasma cells were found frequently in the 50% of patients who manifest CSF pleocytosis.82 An adult who was seropositive for contactin-associated protein-2 (CASPR) antibodies without a detected tumor showed increased CSF fractions of CD138+ plasma cells and activated CD8+ T cells.83
Another subset not routinely tested was the CSF CD5+ B cell subset. In pOMS, the frequency of both CD5+ and CD5− B cells was elevated in CSF.84 Increased frequency of CD5+ B cells has also been reported in other neuroimmunological diseases.66
A decreased percentage of CSF CD4+ T cells was found in pOMS and pNPSLE, but not in demyelinating disorders.7,11,28,85 An increased frequency was reported in a study of Rasmussen syndrome.86
The γδ T cell subset, well known for important regulatory functions in neuroinflammation, was not consistently abnormal in frequency, though it was not often measured in CSF. “Double negative” T cells (CD4−CD8−), most of which are γδ T cells, are sometime measured instead.28 An elevated percentage of γδ T cells has been reported in MS,88 and pockets of γδ T cells are found in brain specimens from patients with MS or Rasmussen syndrome.88
In sum, multiple CSF immunophenotypic abnormalities were found, but not all disorders were investigated with equal thoroughness, and many disorders have not been studied with immunophenotyping. The pattern of abnormality was not the same among diseases, though CSF B cell expansion was a common theme. The glaring gaps in our knowledge of the CSF immunophenotype are a challenge to neurologists to fill them in. Flow cytometric immunotyping is nonetheless shown to provide important information, which can be used in personalized medicine.
CSF immunophenotyping reveals age-related and developmental changes
Comparison of the CSF immunophenotype in pediatric and adult control groups affords a fascinating window of the brain and the immune system. Flow cytometric CSF immunophenotyping has been used successful in the analysis of pediatric-onset neuroinflammatory disorders.28,73,76,81,84,90 The need for it stems from concern that different developmental stages of the pediatric immune system (and the brain) and what impact they may have on the appropriateness of treatment decisions. Although lumbar puncture in healthy children is not considered ethical, several sources of non-inflammatory neurological disorders (NIND) are used for comparisons. In contrast, there are multiple sources of the normative blood immunophenotype in children, showing remarkable age-related changes.91,92
A sufficient CSF sample for flow cytometry can be readily obtained from unsedated adults; for children, sedation is necessary, and for toddlers, deep sedation (propofol with LMA) provides compassionate care and an atraumatic sample in a day surgery facility or comparable setting.73,81,89 In infants younger than six months old, CSF immunophenotyping is largely infeasible, due to lower allowable CSF volume (5 – 6 mL total). However, in toddlers and youngsters, 15–20 mL of CSF—depending on exact age—can be safely removed. The risk of post-traumatic headache in young child is about 1–2% using these methods, which is substantially lower than in teens or adults.
In pediatric non-inflammatory neurological disorders, absolute counts of lymphocyte subsets reflect increased T cells (CD3+, CD8+) compared to adults.28 There are also more myeloid and plasmacytoid DC. The absolute counts change over time, resulting in higher B cell and NK counts and lower granulocytes.28 However, the relative portions of most CSF immune cells show more similarities than differences in children and adults, with the except of the age-dependent frequency of CSF γδ T cells. In contrast, the frequency and absolute counts of peripheral blood mononuclear cells (PBMN) changes so dramatically over time91,92 as to complicate the search for immunologic biomarkers. CSF immunophenotyping has revealed that B cell populations discriminate between pediatric- and adult-onset MS.76
Establishing NEDA (immune quiescence)
An important concept, developed in the MS field, is the necessity of proving no evidence of disease activity (NEDA) persists after treatment for neuroinflammation.93 Biomarkers may play an important role in making such a determination. This concept is applicable to neuroinflammatory disorders as a whole, but is only beginning to take hold. Confirming NEDA has been used successfully in pOMS.81 CSF immunophenotyping is an effective means of testing for NEDA.
How CSF immunophenotyping informs on adequacy of treatment response
Pre- vs post-treatment CSF immunophenotyping allows direct correlation of clinical response/outcome and biological response (Table 3).43,45,81,94–100 Certain disease modifying drugs acting primarily on T cells, such as dimethyl fumarate, glatiramer acetate, fingolimod, IFN-β, and natalizumab, are ineffective or induce unanticipated relapse in NMO.101 Actual measurement of CSF B cells and T cells via immunophenotyping would help to prevent potential pitfalls of the trial-and-error approach.
