Abstract
Research on human brain changes during skill acquisition has revealed brain volume expansion in task-relevant areas. However, the large number of skills that humans acquire during ontogeny militates against plasticity as a perpetual process of volume growth. Building on animal models and available theories, we promote the expansion–renormalization model for plastic changes in humans. The model predicts initial increase of gray-matter structure, potentially reflecting growth of neural resources like neurons, synapses, and glial cells, which is followed by a selection process operating on this new tissue leading to complete or partial return to baseline of overall volume after selection has ended. The model sheds new light on available evidence and current debates, and fosters the search for mechanistic explanations.
Keywords: structural brain plasticity, skill acquisition, learning, volume increase, gray matter
Human Brain Plasticity: Expansion and Renormalization?
In 1894, Nobel Prize winner Santiago Ramón y Cajal, considered by many to be the father of modern neuroscience, proposed that mental activity might induce morphological changes in brain structure. Nearly one hundred years later, studies using magnetic resonance imaging (MRI, see Glossary and Box 1) have reported experience-dependent increases in regional estimates of human brain volume and cortical thickness in adulthood. For example, it has been found that London taxi drivers’ gray matter in hippocampus enlarges and adapts to help them store a detailed mental map of the city in which they regularly have to navigate [1]. To date, alternations in brain structure have been reported following various kinds of behavioral interventions on quite different time scales, such as several months of juggling training [2], studying for a medical exam [3], spatial navigation training [4], or foreign language acquisition [5], two weeks of mirror reading training [6], or a few sessions of complex whole-body balancing [7].
Glossary.
Cortical maps. Cortical organization of sensory and motor systems is often described in terms of maps. Cortical maps are collections (areas) of minicolumns in the cortex whose functional topography corresponds to graded variations on an underlying sensory or motor dimension. In the somatotopic map of the motor cortex for example, stimulation of different areas evokes movement of distinct parts of the body. These cortical representations are not static. Cortical map expansion refers to the phenomenon of an increase in cortical representational area, be it within the motor, somatosensory, the visual, or the auditory system.
Flexibility. We define flexibility as the adaptive reconfiguration of the existing behavioral repertoire in the absence of structural change. Hence, according to our definitions, changes in synaptic weights of already established synaptic connections, for example, qualify as flexibility rather than plasticity.
MRI. Magnetic resonance imaging is a non-invasive imaging method that uses a strong electro-magnetic field to align hydrogen atoms of water molecules in tissue. See Box 1 for further details.
Plasticity. We define plasticity as the inherent ability of the brain to undergo lasting structural change in response to altered environmental demands [52,70]. Plastic changes are triggered in the presence of a prolonged mismatch between the functional supply of brain structure and the experiential demands of the environment. Plastic changes thus require plasticity, but are not synonymous with it. We define plasticity as the organism’s potential for plastic change.
Box 1. Magnetic Resonance Imaging in Human Plasticity Studies.
Magnetic resonance imaging is a non-invasive imaging method that uses a strong electromagnetic field to align hydrogen atoms of water molecules in tissue. Radio frequency fields are used to systematically change the alignment of magnetization. This results in a rotating magnetic field created by the hydrogen atoms as they return to baseline that can be detected by the MR scanner. The resulting signal can be used to construct an image of the brain because different tissues (e.g., gray and white matter) have different magnetic properties [88]. Commonly, studies on structural plasticity in humans have used so-called T1-weighted images. The parameter T1 characterizes the exponential curve with which the magnetization of the proton ensemble goes back to its equilibrium value, and differs considerably between white and gray matter. T1-weighted images are therefore commonly used for measures of gray matter morphology.
Although some studies of human plasticity have applied manual segmentation techniques, MR images have most often been processed automatically either using voxel-based morphometry (VBM) or by using computational tools (e.g., FreeSurfer) that outline cortical thickness or estimate regional brain volume. Voxel-based morphometry is based on shifting the MR images into a common stereotactic space and allows identification of regional differences by applying voxel-wise statistics in a combination with Gaussian random fields, identifying each voxel’s probability of belonging to one tissue class (gray matter, white matter, or cerebrospinal fluid) [89]. The resulting measure of gray matter probability reflects a mix of cortical thickness (that can be estimated with techniques such as Freesurfer) and surface area/curvature (which is unlikely to change in short-term plasticity studies on adult humans) [90], and is additionally influenced by alterations in the T1 signal in the absence of volume changes (while estimates of cortical thickness are less sensitive to changes in the signal [91]).
