Abstract
Background
The insertion of a lumbar puncture needle or epidural catheter may be associated with peri‐ and post‐procedural bleeding. People who require this procedure may have disorders of coagulation as a result of their underlying illness, co‐morbidities or the effects of treatment. Clinical practice in some institutions is to mitigate the risk of bleeding in these patients by prophylactically transfusing plasma in order to correct clotting factor deficiencies prior to the procedure. However, plasma transfusion is not without risk, and it remains unclear whether this intervention is associated with reduced rates of bleeding or other clinically‐meaningful outcomes.
Objectives
To assess the effect of different prophylactic plasma transfusion regimens prior to insertion of a lumbar puncture needle or epidural catheter in people with abnormal coagulation.
Search methods
We searched for randomised controlled trials (RCTs), non‐randomised controlled trials (non‐RCT) and controlled before‐after studies (CBAs) in CENTRAL (the Cochrane Library 2016, Issue 11), MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1937), the Transfusion Evidence Library (from 1950), and five other electronic databases as well as ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (ICTRP) for ongoing trials to 9 January 2017.
Selection criteria
We planned to include RCTs, non‐RCTs, and CBAs involving transfusions of plasma given to prevent bleeding in people of any age with a coagulopathy requiring insertion of a lumbar puncture needle or epidural catheter. If identified, we would have excluded uncontrolled studies, cross‐sectional studies and case‐control studies. We would only have included cluster‐RCTs, non‐randomised cluster trials, and CBAs with at least two intervention sites and two control sites. In studies with only one intervention or control site, the intervention (or comparison) is completely confounded by study site making it difficult to attribute any observed differences to the intervention rather than to other site‐specific variables.
We planned to exclude people with haemophilia as they should be treated with the appropriate factor concentrate. We also planned to exclude people on warfarin as guidelines recommend the use of prothrombin complex concentrate for emergency reversal of warfarin.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We identified no completed or ongoing RCTs, non‐RCTs, or CBAs.
Authors' conclusions
There is no evidence from RCTs, non‐RCTs, and CBAs to determine whether plasma transfusions are required prior to insertion of a lumbar puncture needle or epidural catheter, and, if plasma transfusions are required, what is the degree of coagulopathy at which they should be given. We would need to design a study with at least 47,030 participants to be able to detect an increase in the number of people who had bleeding after lumbar puncture or epidural anaesthetic from 1 in 1000 to 2 in 1000.
Plain language summary
Plasma transfusions prior to insertion of lumbar puncture needles for people with abnormal coagulation
Review question We evaluated the evidence about whether people with abnormal coagulation (poor blood clotting) require a plasma transfusion prior to insertion of a lumbar puncture needle or epidural catheter, and if so what is the degree of abnormal coagulation at which a plasma transfusion is required. Background People with abnormal coagulation may require a lumbar puncture or epidural anaesthesia. A lumbar puncture is usually performed by inserting a needle between the bones (vertebrae) of the spine in the lower back into the fluid surrounding the spinal cord (the bundle of nerves that runs down the spine and connects the brain with the body). Lumbar punctures are performed either to obtain a sample of this fluid or to administer treatment into the fluid (chemotherapy or an anaesthetic). The lumbar puncture needle is removed immediately after any fluid samples have been taken or treatment has been administered. An epidural involves inserting a larger diameter needle than a lumbar puncture needle. The epidural needle passes through the same tissues as the lumbar puncture needle but stops short of penetrating the sac of fluid surrounding the spinal cord. Instead any treatment is injected into the space just outside the sac of fluid (called the epidural space). A small tube (an epidural catheter) is often passed through the epidural needle and left in position so that additional local anaesthetic medicines can be given. Current practice in many countries is to give plasma transfusions to prevent serious bleeding due to the procedure if blood tests to assess clotting are abnormal. Although the risk of bleeding appears to be very low, if bleeding does occur, it can be very serious. Correction of clotting abnormalities with a plasma transfusion is not without risks of its own, and it is unclear whether this practice is beneficial or harmful. People may be exposed to the risks of a plasma transfusion without any obvious clinical benefit. The risk that a plasma transfusion can cause serious harm, such as transmission of an infection or severe breathing problems, is very low. Study characteristics We searched scientific databases for clinical studies (randomised controlled trials and well designed non‐randomised studies) of people of any age with abnormal coagulation requiring a lumbar puncture or epidural anaesthesia. The evidence is current to 9 January 2017. In this review, we found no relevant studies. Key results There are no results because we found no relevant studies. The risk from bleeding after a lumbar puncture or epidural is very low and a very large study (with approximately 50,000 people) would be needed to know whether giving plasma transfusions before having these procedures is helpful.
Quality of the evidence
We did not assess the quality of the evidence because there were no included studies.
Background
Description of the condition
Abnormal coagulation refers to the condition in which the blood's ability to clot is impaired (Hunt 2014). People requiring insertion of a lumbar puncture (LP) needle or an epidural catheter often develop abnormal coagulation as a consequence of their underlying illness, co‐morbidities or the effects of treatment.
People requiring LPs and epidurals can have a variety of conditions and include people with liver failure, people who are critically ill and people requiring chemotherapy (Doherty 2014).
An LP is usually performed by inserting a needle into the lower back (underneath the spinal L4 bony process) (Williams 2008). A diagnostic LP is an invasive procedure to obtain samples of cerebrospinal fluid (CSF) (Doherty 2014). CSF is the fluid that bathes and protects the brain and spinal cord. The CSF obtained can then be used for the investigation of haematological malignancies (Vavricka 2003), subarachnoid haemorrhages, meningitis (Riordan 2002), or neurological disorders. LPs are performed by doctors or specially trained nurses. Therapeutic LPs administer drugs into the CSF. This can be for the administration of therapeutics such as chemotherapy or antibiotics, or administration of local anaesthetic to the nerves of the lower spine (spinal anaesthetic) (Doherty 2014). This usually involves inserting a fine needle into the lower back, administration of the therapeutic agent and then removal of the needle (Ng 2004).
An epidural catheter is inserted to administer anaesthetic. Epidural anaesthesia typically involves inserting a larger diameter needle than a spinal needle. The epidural needle passes through the same tissues as a spinal needle but stops short of penetrating the dura (tissue sac that contains CSF). An epidural catheter is often passed through the needle and left in position so that additional local anaesthetic medications can be administered (Ng 2004).The most common indication for epidural anaesthesia is in pregnant women to aid pain relief during labour (Venn 2015). However, epidural anaesthesia can also be used in postoperative pain management especially for people with lower limb ischaemia (narrowing or blockage of the arteries, which markedly reduces blood flow to the legs and feet) (Venn 2015), and people undergoing thoracic surgery (Mendola 2009), as alternatives to general anaesthesia.