Table 3.
Use of CSF immunophenotyping to test the capacity of targeted immunotherapies to rectify the initial abnormalities
| Agent | Indication | Post-treatment Immunophenotyping Results |
|---|---|---|
| Daclizumab | MS | Decreased CD4+CD25+ T cell counts; decreased CD4+ and CD8+ T cell/NK cell ratio; increased CD56(bright) NK cells; no change in CD4+/CD8+ T cell ratio.45,95 |
| Fingolimod | MS | Decreased proportion of CD+ T cells. It had no impact on CSF B cells. The percentage of CSF CD8+ T cells, NK cells, and monocytes increased compared to controls. The CSF CD4+/CD8+ T cell ratio reversed in majority of patients.94 |
| Natalizumab | MS | Lowered CSF B cells and CD4/CD8 ratio.98 Also decreased numbers of CD4+ and CD8+ T cells, and CD138+ plasma cells (Stüve2008).96 |
| Rituximab | MS | CSF B cell counts decreased, as did CD3+ T cell counts.99 In PP-MS (n = 4), CSF B cells were not as depleted as blood B cells, and B cell activation was only temporarily expressed.97 Rituximab is detectable in CSF for up to 24 weeks.100 |
| Rituximab | pOMS | CSF B cell frequency dropped to undetectable levels;12–18 months later, B cells in peripheral blood had repopulated, but the CSF B cell percentage did not exceed control percentages.81 |
| Rituximab-IT | SP-MS | Intrathecal rituximab lacked efficacy on CSF biomarkers.43 Insufficient saturation of CD20, absence of lytic complement, and dearth of cytotoxic NK cells (CD56-dim) may explain the insufficient disease response.43 |
IT = intrathecal
CSF cellular biomarkers can shift target focus to better options
Though anti-CD20 targeting eliminates CD20+ B cells, certain antibody-producing cells in the B cell lineage escape destruction. CSF immunophenotyping has helped unveil the reasons for partial responses and rituximab treatment failures. B cell CD19 expression is broader than that of CD20 during B cell development and initiated earlier (pro-B cell stage).102 Plasma cells are not targeted by anti-CD20.103 The anti-CD19 monoclonal antibody, inebilizumab, has progressed to clinical trials for MS, NMO, and systemic sclerosis.104 Possible risks include chronic B cell depletion.
Pharmacological targeting of Tregs is shown to be advantageous in the treatment of cancer, in which Tregs are manipulated to defeat host anti-cancer immunity, but it may be anticipated to be disadvantageous in neuroimmune disorders, in which boosting Treg cell effect may ameliorate neuroinflammation. Measurement of Tregs in neuroinflammatory disorders is a way of accessing direct data for devising optimal therapeutic strategies.
Longitudinal profiling
Cross-sectional data are important, but longitudinal data allow temporal patterns of lymphocyte recovery after immunotherapy to be discovered.81 The longitudinal approach, facilitated by flow cytometric CSF immunophenotyping, allows distinctions to be made between intrathecal inflammation prior to treatment, during remission, and in the case of partial response and relapse.
Limitations
The CSF immunophenotype represents just the “mobile pool” of CNS-residing immune cells.28 It informs on the phenotype, not function, of the cells. Strict quality control measures are required for valid results.105 CSF sampling must be atraumatic to avoid contamination of CSF with cells from circulating blood. Effective sedation is required in younger children for compassionate care and practical reasons. CSF cells must be collected fresh on ice and brought to the flow cytometry lab promptly for cell labeling. Therefore, immunophenotyping should be planned in advance, not as an afterthought, and cannot be performed reliably on frozen cells. However, most outpatients can be scheduled, and most large hospitals already use flow cytometry for other indications, such as leukemia/lymphoma, so repurposing is possible.