Experience-dependent morphological brain changes have been studied in animals for a long time [92–95]. The discovery that structural alterations are detectable with MR imaging of humans has opened up a new window on brain correlates of human learning, as MR imaging allows for in-vivo investigations of human brain structure with increasingly informative acquisition sequences [77,78]. However, changes detectable with T1-weighted MR images (but also most other types of MR images) are typically difficult to relate to underlying biology. To further complicate matters, the T1 signal most often used in plasticity studies as described above may not only be sensitive to the targeted changes in gray matter structure (e.g., structural changes of neurons, glia cells, and myelin) but may also pick up functional activation changes through concomitant changes in cerebral blood flow [96]. In addition, movement [97] and exact positioning of the head [98] may influence estimates of grey matter volume. Thus, care in design and methods are needed to avoid these confounding interpretations. Additionally, increases in myelination may change the measurement of gray matter probability or cortical thickness, such that an increase in intra-cortical myelination [99] may result in an apparent decrease of estimates of gray matter volume. It therefore seems important to closely observe myelination with appropriate MR sequences when interpreting changes in gray matter. While changes in adjacent white matter may potentially perturb the exact measurement of gray matter in some instances, changes in intra-cortical myelination (such as increased myelination of axons of neurons that have formed new dendritic connections) may exactly be part of the phenomenon of interest. The search for biological mechanisms underlying gray matter changes in humans calls for the use and further development of more quantitative and better interpretable MR sequences [77,78].
However, the existence of learning-related increases in brain volume raises a puzzle that was already recognized by Ramón y Cajal in 1984: “How can the volume of brain remain constant if there is a multiplication and even new formation of terminal branches of protoplasmic appendices and nerve collaterals [induced by mental activity]?” (cited in [8]). Specifically, it seems unfeasible that the vast amount of knowledge and large number of skills that humans acquire during their lifetimes are ultimately represented by continual increases in brain volume. Endless expansion may not be nature’s best solution to the phenomenon of lifelong learning, when in other parts of evolution processes of trimming and selecting the best amongst several candidates has proven immensely useful. Even though previous research on structural plasticity in human adults has not explicitly addressed the issue, it seems likely that the previously observed increases in regional estimates of brain volume do not tell the entire story. Indeed, some of the published studies have also observed decreases, which have often been left unexplained [7,9,10].
Here, we discuss how the expansion–renormalization model, which is rooted in accounts of plasticity from developmental research and animal models, can aid the interpretation of existing human adult data and generate future research questions in research on human structural brain plasticity. According to the expansion–renormalization model, learning-related neural processes often follow a sequence of expansion, selection, and renormalization [11–13]. This resembles the audition process for the cast of a movie, where initially numerous candidates (i.e., computational circuits or ensembles of e.g., neurons, synapses, astrocytes, and glia cells) are called in (overall tissue expansion). These are then tested for suitability for the role (i.e., functional efficiency) and the best candidates (i.e., circuits) are then selected (selection), while the rest are sent home (i.e., pruned away; leading to renormalization). Calling in more candidates (i.e., brain structure growth) improves the quality of the selection, but does not constitute the end product.
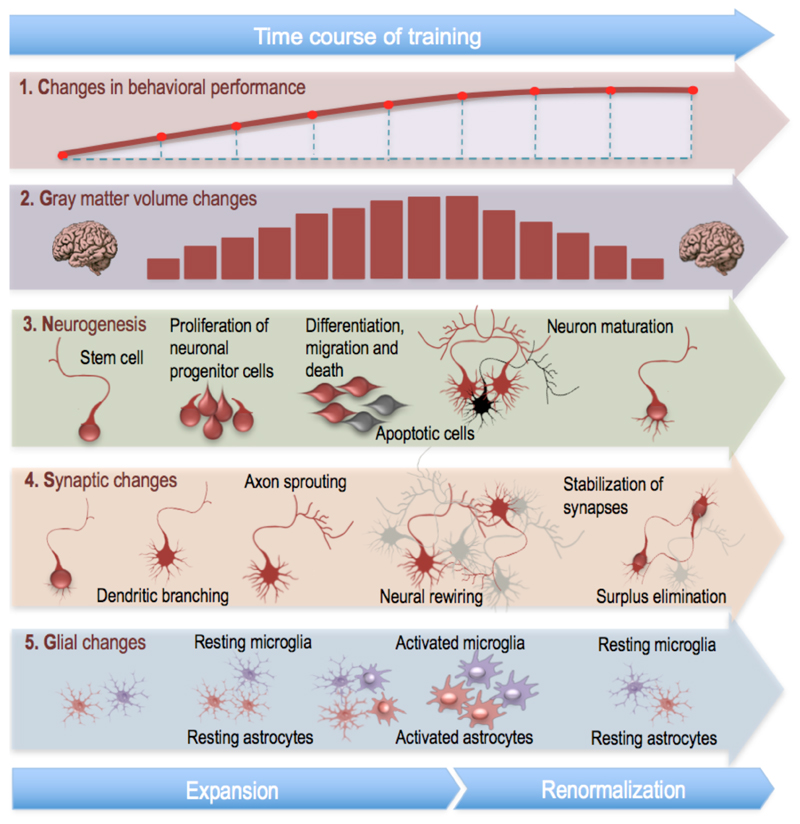
Human skill acquisition and some instances of learning may therefore involve an initial but transient phase of increase of gray matter structure, followed by a partial or even complete return to baseline once a (presumably behaviorally optimal) neural circuitry has been selected. Cellular changes that may underlie the observed macroscopic volume increase and renormalization, and are known to follow this pattern, include adult neurogenesis, synaptic changes, and changes in the number and morphology of glial cells [14]. Together, these potential cellular processes may contribute to experience-induced alterations and renormalization of gray matter structure as observable with MRI. In Figure 1 we have portrayed a schematic illustration of these potential cellular changes.