In the general population, the risk of a spinal haematoma is very low 0.85 per 100,000 (95% confidence interval (CI) 0 to 1.8 per 100,000) (Cook 2009). The risk varies depending upon the type of person undergoing the procedure (1 in 200,000 epidural anaesthetic procedures during labour to 1 in 3600 epidural anaesthetic procedures in older women having knee surgery) (Li 2010; Moen 2004; Ruppen 2006; Vandermeulen 1994). Risk factors for major bleeding are multifactorial and include: increasing age (the procedure is more difficult in older people due to changes to the spine that occur with age), low platelet count, abnormal coagulation (including anticoagulant medication) and traumatic needle or catheter insertion (Erbay 2014; Li 2010; Moen 2004; Vandermeulen 1994). Performing an LP or administration of epidural anaesthesia is a relative contraindication in people with abnormal coagulation due to this perceived higher risk of complications (AAGBI 2013). However, overall, there are no current reliable estimates of the risks of adverse effects such as spinal haematomas in people with abnormal coagulation (AAGBI 2013; Cook 2009).
A large national study of fresh frozen plasma (FFP) use in critical illness reported that 30% of people admitted to the intensive care unit (ICU) developed an abnormality of coagulation (Walsh 2010). The aetiology of coagulopathy in critical illness is complex and multi‐factorial; sepsis, haemodilution, haemorrhage, disseminated intravascular coagulation, hepatic and renal disease and anti‐coagulant medication are all implicated (Hunt 2014). The causes of abnormal coagulation in people who are not critically ill are similarly broad.
Description of the intervention
Current practice in many countries is to correct abnormal coagulation tests ((prolonged prothrombin time (PT) or elevated international normalised ratio (INR)) with transfusion of plasma prior to insertion of an LP needle or epidural catheter, in order to mitigate the risk of serious peri‐ or post‐procedural bleeding (Moiz 2006; NICE 2015; Vlaar 2009; Yaddanapudi 2014).
Plasma is the liquid component of blood (Benjamin 2012). FFP refers to plasma that is frozen within eight hours of removal to ‐30°C, whereas frozen plasma (F24) is that which is frozen within 24 hours. Both contain concentrations of clotting factors equivalent to those found in in vivo blood, although the levels of factor V and VIII fall rapidly on thawing (Stanworth 2007). Current recommendations regarding the correction of coagulopathy prior to invasive procedures reflect expert opinion rather than high‐quality evidence from randomised controlled trials (RCTs) (AAGBI 2013; NICE 2015). An INR greater than or equal to 1.5 is frequently advocated as the threshold above which patients should undergo correction of coagulopathy prior to insertion of an LP needle or epidural catheter (Hunt 2014; NICE 2015). Whilst the use of standard laboratory tests of coagulation to assess bleeding has been criticised, an INR over 1.5 demarcates the level above which the activity of some coagulation factors falls to less than 50% (Hall 2014). An alternative approach to transfusing based on an INR threshold (which only detects low coagulation factor levels) is to use a test such as rotational thromboelastometry (ROTEM) or thromboelastography (TEG) that assesses how well a blood clot forms in whole blood (haemostasis) (Kinnaird 2013). ROTEM and TEG not only assess coagulation factor function, but also platelet function, strength of the clot and whether the clot is rapidly broken down (Whiting 2014).
Recent studies report that 15% to 26% of non‐bleeding critically ill patients receive prophylactic FFP transfusions prior to an invasive procedure (Dara 2005; Stanworth 2010; Stanworth 2011). However, there remains substantial heterogeneity in clinicians' views about the effectiveness of this intervention, with doubts over its effectiveness and the balance of the risk‐benefit ratio (Watson 2011).
How the intervention might work
Plasma transfusion is administered to people with abnormal coagulation in order to correct multiple clotting factor deficiencies and therefore reduce the incidence of bleeding. A dose of at least 10 mL to 15 mL/kg is required to significantly improve the INR (O'Shaughnessy 2004). However, clinical studies indicate that the INR is often minimally reduced following administration of FFP, especially when only modestly increased pre‐transfusion (Abdel‐Wahab 2006; Stanworth 2011). It remains unclear whether plasma transfusion in people with abnormal coagulation, despite improving standard laboratory tests of coagulation, reduces the incidence of clinically important bleeding or improves other meaningful patient‐oriented outcomes such as mortality.
Risks associated with the intervention
If plasma transfusions are ineffective, people are exposed to the risks associated with plasma transfusion unnecessarily. These include transfusion‐associated lung injury (Khan 2007; Rana 2006), transfusion‐associated circulatory overload (Narick 2011), multi‐organ failure (Watson 2009), and sepsis (Sarani 2008).
The requirement to administer plasma to correct coagulopathy prior to insertion of an LP needle or epidural catheter may additionally delay the start of a treatment. This could lead to unnecessary delays and cancellations of procedures, which may be time‐critical in an emergency situation. Delays in initiating treatment may lead to poorer patient outcomes (increased morbidity and mortality). It may also mean that a person does not receive regional anaesthesia, but instead receives a general anaesthetic that may place them at greater risk of complications (Amini 2015; Sanford 2015).
Why it is important to do this review
It is uncertain whether plasma transfusions are effective at preventing bleeding in patients with abnormal coagulation undergoing an invasive procedure (Desborough 2012; Hunt 2014; Segal 2005; Stanworth 2007). If effective, the INR threshold above which plasma transfusions are clinically effective is also uncertain. Wide variation in the use of FFP prior to invasive procedures exists, indicating significant clinician uncertainty and potentially exposing patients to varying risks (Watson 2011).
Previous systematic reviews have either only assessed the evidence from RCTs (Stanworth 2004; Yang 2012); only assessed the evidence associated with one or two outcomes (all‐cause mortality and multi‐organ failure) (Murad 2010); or were performed more than 10 years ago (Segal 2005; Stanworth 2004). In these previous systematic reviews, there was no RCT evidence for the use of plasma transfusions prior to insertion of an LP needle or epidural catheter (Murad 2010; Segal 2005; Stanworth 2004; Yang 2012). This review addresses an important question for clinicians and the best available evidence needs to be summarised. This review will therefore summarise the evidence from a broad range of studies and include a broad range of outcomes.