Clinical Implications
Exploration of the full potential of CSF biomarkers is only beginning. CSF provides an invaluable, unique portal into CNS immunopathology, not afforded by circulating blood.28 It reveals the biology of intrathecal inflammation and potentially guides selection of optimal immunotherapies for individual patients while monitoring their biological efficacy.28 Management of neuroimmunologic diseases has been hindered by the inability to measure intrathecal inflammation reliably using decades-old standard laboratory measures.11 Multiparameter flow cytometry has become a powerful tool to correlate clinically-relevant factors, such as stage of diseases activity, relapse, and remission, with CSF cellular subsets.85 While the CSF cell pellet is consumed for flow cytometry, the very substantial cell-free CSF supernatant can be aliquoted, frozen, optimally banked,106 and used for many other biomarker studies. Biomarkers need to be incorporated into clinical trials for surrogate data to the clinically-relevant outcomes,11 and there are now available biomarker-guided clinical trial designs.107 It is easy to envisage an entirely different way of evaluating and interpreting neuroinflammation in patients of all ages with neuroinflammatory disorders. Neurologists bold enough to adopt and implement the biomarker approach will be able to step into the promising new era.
Acknowledgments
Grant Support
The work of BB was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH). MP had no targeted funding.
Prof. Dr. Pranzatelli is President and Founder of the National Pediatric Neuroinflammation Organization, Inc., a Florida charitable organization and a registered 501(c)(3) organization.
Abbreviations
- CSF
cerebrospinal fluid
- CNS
central nervous system
- ELEs
contrast-enhancing lesions
- immunophenotyping
immune cell phenotype profiling
- NIND
non-inflammatory neurological disorders
- MS
multiple sclerosis
- OMS
opsoclonus myoclonus syndrome
- NMO
neuromyelitis optica
- NPSLE
neuropsychiatric system lupus erythematosus
- DC
dendritic cell
- NK cell
natural killer cell
Footnotes
Disclosure of interests: B.B. is co-inventor on the U.S. Patent Application No. 62/038,530: Biomarkers for Diagnosis and Management of Neuroimmunological Diseases, but received no financial gains from this patent. M.P. has no commercial, proprietary, or financial interest in any products or companies described in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIH Definitions Working Group. Biomarkers and surrogate endpoints in clinical research: definitions and conceptual model. In: Downing GJ, editor. Biomarkers and Surrogate Endpoints. Amsterdam: Elsevier; 2000. pp. 1–9. [Google Scholar]
- 2.Paone JF, Waalkes TP, Baker RR, Shaper JH. Serum UDP-galactosyl transferase as a potential biomarker for breast cancer. J Surg Oncol. 1980;15:59–66. doi: 10.1002/jso.2930150110. [DOI] [PubMed] [Google Scholar]
- 3.Schisterman EF, Albert PS. The biomarker revolution. Stat Med. 2012;31(22):2513–2515. doi: 10.1002/sim.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson JK. Biomarkers and surrogate endpoints. Br J Clin Pharmacol. 2005;59(5):491–494. doi: 10.1111/j.1365-2125.2005.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnefoy J-Y. The biomarker revolution: a step toward personalized medicine. Personalized Medicine. 2008;5(6):553–556. doi: 10.2217/17410541.5.6.553. [DOI] [PubMed] [Google Scholar]
- 6.Amur S, Frueh FW, Lesko LJ, Huang SM. Integration and use of biomarkers in drug development, regulation and clinical practice: a US regulatory perspective. Biomark Med. 2008;2(3):305–311. doi: 10.2217/17520363.2.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;27(Pt 7):1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]
- 8.Kowarik MC, Grummel V, Wemlinger S, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol. 2014;261:130–143. doi: 10.1007/s00415-013-7145-2. [DOI] [PubMed] [Google Scholar]
- 9.Woo J, Baumann A, Arguello V. Recent advances of flow cytometry: new applications in hematology and oncology. Expert Rev Mol Diagn. 2014;14:67–81. doi: 10.1586/14737159.2014.862153. [DOI] [PubMed] [Google Scholar]
- 10.Masdeu JC, Gadhia R, Faridar A. Brain CT and MRI. differential diagnosis of imaging findings. Handb Clin Neurol. 2016;136:1037–1054. doi: 10.1016/B978-0-444-53486-6.00054-5. [DOI] [PubMed] [Google Scholar]
- 11.Komori M, Blake A, Greenwood M, et al. CSF markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann Neurol. 2015;78(1):3–20. doi: 10.1002/ana.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Amer J Psychiatr. 2015;28 doi: 10.1176/appi.ajp.2015.15060753. appiajp201515060753. [DOI] [PubMed] [Google Scholar]