Figure 1. Schematic illustration of potential cellular changes underlying gray matter volume expansion and renormalization as detectable with MR images.
In the course of training, behavioral performance continuously increases until it stabilizes (1). Estimates of regional gray matter volume increases during initial phases of learning and renormalizes either partially or completely to baseline levels during later phases when high task proficiency is reached (2). Adult neurogenesis (restricted to some regions of the brain) (3) may accompany this process of learning and is characterized by initial proliferation of neuronal progenitor cells, some of which further undergo apoptosis. Some of these new cells survive while most die during their differentiation, migration, and neuronal maturation. Synaptic changes (4) may follow a comparable pattern with dendritic branching and axonal sprouting increasing the number of synapses during initial phases of learning but then returning to baseline levels. Importantly, the selective elimination of dendritic branches, axonal projections, and synaptic connections – a process typically referred to as pruning – along with the stabilization of synapses allows for particularly efficient neural rewiring. Finally, changes in the number and morphology of glial cells (5) may again follow a comparable pattern, as they can proliferate and shift from resting to activated states and return to resting state again, once the skill is acquired. Together, these potential cellular processes may contribute to experience-induced alterations and renormalization of gray matter structure as observable with MRI.
In the sections that follow, we show that the inverted-U shape of plastic change seems to hold true across different spatial extensions and timescales of plasticity. We have recently shown this pattern for structural changes in human gray matter structure [15], as observable with MRI. We give a brief overview of the potential cellular mechanisms that may contribute to this observable volume changes on the macroscopic level. We then summarize evidence on the inverted-U shape of plastic changes for cortical map dynamics [16–18], and synaptic changes [19–21], as well as for developmental processes, and conclude with an outlook on future research directions in this field.
Expansion and Renormalization of Human Gray Matter Structure
Research has documented changes in human gray matter structure after a few months of training (e.g., [2,5] as noted above) and has also indicated that such changes can emerge quite early during the learning process [7,22–24]. However, the fate and durability of these changes has not been tracked in the course of continuous learning in humans. Recently, we acquired up to 18 T1-weighted structural MR images over a 7-week period, during which 15 right-handed adult participants practiced left-hand writing and drawing [15]. The images were analyzed with voxel-based morphometry (VBM), yielding estimates of gray matter volume, based on the T1-weighted signal (see Box 1 on MRI for further information). After four weeks, increases were observed in the estimates of gray matter volume in both left and right primary motor cortices relative to a control group; another three weeks later, these differences were no longer reliable. Time-series analyses showed that the estimates of gray matter volume in both primary motor cortices increased during the first four weeks and then partially renormalized, particularly in the right hemisphere, in the presence of continued practice and increasing task proficiency.
These results are in line with findings from a series of studies investigating effects of whole-body balancing training – even though on a slightly different time scale [7,24]. Rapid increases in cortical thickness in primary motor cortex were observed after approximately 45 minutes of training that were not detectable after 2 weeks of training. Importantly, the above described results are also in line with results from an animal study, in which three adult macaque monkeys were scanned on multiple occasions before, during, and after learning how to use a rake to retrieve food [25]. There were learning-related increases in task-relevant brain regions that also mapped onto the learning curves. Crucially, despite continued training, the observed increases in gray matter structure reversed after the monkey’s performance reached asymptote. After training, the volume was still greater than the volume before training, but much smaller in magnitude than the peak volume observed before asymptotic performance was reached.
Learning-related expansion–renormalization changes differ from volume decreases induced by disuse or lack of sensory input, observed for example following hand immobilization or limb amputation [26,27]. In stark contrast to the latter, task performance continues to improve or stabilizes at high proficiency levels during the renormalization period.
Cellular processes potentially underlying gray matter changes
Increases and decreases of estimates of localized volume during learning as observed with MRI are most likely the result of a conglomerate of different cellular processes [14,28]. Figure 1 schematically illustrates these cellular processes. Several candidate mechanisms on the cellular and molecular level have been proposed that could account for MRI observations [14], including neurogenesis, synaptic changes, dendritic branching, and axon sprouting, as well as changes in glial number and morphology (for both astrocytes and microglia). It should be noted that changes observed outside of the hippocampus are probably not due to neurogenesis, as growth of new neurons in adults has only been established in the dentate gyrus and the olfactory bulb (e.g., [29,30]) and, more recently, in humans in the striatum [31]. Synaptogenesis and changes in spine morphology have been discussed in the context of learning and gray matter alterations. In animal work, synapse formation and synaptic turnover have been implicated in supporting learning-dependent changes in cortical function [32–35]. Changes in dendritic length and branching or in the actual number of dendritic spines per neuron are likely to contribute to experience-dependent volumetric changes in gray matter (e.g., [11,36,37]. Glia cells maintain ion homeostasis, regulate blood flow in response to neuronal activity, form myelin, and provide support and protection for neurons [38,39]. They are highly plastic, can increase in number, and display a number of morphological changes in response to altered experience, including increased volume fraction, increased cell surface, and proliferation of their processes, all of these can be summarized under the term gliogenesis [40–42]. Glial processes could in theory increase to support new synapses, or to compensate for neuronal process loss [43]. Thus, increases in gliogenesis could to some extent account for gray matter changes observed with MRI [14]. Figure 1 sketches out how changes on the cellular level for the different cell types mentioned above could hypothetically contribute to the overall increase of gray matter volume followed by its decrease. It is likely that the structural changes uncovered by MRI represent the net outcome of a complex mixture of changes across these different cell types.