Objectives
To assess the effect of different prophylactic plasma transfusion regimens prior to insertion of a lumbar puncture needle or epidural catheter in people with abnormal coagulation.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), non‐randomised controlled trials (non‐RCTs) and controlled before‐after studies (CBAs), irrespective of language or publication status. If identified, we planned to exclude uncontrolled studies, cross‐sectional studies and case‐control studies.
We would only have included cluster‐RCTs, non‐randomised cluster trials, and CBAs with at least two intervention sites and two control sites. This was because in studies with only one intervention or control site, the intervention (or comparison) is completely confounded by study site making it difficult to attribute any observed differences to the intervention rather than to other site‐specific variables.
Types of participants
People, of any age, with abnormal coagulation (as defined by the included studies) requiring insertion of a lumbar puncture needle or epidural catheter.
If identified, we would not have included studies involving people with haemophilia as they should be treated with the appropriate factor concentrate (WFH 2012). Similarly, we would not have included studies involving people on warfarin as guidelines recommend the use of prothrombin complex concentrate for emergency reversal of warfarin (Keeling 2011; Tran 2013).
Types of interventions
Comparison 1: Plasma transfusion (for example, when an international normalised ratio (INR) is 1.5 or above; INR 2 or above; INR 3 or above; or other study‐specified INR or prothrombin time (PT) ratio threshold; or thromboelastography (TEG)‐guided) versus no plasma transfusion.
Comparison 2: Plasma transfusion when an INR is at a higher threshold (for example, INR 2 or above or INR > 3 or TEG‐guided) versus plasma transfusion when INR is at a lower threshold (for example INR 1.5 or above).
Types of outcome measures
Primary outcomes
Major procedure‐related bleeding within 24 hours of the procedure (as defined by 24 hours after removal of lumbar puncture needle or catheter in the case of epidural anaesthesia). For example: spinal haematoma; intraventricular, intracerebral or subarachnoid haemorrhage; or major bleeding (not further defined), as reported by individual studies.
-
Serious adverse events:
transfusion‐related complications within 24 hours of the procedure (including transfusion‐related acute lung injury (TRALI), transfusion‐transmitted infection, transfusion‐associated circulatory overload (TACO), transfusion‐associated dyspnoea (TAD), acute transfusion reactions);
venous and arterial thromboembolism (including deep vein thrombosis; pulmonary embolism; stroke; myocardial infarction) (up to 30 days);
lumbar puncture (LP)‐related or epidural anaesthetic‐related complications within seven days of the procedure (infection, headache, cerebral herniation, neurological symptoms such as radicular pain or numbness, back pain).
Secondary outcomes
All‐cause mortality (up to 24 hours and up to 30 days).
Minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (defined as prolonged bleeding at the insertion site that only required treatment with a pressure bandage) or minor bleeding (not further defined) as reported by individual studies.
Total number of days in hospital.
Proportion of patients receiving plasma transfusions within 24 hours of the procedure.
Change in baseline coagulation test abnormalities PT ratio, INR or as defined by the study within 24 hours after the plasma transfusion.
Quality of life, as defined by the individual studies.
Search methods for identification of studies
The Systematic Review Initiative’s Information Specialist (CD) formulated the search strategies in collaboration with the Cochrane Haematological Malignancies Group.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, 2016, Issue 11) (http://www.cochranelibrary.com/) (Appendix 1).
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE 1946 to 9th January 2017) (Appendix 2).
Embase (OvidSP, 1974 to 9th January 2017) (Appendix 3).
CINAHL (EBSCOHost, 1937 to 9th January 2017) (Appendix 4).
PubMed (e‐publications ahead of print only) (http://www.ncbi.nlm.nih.gov/pubmed) (Appendix 5).
Transfusion Evidence Library (1950 to 9th January 2017) (www.transfusionevidencelibrary.com), this includes a search of grey literature (Appendix 6).
LILACS (1980 to 9th January 2017) (http://lilacs.bvsalud.org/en/) (Appendix 7).
IndMed (1986 to 9th January 2017) (http://indmed.nic.in/indmed.html) (Appendix 8).
KoreaMed (1958 to 9th January 2017) (http://koreamed.org/) (Appendix 9).
PakMediNet (1995 to 9th January 2017) (http://www.pakmedinet.com/) (Appendix 10).
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (Thomson Reuters, 1990 to 9th January 2017) (Appendix 11).
Transfusion Evidence Library (1950 to 9th January 2017) (www.transfusionevidencelibrary.com), this includes a search of grey literature (Appendix 6).
LILACS (1980 to 9th January 2017) (http://lilacs.bvsalud.org/en/) (Appendix 7).
IndMed (1986 to 9th January 2017) (http://indmed.nic.in/indmed.html) (Appendix 8).
KoreaMed (1958 to 9th January 2017) (http://koreamed.org/) (Appendix 9).
PakMediNet (1995 to 9th January 2017) (http://www.pakmedinet.com/) (Appendix 10).
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (Thomson Reuters, 1990 to 9th January 2017) (Appendix 11).
We searched for ongoing trials in the following clinical trial registers to 9th January 2017.
ClinicalTrials.gov (https://www.clinicaltrials.gov/) (Appendix 12).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) (Appendix 13).
We combined searches in MEDLINE and Embase with the recommended Cochrane RCT search filters (Lefebvre 2011) and with systematic review and observational studies filters based on those of the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html). Searches in CINAHL were combined with SIGN systematic review, observational studies and RCT filters. We did not limit searches by language, year of publication or publication type.
If we had identified studies for inclusion we had planned to search MEDLINE (Ovid) for errata or retraction statements for the reports of these studies.
Searching other resources
We conducted handsearching of the reference lists of any relevant systematic reviews to identify further relevant studies. For future iterations of this review, we will make contact with lead authors of relevant studies to identify any unpublished material, missing data or information regarding ongoing studies.
Data collection and analysis
We summarised data in accordance with standard Cochrane methodologies.
Selection of studies
We selected studies with reference to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Systematic Review Initiative’s Information Specialist (CD) initially screened all search hits for relevance against the eligibility criteria and discarded all those that were clearly irrelevant. Thereafter, two review authors (LE, MD) independently screened all the remaining references for relevance against the full eligibility criteria.
Full‐text papers were retrieved for all references for which a decision on eligibility could not be made from title and abstract alone. We did not request additional information from study authors because it was not necessary to assess the eligibility for inclusion of individual studies. The two review authors discussed the results of study selection and resolved any discrepancies between themselves without the need for a third review author.