- 13.Mir F, Lee D, Ray H, Sadiq SA. CSF isoprostane levels are a biomarker of oxidative stress in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2014;1(2):e21. doi: 10.1212/NXI.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gackowski D, Rozalski R, Siomek A, et al. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J Neurol Sci. 2008;266(1–2):57–62. doi: 10.1016/j.jns.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Ferreiro E, Baldeiras I, Ferreira IL, et al. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers. Internat J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darwish RS, Amiridze N, Aarabi B. Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury. J Trauma. 2007;63(2):439–442. doi: 10.1097/TA.0b013e318069178a. [DOI] [PubMed] [Google Scholar]
- 17.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38(3–4):409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009;12(4):379–385. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YT, Wu SB, Lee WY, Wei YH. Mitochondrial respiratory dysfunction-elicited oxidative stress and posttranslational protein modification in mitochondrial diseases. Ann N Y Acad Sci. 2010;1201:147–156. doi: 10.1111/j.1749-6632.2010.05631.x. [DOI] [PubMed] [Google Scholar]
- 21.Lenaz G, Bovina C, D'Aurelio M, et al. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese V, Lodi R, Tonon C, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci. 2005;233(1–2):145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.McMahon J, McQuaid S, Reynolds R, Fitzgerald U. Expression of Er Stress- and Hypoxia- Associated Molecules in Grey Matter Lesions in Multiple Sclerosis. Glia. 2011;59:S62–SS3. doi: 10.1177/1352458512438455. [DOI] [PubMed] [Google Scholar]
- 24.Lu FM, Yuan Z. PET/SPECT molecular imaging in clinical neuroscience: recent advances in the investigation of CNS diseases. Quant Imaging Med Surg. 2015;5(3):433–447. doi: 10.3978/j.issn.2223-4292.2015.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosconi L, McHugh PF. FDG- and amyloid-PET in Alzheimer's disease: is the whole greater than the sum of the parts? Q J Nucl Med Mol Imaging. 2011;55(3):250–264. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Mias GI, Li-Pook-Than J, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148(6):1293–1307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S, Lin YC, Wu T, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol. 2014;192(6):2551–2563. doi: 10.4049/jimmunol.1302884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards KR, Goyal J, Plavina T, et al. Feasibility of the use of combinatorial chemokine arrays to study blood and CSF in multiple sclerosis. PLoS ONE. 2013;8(11):e81007. doi: 10.1371/journal.pone.0081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann ClinTransl Neurol. 2016;3(3):216–225. doi: 10.1002/acn3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergman J, Dring A, Zetterberg H, et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflammation. 2016;3(5):e271. doi: 10.1212/NXI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):155–159. doi: 10.1177/1352458515623365. [DOI] [PubMed] [Google Scholar]
- 33.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids and barriers of the CNS. 2014;11(1):26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang YM, Zhang X, Wagner UG, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195(10):1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu C, Miller MC, Caralopoulos IN, et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids and barriers of the CNS. 2012;9(1):3. doi: 10.1186/2045-8118-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Current topics in developmental biology. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- 37.Rech TH, Vieira SR, Nagel F, Brauner JS, Scalco R. Serum neuron-specific enolase as early predictor of outcome after in-hospital cardiac arrest: a cohort study. Crit Care. 2006;10(5):R133. doi: 10.1186/cc5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertolotto A, Malentacchi M, Capobianco M, et al. The use of the 25 Sprotte needle markedly reduces post-dural puncture headache in routine neurological practice. Cephalalgia. 2016;36(2):131–138. doi: 10.1177/0333102415583983. [DOI] [PubMed] [Google Scholar]