Cortical Map Dynamics and Functional Changes
Similar to macroscopic changes in estimates of regional gray matter volume, cortical map plasticity follows a comparable pattern of expansion followed by renormalization during learning [16–18,44–50]. For example, it has been found that rats training to perform a skilled reaching task exhibit expanded cortical maps after three days of training [16]. After eight days of training, however, these expansions had subsided, while behavioral performance remained stable. A similar pattern was observed in a different study where the global tonotopic representation in the auditory cortex of rats changed progressively depending on the stage of training in auditory operant conditioning [47]. At the early stage, tone-responsive areas in the core cortex expanded, while both the core and belt cortices shrank at later stages as behavior became conditioned. Similar renormalization occurs in the human visual cortex after learning a visual discrimination task [44]. Within the first few weeks of training, brain activation in a V1 subregion corresponding to the trained visual field quadrant increased alongside task performance. While improvements in task performance then saturated and performance was maintained at a stable level, brain activation in the corresponding areas renormalized to pre-training levels. The same pattern has been observed during human motor skill acquisition [46]. Ten subjects performing a finger movement task daily for four weeks showed increases in regional activity in primary and supplementary motor areas as observed with functional MR imaging after two weeks of training. However, activity decreased from week two to week four [46]. More generally, motor sequence learning has been shown to be associated with increasing motor system activity in the early stages of learning, followed by a reduced level of activity during execution of highly practiced motor behavior [51].
In an important study, an extension of the auditory cortical map in rats was experimentally created using a technique called nucleus basalis stimulation–tone pairing [17]. Subsequently, animals were trained in an auditory discrimination task, where improved discrimination learning was observed in animals with an expanded cortical map. Auditory cortex map expansion diminished over the following weeks but left tone discrimination performance unaffected. Thus, the expansion of the maps was related to the trajectory of learning, but was not necessary for maintenance of the learnt skill. Later work indicates that microstructural changes accompany such functional reorganization. The pattern of cortical map expansion and retraction was also investigated with functional MRI and – on a more cellular level – with paired electrophysiological recordings in the periphery of expanded whisker representation [48]. It was shown that pyramidal neurons are initially recruited to local excitatory networks and then eliminated from them, resulting in bidirectional rewiring in somatosensory cortex following deprivation of its main whisker input.
An expansion–renormalization model has been proposed to account for these findings [12,17]. Initial fast, flexibility-based [52] learning may involve the selection of a pre-existing efficient circuit [12]. Subsequent slower learning may then evolve through a transient phase of map expansion that serves to temporarily augment the pool of circuits to be used in an “explorative” manner, until the most efficient circuitry has been selected to perform the task. Later in learning, performance relies on the selected circuitry that is further stabilized through practice, and thus the cortical map renormalizes.
Formation and Elimination of Synapses
Research regarding learning-related changes in dendritic spines is consistent with the hypothesis that the memory trace serving skilled performance is often localized in rewired specific circuitry rather than in any large-scale expansion of tissue in the whole region [11,36,53]. During motor skill acquisition or new sensory experiences, novel dendritic spines rapidly grow to form synapses in sensory and motor cortices of rodents [19–21]. In this process, the dendrites are not merely passive couriers of synaptic currents to the soma, but rather play a crucial role for synaptic plasticity, as they generate several-fold more spikes than the soma and are therefore also a unit of neural computation [54]. However, with continued training or experience, the phase of growth is followed by a slower process of stabilization of the new synapses and elimination of spines that had existed before training (and potentially formed early in life), almost returning the overall number of spines to pre-training levels, while performance on the trained task remains high [19,20]. A small fraction of the new spines remain stable over a long period of time [20,21] and might serve as a form of memory of the skill, rapidly becoming active as soon as the task is repeated. However, no new spines are grown unless the task is a novel one [19–21,55].
Plastic Changes: A Darwinian Learning Process
The pruning model of early development [56–58] posits the same general pattern of increase followed by decrease as described above, only on an ontogenetic timescale. The rapid increase of synapses after birth is followed by experience-dependent selective stabilization of behaviorally relevant connections and the elimination of those connections that prove to be non-functional [59], resulting in an overall trajectory of decrease from childhood to adolescence [60,61] (but see also [62] for other potential mechanisms next to pruning resulting in this shape of change).