The results of study selection were reported using a PRISMA flow diagram (Moher 2009). We recorded the reasons for excluding studies based on full‐text assessment and added those to the Characteristics of excluded studies table.
We planned to collate multiple reports of one study, so that the study, and not the report, was the unit of analysis.
Data extraction and management
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions, two review authors (LE, MD) planned to independently extract data onto standardised forms and perform a cross‐check (Higgins 2011a). However, no completed or ongoing study was included in this review.
We planned to extract the following information for each study.
Source: Study ID; report ID; review author ID; date of extraction; ID of author checking extracted data; citation of paper; contact authors details.
General study information: Publication type; study objectives; funding source; conflict of interest declared; other relevant study publication reviewed.
Study details and methods: Location; country; setting; number of centres; total study duration; recruitment dates; length of follow‐up; power calculation; primary analysis (and definition); stopping rules; method of sequence generation; allocation concealment; blinding (of clinicians, participants and outcome assessors); any other concerns regarding bias; inclusion and exclusion criteria.
Characteristics of interventions: Number of study arms; description of experimental arm; description of control arm;and other relevant information.
Characteristics of participants: Age; gender; primary diagnosis; subgroup classification of primary disease type where appropriate, severity of primary disease, where appropriate, prognostic classification of primary disease where appropriate; additional therapy received; risk of alloimmunisation; baseline haematology laboratory parameters; confounders reported.
Participant flow: Total number screened for inclusion; total number recruited; total number excluded; total number allocated to each study arm; total number analysed (for review outcomes); number of allocated patients who received planned treatment; number of dropouts with reasons (percentage in each arm); protocol violations; missing data.
Outcomes: Major procedure‐related bleeding within 24 hours of the procedure; serious adverse events (transfusion‐related complications within 24 hours of the procedure; venous and arterial thromboembolism; LP‐related or epidural anaesthetic‐related complications within seven days of the procedure); all‐cause mortality (up to 24 hours and up to 30 days); minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure; total number of days in hospital; proportion of patients receiving plasma transfusions within 24 hours of the procedure; venous and arterial thromboembolism; change in baseline coagulation test abnormalities; quality of life, as defined by the individual studies.
For interventional cohort and pre‐post single arm or multiple arms studies, we also planned to collect data if available on: confounding factors, the comparability of groups on confounding factors; methods used to control for confounding and on multiple effect estimates (both unadjusted and adjusted estimates) as recommended in chapter 13 of theCochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
Assessment of risk of bias in included studies
Randomised controlled trials (RCTs)
We planned to assess the risk of bias for all included RCTs using the Cochrane 'Risk of bias' tool according to chapter eight of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). However, no completed study was included in this review.
In future updates of this review, if there are included RCTs, two review authors will work independently to assess each element of potential bias listed below as 'high', 'low' or 'unclear' risk of bias. We will report a brief description of the judgement statements upon which the authors have assessed potential bias in the Characteristics of included studies table. We will ensure that a consensus on the degree of risk of bias is met through comparison of the review authors’ statements and where necessary, through consultation with a third review author (SS). We will use Cochrane's tool for assessing risk of bias, that will include the following domains.
Selection bias
We will describe for each included study if and how the allocation sequence was generated and if allocation was adequately concealed prior to assignment. We will also describe the method used to conceal the allocation sequence in detail and determine if intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Performance bias
We will describe for each included study, where possible, if the study participants and personnel were adequately blinded from knowledge of which intervention a participant received. We will judge studies as low risk of bias if they were blinded, or if we judge that lack of blinding could not have affected the results.
Detection bias:
Was blinding of the outcome assessors effective in preventing systematic differences in the way in which the outcomes were determined?
Attrition bias
We will describe for each included study the attrition bias due to amount, nature or handling of incomplete outcome data. We will also try to evaluate whether intention‐to‐treat (ITT) analysis was performed or could be performed from published information.
Reporting bias
We will describe for each included study the possibility of selective outcome reporting bias.
Other issues
Was the study apparently free of other problems that could put it at risk of bias?
We will summarise the risk of bias for each key outcome for each included study. We will judge studies with at least one domain of high risk at high risk of bias overall etc.
Non‐randomised studies
We planned to use ROBINS‐I tool (formerly known as ACROBAT‐NRSI) to rate the quality of non‐randomised controlled trials (non‐RCTs) and controlled before‐after studies (CBAs) studies (Sterne 2014). This tool is based on the Cochrane 'Risk of bias' tool for rating the quality of RCTs (Higgins 2011c). The tool covers seven domains and the quality of evidence is rated as low, moderate, serious, critical or no information (see Appendix 14 for a copy of the tool), and uses signalling questions for the assessment of:
Bias due to confounding;
Bias in the selection of participants;
Bias in measurement of interventions;
Bias due to departure from intended interventions;
Bias due to missing data;
Bias in measurement of outcomes;
Bias in the selection of the reported result.
However, no completed study was included in this review.
We planned to resolve disagreements on the assessment of quality of an included study by discussion until we reach consensus or failing that by consulting a third review author.
We pre‐specified the following main potential confounding factors.
Primary diagnosis of patient (e.g. liver disease; critical illness; pregnancy).
Age: variability in the age of patients included, e.g. paediatric (less than 16 years) versus adult (> 16 years) versus older adult (> 60 years).
Gender: male to female ratio.
Previous severe bleeding (e.g. World Health Organization (WHO) grade 3 or 4 or equivalent).
Measures of treatment effect
We did not perform any of the planned analyses because no completed study was included in this review.
In future updates of this review we will perform the following.
-
Randomised controlled trials (RCTs)
For continuous outcomes, we will record the mean, standard deviation (SD) and total number of participants in both the treatment and control groups. For dichotomous outcomes, we will record the number of events and the total number of participants in both the treatment and control groups.
For continuous outcomes using the same scale, we will perform analyses using the mean difference (MD) with 95% confidence intervals (CIs). If continuous outcomes are reported using different scales we will use standardised mean difference (SMD).
If available, we will extract and report hazard ratios (HRs) for time‐to‐event‐data (mortality or time in hospital) data. If HRs are not available, we will make every effort to estimate as accurately as possible the HR using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007). If sufficient studies provide HRs, we will use HRs in favour of risk ratios (RRs) or MDs in a meta‐analysis, but for completeness, we will also perform a separate meta‐analysis of data from studies providing only RRs or MDs for the same outcome.