- 39.Chiasserini D, van Weering JR, Piersma SR, et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. 2014;106:191–204. doi: 10.1016/j.jprot.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Baird GS, Nelson SK, Keeney TR, et al. Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am J Pathol. 2012;180(2):446–456. doi: 10.1016/j.ajpath.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci. 2007;8(10):743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 42.Mandal R, Guo AC, Chaudhary KK, et al. Multi-platform characterization of the human cerebrospinal fluid metabolome: a comprehensive and quantitative update. Genome Med. 2012;4(4):38. doi: 10.1186/gm337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komori M, Lin YC, Cortese I, et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol. 2016;3(3):166–179. doi: 10.1002/acn3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin YC, Winokur P, Blake A, Wu T, Romm E, Bielekova B. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann Clin Transl Neurol. 2015;2(5):445–455. doi: 10.1002/acn3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bielekova B, Richert N, Herman ML, et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology. 2011;77(21):1877–86. doi: 10.1212/WNL.0b013e318239f7ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pranzatelli MR, Tate ED, McGee NR, et al. Key role of CXCL13/CXCR5 axis for cerebrospinal fluid B cell recruitment in pediatric OMS. J Neuroimmunol. 2012;243(1–2):81–88. doi: 10.1016/j.jneuroim.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Ioannidis JP, Khoury MJ. Improving validation practices in “omics” research. Science. 2011;334(6060):1230–1232. doi: 10.1126/science.1211811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ioannidis JP, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–175. doi: 10.1016/S0140-6736(13)62227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ioannidis JP. A roadmap for successful applications of clinical proteomics. Proteomics Clin Appl. 2011 Jun;5(5–6):241–247. doi: 10.1002/prca.201000096. [DOI] [PubMed] [Google Scholar]
- 50.Bielekova B, Vodovotz Y, An G, Hallenbeck J. How implementation of systems biology into clinical trials accelerates understanding of diseases. Front Neurol. 2014;5:102. doi: 10.3389/fneur.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosa P, Komori M, Waters R, et al. Novel composite MRI scale correlates highly with disability in multiple sclerosis patients. Mult Scler Relat Disord. 2015;4(6):526–535. doi: 10.1016/j.msard.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bielekova B. Perspective: Who dares, wins. Nature. 2016;540(7631):S10. doi: 10.1038/540S10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaminski DA, Wei C, Qian Yu, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Frontiers Immunol. 2012;3:1–15. doi: 10.3389/fimmu.2012.00302. article 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemecek A, Zimmermann H, Rübenthaler J, et al. Flow cytometric analysis of T cell/monocyte ratio in clinically isolated syndrome identifies patients at risk of rapid disease progression. Mult Scler. 2016;22(4):483–493. doi: 10.1177/1352458515593821. [DOI] [PubMed] [Google Scholar]
- 55.Virgo PF, Gibbs GJ. Flow cytometry in clinical pathology. Ann Clin Biochem. 2012;49(Pt 1):17–28. doi: 10.1258/acb.2011.011128. [DOI] [PubMed] [Google Scholar]
- 56.Svenningson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol. 1995;63:39–46. doi: 10.1016/0165-5728(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 57.Kivasäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2013;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitby L, Whitby A, Fletcher M, Barnett D. Current laboratory practices in flow cytometry for the enumeration of CD4+ T-lymphocyte subsets. Cytometry B Clin Cytom. 2015 Mar 31; doi: 10.1002/cyto.b.21241. doi:10.1002/cyto.b.21241. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Yamanka YJ, Gierahn TM, Love JC. The dynamic lives of T cells: new approaches and themes. Trends Immunol. 2013;34:59–66. doi: 10.1016/j.it.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T-cell mediated tissue damage. Nature Medicine. 2007;13(2):139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 61.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci. 2013;333(0):76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thom V, Schmid S, Gelderblom M, et al. IL-17 production by CSF lymphocytes as a biomarker for cerebral vasculitis. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e214. doi: 10.1212/NXI.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 64.Gobel K, Bittner S, Melzer N, et al. CD4(+) CD25(+) FoxP3(+) regulatory T cells suppress cytotoxicity of CD8(+) effector T cells: implications for their capacity to limit inflammatory central nervous system damage at the parenchymal level. J Neuroinflammation. 2012;9:41. doi: 10.1186/1742-2094-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez-Martin E, Picón C, Cross-Frossard L, et al. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol. 2015;180(2):243–249. doi: 10.1111/cei.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Correale J, Mix E, Olsson T, et al. CD5+ B cells and CD4-8- T cells in neuroimmunological diseases. J Neuroimmunol. 1991;32:123–132. doi: 10.1016/0165-5728(91)90004-q. [DOI] [PubMed] [Google Scholar]