In our view, learning and development, while operating at different spatial and temporal scales, both integrate constructivist and selectionist features. The coordination of construction and selection permits directed growth in response to new experiences, followed by stabilization of functional synaptic constellations and an elimination of the surplus (see also [20]). A process like this, with initial production of diversity followed by selection and stabilization, has the features typically ascribed to Darwinian models of cortical plasticity and neural development [12,63–66]. The expansion–renormalization model of plasticity is therefore in agreement with the Darwinian framework, exhibiting these three major traits: diversity (initially calling in a large number of cells of various types), selection, and stabilization [12,65,66]. Within our previous analogy of auditions for the cast of a movie, numerous candidates are first progressively called in, then the best are selected, and the rest are sent home, that is, “pruned away.” Calling in more candidates may improve the outcome by increasing the likelihood of finding a global rather than local maximum of optimality, but a larger cast (i.e., more gray matter) is not the targeted end product. Metabolic efficiency might be a driving force behind this process [13], as learnt information can be represented by fewer spikes (or neurons) after skill acquisition than before [67,68]. While more neurons and synapses are being used during initial stages of learning, potentially entailing an expansion of tissue in these regions due to metabolic demands, later on, the most efficient wiring is selected, resulting in fewer, but highly specialized and stable neurons and synapses [13]. Given the efficiency and robustness of such a process and its past application to related plasticity phenomena, it is surprising that it has not been seriously considered so far in the field of human experience-dependent structural changes of gray matter.
Future Perspectives
We have argued that the concepts of expansion, selection, and renormalization are consistent with animal models and theoretical accounts of skill acquisition and development, and together contribute to a mechanistic understanding of human plasticity. Importantly, the expansion–renormalization model opens up several new research directions, informs predictions for work on experience-dependent and developmental changes in human brain structure (see Outstanding Questions Box), and calls for a critical examination of the current evidence [69]. Much of the evidence supporting the expansion–renormalization model is related to sensory and motor skills. It remains to be investigated how general of a principle this pattern of expansion and renormalization is and whether increasing proficiency in tasks tapping into higher-order cognitive abilities are characterized by a different shape of plastic brain changes, with growth possibly being maintained for longer.
Outstanding Questions Box.
How can we better bridge the gap between microscopic methods used in animal models and macroscopic methods used in human research?
Are expansion–renormalization patterns observed at different spatial and temporal scales sharing common biological mechanisms or have they been shaped independently and happen to unfold in a similar way by common requirements to form complex but robust dynamic systems?
What is the trigger that initiates expansion? Are top-down signals coding for the mismatch between task demands and available cortical resources, or does increased reliance on a specific brain region alone suffice to begin the process? Through which mechanisms does expansion stop and renormalization start? Can these top-down signals be related to theories of model-based and model-free learning [100]?
Which methods are adequate to investigate functional correlates of structural reorganization in the course of expansion and renormalization (e.g., high-resolution multivoxel pattern analysis)?
How does reactivation of a previously acquired skill differ from initial skill acquisition?
Much of the evidence supporting the expansion–renormalization model is related to sensory and motor skills and focused on changes in cerebral cortex areas. Is increasing proficiency in tasks tapping into higher-order cognitive abilities also associated with expansion–renormalization patterns in task-relevant areas and are the mechanisms the same for subcortical regions?
Can the expansion-renormalization model guide research on transfer of training? Should transfer tasks be introduced prior to renormalization to enhance generalization across skills?
Do associations between behavioral improvements and structural changes differ across individuals?
Can the expansion–renormalization model help to better understand age-related differences in cortical plasticity from infancy to old age?
To appropriately capture the temporal dynamics of plastic changes, it is necessary to include more than two measurement time points when investigating any training, intervention, or developmental process. Interestingly, previous studies with measurements only before and after training have not only reported increases in gray matter volume, but also some decreases [7,9,10], which were often left mechanistically unexplained. The expansion–renormalization model predicts decreases if the first MR measurement of participants is taken after volume expansion has already occurred; that is, at a point in time when individuals have attained some proficiency in performing the target task, either in the course of the experiment or outside the laboratory.
Generally, adopting the expansion–renormalization model requires new study designs with multiple measurement time points to investigate associations between behavioral improvements and structural changes in humans (which have been notoriously difficult to detect, see [70]). Such associations are expected to depend on the timing of measurements and individual differences in learning progression. For example, reductions (or smaller increases) in volume may relate to greater behavioral improvements if post-test measurements are taken during the renormalization phase. Our line of reasoning also resonates well with findings regarding the physiological basis of the effects of earlier episodes of plasticity on later learning. The malleability and later preservation of post-synaptic spines on apical dendrites of pyramidal neurons in Layer V have been found to serve as mechanisms to encode and store memory in cortical circuits [20,21,55,71,72]. Experiences triggering plastic changes therefore leave a trace in cortical circuits that are selected for the learned skill and that provide a structural substrate for memory retention. Based on this evidence, we can speculate that the expansion–renormalization pattern is reduced or even absent when a previously acquired skill is reactivated, for instance, when training on a task that has previously elicited plastic change is resumed after a break. The most efficient cortical circuit is already selected and the skill in question is represented as “trace” (i.e., most likely within previously acquired synaptic connections) in this circuit, which just needs to be reactivated. Therefore, no new circle of expansion and renormalization is needed [19–21,53].