For dichotomous outcomes, we will report the pooled RR with a 95% CI. (Deeks 2011). Where the number of observed events is small (< 5% of sample per group), and where trials have balanced treatment groups, we will report the Peto’s Odds Ratio (OR) with 95% CI (Deeks 2011).
For cluster‐randomised trials, we will extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We will obtain statistical advice (MT) to ensure the analysis is appropriate. If appropriate analyses are not available, we will make every effort to approximate the analysis following the recommendations in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
If data allow, we will undertake quantitative assessments using Review Manager 5 (RevMan 2014).
-
Non‐randomised studies
For dichotomous outcomes, if available we will extract and report the RR with a 95% CI from statistical analyses adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of RRs (i.e. the RR post intervention/RR pre intervention).
For continuous variables, if available we will extract and report the absolute change from a statistical analysis adjusting for baseline differences (such as regression models, mixed models or hierarchical models), or the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute post‐intervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups/the post‐intervention level in the control group) (EPOC 2015).
If data allow, we will undertake quantitative assessments using Review Manager 5 (RevMan 2014).
-
All studies
Where appropriate, we will report the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs.
If we cannot report the available data in any of the formats described above, we will perform a narrative report, and if appropriate, we will present the data in tables.
Unit of analysis issues
We planned to treat any unit of analysis issues in accordance with the advice given in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). However, no completed study was identified in this review and there were therefore no unit of analysis issues.
Dealing with missing data
We did not need to contact any study authors directly to enable us to make a decision on whether a study should be excluded.
Assessment of heterogeneity
We did not perform any of the planned analyses because no completed study was included in this review.
In future updates of this review we will:
Combine the data to perform a meta‐analysis, if the clinical and methodological characteristics of individual studies are sufficiently homogeneous. We will analyse the data in RCTs, non‐RCTs, and CBA studies separately;
Evaluate the extent of heterogeneity by visual inspection of forest plots as well as by utilising statistical methods;
Assess statistical heterogeneity of treatment effects between studies using a Chi2 test with a significance level at P < 0.1. We will use the I2 statistic to quantify the degree of potential heterogeneity and classify it as low if the I2 is ≤ 50%, moderate if the I2 is 50% to 80% or considerable if the I2 is > 80%. We will use the random‐effects model for low to moderate heterogeneity. If statistical heterogeneity is considerable, and we cannot identify a cause for the heterogeneity, the overall summary statistic will not be reported. Potential causes of heterogeneity will be assessed by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
We were unable to perform a formal assessment of potential publication bias (small‐trial bias) by generating a funnel plot and statistically test using a linear regression test (Sterne 2011), because there were no completed trials within this review.
Data synthesis
We planned to perform analyses according to the recommendations of Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions, using aggregated data for analysis (Deeks 2011). We did not perform any of the planned analyses because no completed study was included in this review.
In future updates of this review we will perform the following:
If studies are sufficiently homogenous in their study design, we will conduct meta‐analyses according to Cochrane recommendations (Deeks 2011). We will not conduct meta‐analyses that include both RCTs and non‐RCTs. We will conduct separate meta‐analyses for each comparison. Different thresholds within the comparisons will only be grouped together if they are considered to be clinically similar.
-
Randomised controlled trials (RCTs)
For RCTs where meta‐analysis is feasible, we will use the random‐effects model for pooling the data. For binary outcomes, we will base the estimation of the between‐study variance using the Mantel‐Haenszel method. We will use the inverse‐variance method for continuous outcomes, outcomes that include data from cluster‐RCTs, or outcomes where HRs are available. If heterogeneity is found to be above 80%, and we identify a cause for the heterogeneity, we will explore this with subgroup analyses. If we cannot find a cause for the heterogeneity then we will not perform a meta‐analysis, but comment on the results as a narrative with the results from all studies presented in tables.
-
Non‐randomised studies
If meta‐analysis is feasible for non‐RCTs or CBA studies, we will analyse non‐RCTs and CBA studies separately. We will only analyse outcomes with adjusted effect estimates if these are adjusted for the same factors using the inverse‐variance method as recommended in chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
-
All studies
We will use the random‐effects model for all analyses as we anticipate that true effects will be related but will not be the same for included studies. If we cannot perform a meta‐analysis, we will comment on the results as a narrative with the results from all studies presented in tables.
Summary of Findings
We planned to use the GRADE tool (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence for each outcome. We planned to present a 'Summary of 'findings' table as suggested in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011a; Schunemann 2011b). The outcomes we planned to include are listed below in order of most relevant endpoints for patients.
Major procedure‐related bleeding.
Serious adverse events ‐ transfusion‐related.
Serious adverse events ‐ venous and arterial thromboembolism.
Serious adverse events ‐ LP or epidural‐related.
All‐cause mortality.
Total number of days in hospital.
Quality of life, as defined by the individual studies.
Subgroup analysis and investigation of heterogeneity
If adequate data were available, we planned to perform subgroup analyses for each of the following outcomes in order to assess the effect on heterogeneity.
Type of participant (such as obstetric, intensive care, liver disease, etc.).
Age of participants grouped as infant (nought to one year); paediatric (one to 16 years) adult (17 years to 60 years) elderly adult (greater than 60 years).
Underlying bleeding tendencies (e.g. associated thrombocytopenia, platelet dysfunction).
Type of procedure (diagnostic LP; therapeutic LP; epidural catheter).
Type of plasma component.
Dose of plasma component.
However, no completed study was identified in this review and therefore we could not perform any subgroup analyses.
Sensitivity analysis
We planned to assess the robustness of our findings by performing the following sensitivity analyses according to the recommendations of Cochrane (Deeks 2011) where appropriate:
including only those studies with a ‘low risk of bias’ (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation);
including only those studies with less than a 20% dropout rate.
However, no completed study was identified in this review and therefore we could not perform any sensitivity analyses.
Results
Description of studies
See Characteristics of excluded studies.
Results of the search
The search (conducted 9th January 2017) identified a total of 2173 potentially‐relevant records. There were 1377 records after duplicates were removed. Two review authors (LE; MD) excluded 1373 records on the basis of the abstract. Four full‐text articles were retrieved for assessment by the same two review authors. All four studies were excluded (Figure 1).
1.