- 67.Hardy RR, Hayakawa K. Perspectives on fetal derived CD5+ B1 cells. Eur J Immunol. 2015;45(11):2978–2984. doi: 10.1002/eji.201445146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jackson SW, Kolhatkar NS, Rawlings DJ. B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol. 2015;33:70–77. doi: 10.1016/j.coi.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–176. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 70.Chihara N, Aranami T, Oki S, et al. Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One. 2013;8(12):e83036. doi: 10.1371/journal.pone.0083036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pashenkov M, Huang YM, Kostulas V, et al. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(Pt 3):480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 72.Njemini R, Onyema OO, Remans W, et al. Shortcomings in the application of multicolour flow cytometry in lymphocyte subsets enumeration. Scand J Immunol. 2014;79:75–89. doi: 10.1111/sji.12142. [DOI] [PubMed] [Google Scholar]
- 73.Pranzatelli MR, Travelstead AL, Tate ED, et al. B- and T-cell markers in opsoclonus-myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology. 2004;62:1526–1532. doi: 10.1212/wnl.62.9.1526. [DOI] [PubMed] [Google Scholar]
- 74.De Graaf MT, de Jongste AHC, Kraan J, et al. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry Part B (Clinical Cytometry) 2011;80B:271–281. doi: 10.1002/cyto.b.20603. [DOI] [PubMed] [Google Scholar]
- 75.De Jongste AH, de Graaf MT, van den Broek PD, et al. Elevated numbers of regulatory T cells, central memory T cells and class-switched B cells in cerebrospinal fluid of patients with anti-Hu antibody associated paraneoplastic neurological syndromes. J Neuroimmunol. 2013;258(1–2):85–90. doi: 10.1016/j.jneuroim.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz A, Balint B, Korporal-Kuhnke M, et al. B-cell populations discriminate between pediatric- and adult-onset multiple sclerosis. Neurol Neurimmunol Neuroinflamm. 2017;4:e309. doi: 10.1212/NXI.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dale RC, Tantsis E, Merheb V, Brilot F. Cerebrospinal fluid B-cell expansion in longitudinally extensive transverse myelitis associated with neuromyelitis optica immunoglobulin G. Dev Med Child Neurol. 2011;53(9):856–860. doi: 10.1111/j.1469-8749.2011.03975.x. [DOI] [PubMed] [Google Scholar]
- 78.Dale RC, Pillai S, Brilot F. Cerebrospinal fluid CD19(+) B-cell expansion in N-methyl-D-aspartate receptor encephalitis. Dev Med Chil Neurol. 2013;55(2):191–193. doi: 10.1111/dmcn.12036. [DOI] [PubMed] [Google Scholar]
- 79.Pranzatelli MR, McGee NR, Wang ZY, Agrawal BK. Characteristics and pharmacodynamics of severe neuroinflammation in a child with neurolupus. Neurol Neuroimmunol Neuroinflamm. 2016;4(2):e316. doi: 10.1212/NXI.0000000000000316. eCollection 4:e316; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albert ML, Darnell JC, Bender A, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 81.Pranzatelli MR, Tate ED, Travelstead AL, et al. Long-term cerebrospinal fluid and blood lymphocyte dynamics after rituximab for pediatric opsoclonus-myoclonus. J Clin Immunol. 2010;30:106–113. doi: 10.1007/s10875-009-9335-3. [DOI] [PubMed] [Google Scholar]
- 82.Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306(1–2):82–90. doi: 10.1016/j.jns.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 83.Melzer N, Golombeck KS, Gross CC, Meuth SG, Wiendl H. Cytotoxic CD8+ T cells and CD138+ plasma cells prevail in cerebrospinal fluid in non-paraneoplastic cerebellar ataxia with contactin-associated protein-2 antibodies. J Neuroinflammation. 2012;9:160. doi: 10.1186/1742-2094-9-160. doi:10:1186/1742-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pranzatelli MR, Travelstead AL, Tate ED, et al. CSF B-cell expansion in opsoclonus-myoclonus syndrome: a biomarker of disease activity. Mov Disord. 2004;19:770–777. doi: 10.1002/mds.20125. [DOI] [PubMed] [Google Scholar]
- 85.Alvermann S, Hennig C, Stüve O, et al. Immunophenotyping of cerebrospinal fluid cells in multiple sclerosis: in search of biomarkers. JAMA Neurol. 2014;71:905–912. doi: 10.1001/jamaneurol.2014.395. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi Y, Mine J, Kubota Y, et al. A substantial number of Rasmussen syndrome patients have increased IgG, CD4+ T cells, TNFα, and granzyme B in CSF. Epilepsia. 2009;50(6):14199–1431. doi: 10.1111/j.1528-1167.2008.01977.x. [DOI] [PubMed] [Google Scholar]