Training-dependent brain changes in both animals and humans are typically paralleled by behavioral performance improvements on the practiced tasks (e.g., [15,19,25]; for review, see [70]). It is tempting to conclude that structural brain changes “reflect” improvement in behavioral proficiency. However, structural brain changes may also be practice-related epiphenomena, reflecting adaptive changes of the brain to metabolic demands driven by increased local neural activity. Thus, the critical question is whether supervisory learning signals are required to trigger practice-related structural brain changes, or whether such changes also occur in the absence of such a signal, that is, during use of the region but without learning [73]. The example of prior cortical map extension improving later learning in an auditory discrimination task [17] indicates that cortical map plasticity does indeed enhance learning, but more studies tackling the difference between learning-dependent or use-dependent brain changes are needed. To shed light on this issue, it is crucial to investigate the link between functional activation and structural brain changes, including their roles in cortical map plasticity, and how they relate to behavioral changes.
A related point of debate concerns the transfer of training effects to untrained functions. The neural overlap hypothesis predicts that brain changes induced by training a specific function might also affect untrained functions relying on the same or at least partially overlapping neural networks [74]. Still, many cognitive intervention studies show scarce evidence for generalizable performance improvements, that is, transfer of skills to untrained tasks (for a review, see [75,76]). We may speculate that within the expansion–renormalization model transfer effects could be most likely to occur prior to the renormalization phase of structural changes, that is, at or just before volume expansion has reached its peak. At this point, in terms of the movie casting analogy, several candidates are called in for the audition, that is, numerous neurons, synapses and glia cells are around anyway. Some of them may not be kept for the initially intended production, that is, the initially trained task (i.e., they are destined to be “pruned away” during the renormalization phase), but may end up taking over “roles” in other productions, that is, may actually be recruited for another task and are therefore kept anyway. Hence, we contend that transfer to related skills may be most likely in the time window after expansion and before renormalization. If this hypothesis is correct, broadening the range of trained skills in the course of the intervention may prove to be effective in creating lasting transfer effects.
Finally, identifying the nature of the cellular processes contained in the MRI signal and their timing and coordination during skill acquisition [77,78] continues to be notoriously difficult. Changes detectable with MRI are likely to represent a mixture of coordinated changes in different cell types (see Figure 1) and possibly changes in intra-cortical myelin [79]. Imaging techniques that allow for a closer link to biological underpinnings hold the potential to evaluate different hypotheses regarding cellular mechanisms underlying gray matter changes. MR sequences like myelin-water fraction [80] or multi-parameter mapping [81] come to mind, as well as proton MR spectroscopy which has already been used to characterize neurochemical concentrations in aging [82] and could potentially also be useful in the context of learning-dependent brain changes [83]. Recently, a new radioligand has been used in positron emission tomography (PET) to quantify synaptic density in the living brain [84], which opens insights into cellular processes. Combining experimental work in humans with comparable animal models holds the potential to relate changes visible with MR techniques to underlying biological mechanisms that are normally only observable with more invasive methods [10,77,85–87]. This work will help to delineate the concerted succession of changes in different cell types in greater detail, and to decompose the processes that give rise to the expansion–renormalization trajectory [15].
Trends Box.
Research on human plasticity has been energized by the discovery of experience-dependent growth of brain volume in adulthood. However, is it really feasible to portray the vast amount of knowledge and the large number of skills that humans acquire in terms of a perpetual process of brain volume growth?
Prominent theoretical accounts of plasticity, developmental data and animal models suggest a sequence of learning-related expansion, selection, and renormalization of brain activity and structure.
Recent studies on experience-dependent changes in gray matter structure support this model also for human learning. Estimates of the volume of human primary motor cortices were recently shown to increase during the first weeks of motor learning, and then partially renormalize during continued practice.
Acknowledgements
The authors would like to thank Simone Kühn and Benjamín Garzón for valuable discussions, Ludmila Müller for rendering the figure, and Julia Delius und Lana Riccius for comments on the text. The work by ML on this manuscript was done in the context of research supported by the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013) / ERC Grant agreement n° 617280 – REBOOT and the Swedish Research Council (446-2013-7189). Work by UL profited from a research stay as a Fernand Braudel senior fellow at the European University Institute, San Domenico di Fiesole, Italy. Work by CB was supported by the Agence Nationale de la Recherche / ANR-JC Grant n° ANR-16-CE28-0008-01 and the Swedish Research Council (2015-01717). The authors declare no competing financial interests.