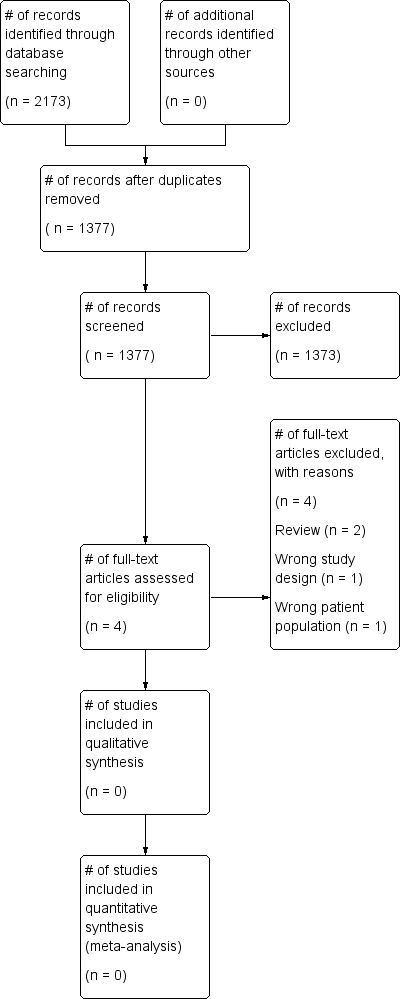
Study flow diagram.
Included studies
No completed or ongoing trials were included in this review. No studies that fitted the criteria were found.
Excluded studies
See Characteristics of excluded studies for further details. • Two studies were reviews (Bellini 2014; Tryba 1989). • One study did not include people with abnormal coagulation prior to insertion of the epidural catheter (Lim 2006). • One study was a single‐centre retrospective observational study (Friedman 1989).
Risk of bias in included studies
No trials were identified for inclusion in the review.
Effects of interventions
No trials were identified for inclusion in the review.
Discussion
Summary of main results
There were no completed or ongoing studies that were relevant to this review.
Overall completeness and applicability of evidence
This review did not identify any completed randomised controlled trials (RCTs), non‐randomised controlled trials (non‐RCTs) or controlled before‐after studies (CBAs) eligible for inclusion and therefore there is no evidence that can be assessed.
Any future study would need to be very large to detect a difference in the risk of bleeding. For example, if we assumed that major bleeding occurred in 1 out of 1000 people who had an LP when their INR was 1.5 or below, and that the risk of major bleeding doubled to 2 out of 1000 when their INR was 3, we would need to design a study with at least 47,030 participants to be able to detect this difference with 80% power and 5% significance (calculated using a power calculator at Sealed Envelope).
Quality of the evidence
This review did not identify any completed studies and therefore there is no evidence that could be assessed.
Potential biases in the review process
To our knowledge, our review process was free from bias. We conducted a comprehensive search, searching data sources (including multiple databases and clinical trial registries) to ensure that all relevant studies would be captured. The relevance of each paper identified was carefully assessed and all screening and data extractions were performed in duplicate. We prespecified all outcomes and subgroups prior to analysis.
Agreements and disagreements with other studies or reviews
Of the four systematic reviews that addressed the use of plasma transfusion prior to a procedure (Murad 2010; Segal 2005; Stanworth 2004; Yang 2012), only one contained a study that assessed the use of plasma transfusions in people who required a lumbar puncture (Segal 2005). This study was the single‐centre retrospective study excluded from this review because it was the wrong study design (Friedman 1989). No other studies were identified.
Authors' conclusions
Implications for practice.
As no studies were found for inclusion in the review, it is not possible to comment on the evidence for or against the efficacy of plasma transfusions prior to lumbar punctures and epidural catheters for people with abnormal coagulation.
Implications for research.
It is unlikely that any future randomised controlled trials (RCTs), non‐randomised controlled trials (non‐RCTs) or controlled before‐after studies (CBAs) will be able to answer this review's primary outcome of major bleeding because the event is rare and there is a potential risk to patient safety. This review also showed no good quality non‐randomised studies have been performed to assess the use of plasma transfusions prior to lumbar punctures or epidural catheters in people with abnormal coagulopathy. With the emergence of electronic health data around the world, there is the potential to design a study using 'big data' that could answer this review's questions.
Notes
The methods section of this review are based on a template designed by the study authors in association with the Cochrane Haematological Malignancies Group.
Acknowledgements
We thank the editorial base of the Cochrane Haematological Malignancies Review Group.
We thank the peer and consumer reviewers of this review.
We thank NHS Blood and Transplant.
We thank the National Institute of Health Research (NIHR). This review is part of a series of reviews that have been funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the NIHR Oxford Biomedical Research Centre Programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Plasma] this term only #2 MeSH descriptor: [Blood Component Transfusion] this term only #3 plasma #4 #2 and #3 #5 (plasma near/5 (transfus* or prophyla* or fresh* or frozen or freez* or prefrozen or prefreez* or thaw* or prethaw* or infus* or treatment* or therap* or administ* or donor* or donat*)) #6 ((autologous or homolog* or allogen* or allo‐gen*) next plasma) #7 (FFP or SDFFP or MBFFP or uniplas* or octaplas* or FP24 or frischplasma or "clinical plasma") #8 (plasma near/3 (pathogen inactivated or pathogen reduced or universal or donor*)) #9 ((pasteurized or pasteurised or methylene or solvent or detergent or cryoprecipitate or supernatant or cryosupernatant or thawed) near/5 plasma) #10 #1 or #4 or #5 or #6 or #7 or #8 or #9 #11 MeSH descriptor: [Spinal Puncture] this term only #12 MeSH descriptor: [Anesthesia, Epidural] explode all trees #13 MeSH descriptor: [Anesthesia, Spinal] this term only #14 MeSH descriptor: [Injections, Spinal] explode all trees #15 MeSH descriptor: [Myelography] this term only #16 MeSH descriptor: [Nerve Block] explode all trees #17 ((spine or spinal or intraspinal* or dura* or intradural* or extradural* or lumbar* or intralumbar* or theca* or intrathecal or subarachnoid* or peridural* or caudal*) near/6 (punctur* or inject* or infus* or anesth* or anaesth* or needle* or tap* or catheter* or block* or drug* or administ*)) #18 ((intrathecal or theca*) near/6 (treatment* or chemotherapy or antibiotic* or therapy or inject*)) #19 (epidural or nerve block* or chemical neurolys* or chemoneurolys* or chemodenervation* or myelogra*) #20 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 #10 and #20