- 87.Bielganowski P, Bieganowski K, Zaborski J, Czlonkowska A. Oligoclonal expansion of gamma delta T-cells in cerebrospinal fluid of multiple sclerosis patients. Mult Scler. 1996;2:78–82. doi: 10.1177/135245859600200203. [DOI] [PubMed] [Google Scholar]
- 88.Owens GC, Erickson KL, Malone CC, et al. Evidence for involvement of gamma delta T cells in the immune reponse in Rasmussen encephalitis. J Neuroinflammation. 2015;12:134. doi: 10.1186/s12974-015-0352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pranzatelli MR. Going with the flow: Neuroinflammation. Dev Med Child Neurol. 2011;53:782. doi: 10.1111/j.1469-8749.2011.03997.x. [DOI] [PubMed] [Google Scholar]
- 90.Häusler M, Sellhaus B, Schweizer K, et al. Flow cytometric cerebrospinal fluid analysis in children. Pathol Res Pract. 2003;199:667–675. doi: 10.1078/0344-0338-00478. [DOI] [PubMed] [Google Scholar]
- 91.Comans-Bitter WM, De Goot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1996;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 92.Duchamp M, Sterlin D, Diabate A, et al. B-cell subpopulations in children: National reference values. Immunity Inflamm Dis. 2014;2(3):131–140. doi: 10.1002/iid3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Havrdova E, Galetta S, Stefoski D, Comi G. Freedom from disease activity in multiple sclerosis. Neurology. 2010;74:S3–7. doi: 10.1212/WNL.0b013e3181dbb51c. [DOI] [PubMed] [Google Scholar]
- 94.Kowarik MC, Pellkofer HL, Cepok S, et al. Differential effects of fingolimod (FTY7200) on immune cells in the CSF and blood of patients with MS. Neurology. 2011;76(14):1214–1221. doi: 10.1212/WNL.0b013e3182143564. [DOI] [PubMed] [Google Scholar]
- 95.Lin YC, Winokur P, Blake A, et al. Daclizamab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann Clin Transl Neurol. 2015;2(5):445–455. doi: 10.1002/acn3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stüve O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J Neurol Sci. 2008;274(1–2):39–41. doi: 10.1016/j.jns.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 97.Monson NL, Cravens PD, Frohman EM, et al. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol. 2005;62(2):258–264. doi: 10.1001/archneur.62.2.258. [DOI] [PubMed] [Google Scholar]
- 98.Warnke C, Stettner M, Lehmensiek V, et al. Natalizumab exerts a suppressive effect on surrogates of B cell function in blood and CSF. Mult Scler. 2015;21(8):1036–1044. doi: 10.1177/1352458514556296. [DOI] [PubMed] [Google Scholar]
- 99.Piccio L, Naismith RT, Trinkaus K, et al. Changes in B- and T-lymphocyte and chemokine levels with rutuximab treatment in multiple sclerosis. Arch Neurol. 2010;67(6):707–714. doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009;15(2):189–192. doi: 10.1177/1352458508098268. [DOI] [PubMed] [Google Scholar]
- 101.Kira JI. Unexpected exacerbations following initiation of disease-modifying drugs in neuromyelitis optica spectrum disorder: Which factor is responsible, anti-aquaporin 4 antibodies, B cells, TH1 cells, Th2 cells, Th17 cells, or others? Mult Scler. 2017 Apr 1; doi: 10.1177/1352458517703803. 1352458517703803. [DOI] [PubMed] [Google Scholar]
- 102.Chen D, Gallegher S, Monson NL, et al. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. 2016;5(12):107. doi: 10.3390/jcm5120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stüve O, Arnke WC, Deason K, et al. CD19 as a molecular target in CNS autoimmunity. Acta Neuropathol. 2014;128(2):177–190. doi: 10.1007/s00401-014-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schiopu E, Chatterjee S, Hsu V, et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study. Arthr Res Ther. 2016;18:131. doi: 10.1073/pnas.0505539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCoy JP, Jr, Overton WR. Quality control in flow cytometry for diagnostic pathology: II. A conspectus of reference ranges for lymphocyte immunophenotyping. Cytometry. 1994;18:129–139. doi: 10.1002/cyto.990180304. [DOI] [PubMed] [Google Scholar]
- 106.Teunissen CE, Tumani H, Engelborghs S, Mollenhauer B. Biobanking of CSF: international standardization to optimize biomarker development. Clin Biochem. 2014;47(4–5):288–292. doi: 10.1016/j.clinbiochem.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 107.Antonio M, Kolamunnage-Dona R, Jorgensen AL. Biomarker-guided non-adaptive trial designs in phase II and phase III: a methodological review. J Pers Med. 2017;7(1) doi: 10.3390/jpm7010001. pii: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]