References
- 1.Maguire EA, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draganski B, et al. Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 3.Draganski B, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lövdén M, et al. Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiol Aging. 2012;33:620.e9–620.e22. doi: 10.1016/j.neurobiolaging.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Mårtensson J, et al. Growth of language-related brain areas after foreign language learning. Neuroimage. 2012;63:240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Ilg R, et al. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taubert M, et al. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azmitia EC. Cajal and brain plasticity: Insights relevant to emerging concepts of mind. Brain Res Rev. 2007;55:395–405. doi: 10.1016/j.brainresrev.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi H, et al. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One. 2011 doi: 10.1371/journal.pone.0023175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholz J, et al. Rotarod training in mice is associated with changes in brain structure observable with multimodal MRI. Neuroimage. 2015;107:182–189. doi: 10.1016/j.neuroimage.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino H, et al. Circuit mechanisms of sensorimotor learning. Neuron. 2016;92:705–721. doi: 10.1016/j.neuron.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zatorre RJ, et al. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenger E, et al. Repeated structural imaging reveals nonlinear progression of experience-dependent volume changes in human motor cortex. Cereb Cortex. 2017;27:2911–2925. doi: 10.1093/cercor/bhw141. [DOI] [PubMed] [Google Scholar]
- 16.Molina-Luna K, et al. Motor learning transiently changes cortical somatotopy. Neuroimage. 2008;40:1748–1754. doi: 10.1016/j.neuroimage.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Reed A, et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Pascual-Leone A, et al. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 19.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, et al. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofer SB, et al. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driemeyer J, et al. Changes in gray matter induced by learning — Revisited. PLoS One. 2008 doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamzei F, et al. Dynamic gray matter changes within cortex and striatum after short motor skill training are associated with their increased functional interaction. Neuroimage. 2012;59:3364–3372. doi: 10.1016/j.neuroimage.2011.10.089. [DOI] [PubMed] [Google Scholar]
- 24.Taubert M, et al. Rapid and specific gray matter changes in M1 induced by balance training. Neuroimage. 2016;133:399–407. doi: 10.1016/j.neuroimage.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Quallo MM, et al. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad Sci U S A. 2009;106:18379–18384. doi: 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granert O, et al. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage. 2011;54:32–41. doi: 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Draganski B, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;31:951–957. doi: 10.1016/j.neuroimage.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Kassem MS, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 29.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 30.Huart C, et al. Plasticity of the human olfactory system: The olfactory bulb. Molecules. 2013;18:11586–11600. doi: 10.3390/molecules180911586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst A, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 32.Kleim JA, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 33.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 34.Wilbrecht L, et al. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J Neurosci. 2010;30:4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtmaat A, et al. Imaging of experience-dependent structural plasticity in the mouse neocortex in vivo. Behav Brain Res. 2008;192:20–25. doi: 10.1016/j.bbr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 37.Kolb B, et al. Contrasting effects of motor and visual spatial learning tasks on dendritic arborization and spine density in rats. Neurobiol Learn Mem. 2008;90:295–300. doi: 10.1016/j.nlm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Brodal P. The Central Nervous System: Structure and Function. Oxford University Press; 2010. Glia; pp. 19–27. [Google Scholar]
- 39.Wang X, et al. Astrocytic calcium signaling: Mechanisms and implications for functional brain imaging. Methods Mol Biol. 2009;489:93–109. doi: 10.1007/978-1-59745-543-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: Roles in developmental disorders. Ment Retard Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- 41.Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res. 1991;540:273–278. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- 42.Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- 43.Anderson BJ. Plasticity of gray matter volume: The cellular and synaptic plasticity that underlies volumetric change. Dev Psychobiol. 2011;53:456–465. doi: 10.1002/dev.20563. [DOI] [PubMed] [Google Scholar]
- 44.Yotsumoto Y, et al. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tae WS, et al. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- 46.Ma L, et al. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi H, et al. Progressive plasticity of auditory cortex during appetitive operant conditioning. BioSystems. 2010;101:37–41. doi: 10.1016/j.biosystems.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Albieri G, et al. Rapid bidirectional reorganization of cortical microcircuits. Cereb Cortex. 2015;25:3025–3035. doi: 10.1093/cercor/bhu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pruitt DT, et al. Forelimb training drives transient map reorganization in ipsilateral motor cortex. Behav Brain Res. 2016;313:10–16. doi: 10.1016/j.bbr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters AJ, et al. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 51.Wymbs NF, Grafton ST. The human motor system supports sequence-specific representations over multiple training-dependent timescales. Cereb Cortex. 2015;25:4213–4225. doi: 10.1093/cercor/bhu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lövdén M, et al. A theoretical framework for the study of adult cognitive plasticity. Psychol Bull. 2010;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- 53.Hofer SB, Bonhoeffer T. Dendritic spines: The stuff that memories are made of? Curr Biol. 2010;20:157–159. doi: 10.1016/j.cub.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Moore JJ, et al. Dynamics of cortical dendritic membrane potential and spikes in freely behaving rats. Science. 2017;355 doi: 10.1126/science.aaj1497. eaaj1497. [DOI] [PubMed] [Google Scholar]