Appendix 2. MEDLINE (OvidSP) search strategy
1. Plasma/ 2. Blood Component Transfusion/ and plasma.tw,kf. 3. (plasma adj5 (transfus* or prophyla* or fresh* or frozen or freez* or prefrozen or prefreez* or thaw* or prethaw* or infus* or treatment* or therap* or administ* or donor* or donat*)).tw,kf. 4. ((autologous or homolog* or allogen* or allo‐gen*) adj plasma).tw,kf. 5. (FFP or SDFFP or MBFFP or uniplas* or octaplas* or FP24 or frischplasma or clinical plasma).tw,kf. 6. (plasma adj3 (pathogen inactivated or pathogen reduced or universal or donor*)).tw,kf. 7. ((pasteurized or pasteurised or methylene or solvent or detergent or cryoprecipitate or supernatant or cryosupernatant or thawed) adj5 plasma).tw,kf. 8. or/1‐7 9. Spinal Puncture/ 10. exp Anesthesia, Epidural/ 11. Anesthesia Spinal/ 12. exp Injections, Spinal/ 13. Myelography/ 14. exp Nerve Block/ 15. ((spine or spinal or intraspinal* or dura* or intradural* or extradural* or lumbar* or intralumbar* or theca* or intrathecal or subarachnoid* or peridural* or caudal*) adj6 (punctur* or inject* or infus* or anesth* or anaesth* or needle* or tap* or catheter* or block* or drug* or administ*)).tw,kf. 16. ((intrathecal or theca*) adj6 (treatment* or chemotherapy or antibiotic* or therapy or inject*)).tw,kf. 17. (epidural or nerve block* or chemical neurolys* or chemoneurolys* or chemodenervation* or myelogra*).tw,kf. 18. or/9‐17 19. 8 and 18 20. RANDOMIZED CONTROLLED TRIAL.pt. 21. CONTROLLED CLINICAL TRIAL.pt. 22. (randomi* or trial*).tw,kf. 23. (placebo* or randomly or groups).ab. 24. CLINICAL TRIALS AS TOPIC.sh. 25. or/20‐24 26. exp COHORT STUDIES/ 27. (cohort* or controlled trial* or controlled stud* or comparative trial* or comparative stud* or comparison group* or comparator group* or control group*).tw,kf. 28. ((follow up or observational) adj (study or studies)).tw,kf. 29. (longitudinal* or retrospective* or prospective* or cross sectional*).mp. 30. CROSS‐SECTIONAL STUDIES/ 31. CONTROLLED BEFORE‐AFTER STUDIES/ 32. OBSERVATIONAL STUDY/ 33. HISTORICALLY CONTROLLED STUDY/ 34. INTERRUPTED TIME SERIES ANALYSIS/ 35. (nonrandom* or non random*).tw,kf. 36. ((before adj15 (after or during)) or "before‐after" or time series or time point* or repeated measur*).tw,kf. 37. (pre‐post or pre‐test* or pretest* or posttest* or post‐test* or (pre adj5 post)).tw,kf. 38. or/26‐37 39. Meta‐Analysis.pt. 40. (meta analy* or metaanaly*).ab. 41. META‐ANALYSIS/ 42. or/39‐41 43. (studies or trials).ab. 44. 42 and 43 45. (meta analy$ or metaanaly$).ti. 46. (systematic* adj2 (review* or overview*)).tw,kf. 47. (cochrane or medline or pubmed or embase or cinahl or cinhal or lilacs or "web of science" or science citation index or search terms or published articles or search strateg* or reference list* or bibliograph* or handsearch* or hand search* or manual* search*).ab. 48. (additional adj (papers or articles or sources)).ab. 49. (electronic adj (sources or resources or databases)).ab. 50. (relevant adj (journals or articles)).ab. 51. "REVIEW LITERATURE AS TOPIC"/ 52. META‐ANALYSIS AS TOPIC/ 53. or/44‐52 54. Review.pt. 55. exp CLINICAL TRIALS AS TOPIC/ 56. selection criteria.ab. or critical appraisal.ti. 57. (data adj (extraction or analys*)).ab. 58. RANDOMIZED CONTROLLED TRIALS/ 59. OBSERVATIONAL STUDY/ 60. ((cohort* or observational or retrospective*) adj1 (trial* or stud*)).tw,kf. 61. or/55‐59 62. 54 and 61 63. 53 or 62 64. (Comment or Letter or Editorial).pt. 65. 63 not 64 66. 25 or 38 or 65 67. exp animals/ not humans/ 68. 66 not 67 69. 19 and 68
Appendix 3. Embase (OvidSP) search strategy
1. Fresh Frozen Plasma/ 2. Plasma Transfusion/ 3. (plasma adj5 (transfus* or prophyla* or fresh* or frozen or freez* or prefrozen or prefreez* or thaw* or prethaw* or infus* or treatment* or therap* or administ* or donor* or donat*)).tw. 4. ((autologous or homolog* or allogen* or allo‐gen*) adj plasma).tw. 5. (FFP or SDFFP or MBFFP or uniplas* or octaplas* or FP24 or frischplasma or clinical plasma).tw. 6. (plasma adj3 (pathogen inactivated or pathogen reduced or universal)).tw. 7. ((pasteurized or pasteurised or methylene or solvent or detergent or cryoprecipitate or supernatant or cryosupernatant or thawed) adj5 plasma).tw. 8. or/1‐7 9. Lumbar Puncture/ 10. Puncture/ 11. exp Intraspinal Drug Administration/ 12. exp Epidural Anesthesia/ 13. Spinal Anesthesia/ 14. Myelography/ 15. exp Nerve Block/ 16. ((spine or spinal or intraspinal* or dura* or intradural* or extradural* or lumbar* or intralumbar* or theca* or intrathecal or subarachnoid* or peridural* or caudal*) adj6 (punctur* or inject* or infus* or anesth* or anaesth* or needle* or tap* or catheter* or block* or drug* or administ*)).tw. 17. ((intrathecal or theca*) adj6 (treatment* or chemotherapy or antibiotic* or therapy or inject*)).tw. 18. (epidural or nerve block* or chemical neurolys* or chemoneurolys* or chemodenervation* or myelogra*).tw. 19. or/9‐18 20. 