- 55.Caroni P, et al. Synapse rearrangements upon learning: From divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 2014;37:604–614. doi: 10.1016/j.tins.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuldiner O, Yaron A. Mechanisms of developmental neurite pruning. Cell Mol Life Sci. 2015;72:101–119. doi: 10.1007/s00018-014-1729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary DDM. Development of connectional diversity and specificity in the mammalian brain by the pruning of collateral projections. Curr Opin Neurobiol. 1992;2:70–77. doi: 10.1016/0959-4388(92)90165-h. [DOI] [PubMed] [Google Scholar]
- 60.Raznahan A, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez J, et al. Microstructural proliferation in human cortex is coupled with the development of face processing. Science. 2017;355:68–71. doi: 10.1126/science.aag0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edelman GM. Neural Darwinism: The Theory of Neuronal Group Selection. Basic Books; 1987. [DOI] [PubMed] [Google Scholar]
- 64.Changeux JP, Dehaene S. Neuronal models of cognitive functions. Cognition. 1989;33:63–109. doi: 10.1016/0010-0277(89)90006-1. [DOI] [PubMed] [Google Scholar]
- 65.Fernando C, et al. Selectionist and evolutionary approaches to brain function: A critical appraisal. Front Comput Neurosci. 2012 doi: 10.3389/fncom.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernando C, et al. The neuronal replicator hypothesis. Neural Comput. 2010;22:2809–2857. doi: 10.1162/NECO_a_00031. [DOI] [PubMed] [Google Scholar]
- 67.Chu MW, et al. Balancing the robustness and efficiency of odor representations during learning. Neuron. 2016;92:174–186. doi: 10.1016/j.neuron.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makino H, Komiyama T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci. 2015;18:1116–1122. doi: 10.1038/nn.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindenberger U, et al. Towards a stronger science of human plasticity. Nat Rev Neurosci. 2017;18:261–262. doi: 10.1038/nrn.2017.44. [DOI] [PubMed] [Google Scholar]
- 70.Lövdén M, et al. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 2013;37:2296–2310. doi: 10.1016/j.neubiorev.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Meyer D, et al. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 72.Tonegawa S, et al. Memory engram storage and retrieval. Curr Opin Neurobiol. 2015;35:101–109. doi: 10.1016/j.conb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Bellander M, et al. Behavioral correlates of changes in hippocampal gray matter structure during acquisition of foreign vocabulary. Neuroimage. 2016;131:205–213. doi: 10.1016/j.neuroimage.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 74.Dahlin E, et al. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- 75.Noack H, et al. On the validity and generality of transfer effects in cognitive training research. Psychol Res. 2014;78:773–789. doi: 10.1007/s00426-014-0564-6. [DOI] [PubMed] [Google Scholar]
- 76.Simons DJ, et al. Do “brain-training” programs work? Psychol Sci Public Interes. 2016;17:103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 77.Lerch JP, et al. Studying neuroanatomy using MRI. Nat Neurosci. 2017;20:314–326. doi: 10.1038/nn.4501. [DOI] [PubMed] [Google Scholar]
- 78.Tardif CL, et al. Advanced MRI techniques to improve our understanding of experience-induced neuroplasticity. Neuroimage. 2016;131:55–72. doi: 10.1016/j.neuroimage.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 79.Fields RD. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arshad M, et al. Test-retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T 1 w/T 2 w image ratio. Hum Brain Mapp. 2017;38:1780–1790. doi: 10.1002/hbm.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiskopf N, Helms G. Multi-parameter mapping of the human brain at 1mm resolution in less than 20 minutes. Proc Intl Soc Mag Reson Med. 2008;16:2241–2241. [Google Scholar]
- 82.Marjańska M, et al. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neuroscience. 2017;354:168–177. doi: 10.1016/j.neuroscience.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lövdén M, et al. Performance-related increases in hippocampal N-acetylaspartate (NAA) induced by spatial navigation training are restricted to BDNF Val homozygotes. Cereb Cortex. 2011;21:1435–1442. doi: 10.1093/cercor/bhq230. [DOI] [PubMed] [Google Scholar]
- 84.Finnema SJ, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 85.Johansen-Berg H, et al. Human structural plasticity at record speed. Neuron. 2012;73:1058–1060. doi: 10.1016/j.neuron.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lerch JP, et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 87.Sagi Y, et al. Learning in the fast lane: New insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 88.Huettel SA, et al. Functional Magnetic Resonance Imaging. Sinauer; 2004. [DOI] [PubMed] [Google Scholar]
- 89.Ashburner J, Friston KJ. Voxel-based morphometry: The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 90.Hutton C, et al. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chung S, et al. Influence of T1-weighted signal intensity on FSL voxel-based morphometry and FreeSurfer cortical thickness. Am J Neuroradiol. 2017;38:726–728. doi: 10.3174/ajnr.A5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenzweig MR, et al. Cerebral effects of environmental complexity and training among adult rats. J Comp Physiol Psychol. 1964;57:438–439. doi: 10.1037/h0046387. [DOI] [PubMed] [Google Scholar]
- 93.Rosenzweig MR, et al. Variation in environmental complexity and brain measures. J Comp Physiol Psychol. 1962;55:1092–1095. doi: 10.1037/h0042758. [DOI] [PubMed] [Google Scholar]
- 94.Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 95.Greenough WT, et al. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- 96.Franklin TR, et al. A VBM study demonstrating “apparent” effects of a single dose of medication on T1-weighted MRIs. Brain Struct Funct. 2013;218:97–104. doi: 10.1007/s00429-012-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reuter M, et al. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Filippi M, et al. The effect of imprecise repositioning on lesion volume measurements in patients with multiple sclerosis. Neurology. 1997;49:274–276. doi: 10.1212/wnl.49.1.274. [DOI] [PubMed] [Google Scholar]
- 99.Glasser MF, et al. Trends and properties of human cerebral cortex: Correlations with cortical myelin content. Neuroimage. 2014;93:165–175. doi: 10.1016/j.neuroimage.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80:312–325. doi: 10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]