8 and 19 21. MAJOR CLINICAL STUDY/ 22. LONGITUDINAL STUDY/ 23. RETROSPECTIVE STUDY/ 24. OBSERVATIONAL STUDY/ 25. INTERVENTION STUDY/ 26. PROSPECTIVE STUDY/ not RANDOMIZED CONTROLLED TRIAL/ 27. COHORT ANALYSIS/ 28. COMPARATIVE STUDY/ 29. (cohort* or controlled trial* or controlled stud* or comparative trial* or comparative stud* or comparison group* or comparator group* or control group*).tw. 30. ((follow up or observational) adj (study or studies)).tw. 31. (longitudinal* or retrospective* or prospective* or cross sectional*).mp. 32. (nonrandom* or non random*).tw. 33. ((before adj15 (after or during)) or "before‐after" or time series or time point* or repeated measur*).tw. 34. (pre‐post or pre‐test* or pretest* or posttest* or post‐test* or (pre adj5 post)).tw. 35. or/21‐34 36. Meta Analysis/ 37. Systematic Review/ 38. (meta analy* or metaanalys*).tw. 39. (systematic adj2 (review* or overview* or search*)).tw. 40. (literature adj2 (review* or overview* or search*)).ti,ab. 41. (cochrane or embase or cinahl or cinhal or lilacs or BIDS or science citation index or psyclit or psychlit or psycinfo or psychinfo or cancerlit).ti,ab. 42. (electronic* adj (sources or resources or databases)).ab. 43. reference lists.ab. 44. (bibliograph* or handsearch* or hand search* or manual* search*).ab. 45. (hand‐search* or handsearch*).ab. 46. (additional adj (papers or articles or sources)).ab. 47. (relevant adj (journals or articles)).ab. 48. (search term* or published articles or search strateg*).ab. 49. 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 50. data extraction.ab. 51. selection criteria.ab. 52. 50 or 51 53. review.pt. 54. 52 and 53 55. editorial.pt. 56. 49 or 54 57. 56 not 55 58. crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ 59. (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or doubl* blind* or singl* blind* or assign* or allocat* or volunteer*).mp. 60. 58 or 59 61. 35 or 57 or 60 62. 20 and 61
Appendix 4. CINAHL (EBSCOHost) search strategy
S1 (MH "Plasma") S2 (MH "Blood Component Transfusion") S3 TX plasma S4 S2 AND S3 S5 TX (plasma N5 (transfus* or prophyla* or fresh* or frozen or freez* or prefrozen or prefreez* or thaw* or prethaw* or infus* or treatment* or therap* or administ* or donor* or donat*)) S6 TX ((autologous or allogen* or allo‐gen* or homolog*) next plasma) S7 TX (FFP or SDFFP or MBFFP or uniplas* or octaplas* or FP24 or frischplasma or clinical plasma) S8 TX (plasma N3 (pathogen inactivated or pathogen reduced or universal or donor*)) S9 TX ((pasteurized or pasteurised or methylene or solvent or detergent or cryoprecipitate or supernatant or cryosupernatant or thawed) N5 plasma) S10 S1 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S11 (MH "Spinal Puncture") S12 (MH "Anesthesia, Epidural") OR (MH "Anesthesia, Spinal") OR (MH "Nerve Block+") S13 (MH "Injections, Intraspinal+") S14 (MH "Myelography") S15 TX ((spine or spinal or intraspinal* or dura* or intradural* or extradural* or lumbar* or intralumbar* or theca* or intrathecal or subarachnoid* or peridural* or caudal*) N6 (punctur* or inject* or infus* or anesth* or anaesth* or needle* or tap* or catheter* or block* or drug* or administ*)) S16 TX ((intrathecal or theca*) N6 (treatment* or chemotherapy or antibiotic* or therapy or inject*)) S17 TX (epidural or nerve block* or chemical neurolys* or chemoneurolys* or chemodenervation* or myelogra*) S18 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 S19 S10 AND S18 S20 (MH "Prospective Studies+") S21 (MH "Case Control Studies+") S22 (MH "Correlational Studies") OR (MH "Cross Sectional Studies") S23 TI ( (cohort study or cohort studies) ) OR AB ( (cohort study or cohort studies) ) S24 TI ( (observational stud* or retrospective stud*) ) OR AB ( (observational stud* or retrospective stud*) ) S25 S20 OR S21 OR S22 OR S23 OR S24 S26 (MH Clinical Trials+) S27 PT Clinical Trial S28 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*)) S29 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) S30 TI randomi* OR AB randomi* S31 MH RANDOM ASSIGNMENT S32 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three)) S33 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) ) S34 MH PLACEBOS S35 MH META ANALYSIS S36 MH SYSTEMATIC REVIEW S37 TI ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*") OR AB ("meta analys*" OR metaanalys* OR "systematic review" OR "systematic overview" OR "systematic search*") S38 TI ("literature review" OR "literature overview" OR "literature search*") OR AB ("literature review" OR "literature overview" OR "literature search*") S39 TI (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit) OR AB (cochrane OR embase OR cinahl OR cinhal OR lilacs OR BIDS OR science AND citation AND index OR cancerlit) S40 TI placebo* OR AB placebo* S41 MH QUANTITATIVE STUDIES S42 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 S43 S25 OR S42 S44 S19 AND S43
Appendix 5. PubMed (for epublications ahead of print only)
#1 (plasma AND (transfus* OR prophyla* OR fresh* OR frozen OR freez* OR prefrozen OR prefreez* OR thaw* OR prethaw* OR infus* OR treatment* OR therapy OR therapeutic* OR therapies OR administ* OR donor* OR donat* OR autologous OR allogen* OR allo‐gen* OR homolog* OR pathogen inactivated OR pathogen reduced OR universal OR donor* OR pasteurized OR pasteurised OR methylene OR solvent OR detergent OR cryoprecipitate OR supernatant OR cryosupernatant OR thawed)) #2 (FFP OR SDFFP OR MBFFP OR uniplas* OR octaplas* OR FP24 OR frischplasma OR "clinical plasma") #3 #1 OR #2 #4 ((spine OR spines OR spinal OR intraspinal* OR dura OR dural OR intradural OR extradural OR lumbar* OR intralumbar* OR theca* OR intrathecal OR subarachnoid* OR peridural* OR caudal*) AND (punctur* OR inject* OR infus* OR anesth* OR anaesth* OR needle* OR tap OR taps OR catheter* OR block* OR drug* OR administ*)) #5 ((intrathecal OR theca*) AND (treatment* OR chemotherapy OR antibiotic* OR therapy OR therapeutic* OR inject*)) #6 (epidural OR nerve block* OR chemical neurolys* OR chemoneurolys* OR chemodenervation* OR myelogra*) #7 #4 OR #5 OR #6 #8 #3 AND #7 #9 ((random* OR blind* OR "control group" OR placebo OR "controlled trial" OR "controlled study" OR groups OR trials OR "systematic review" OR "systematic overview" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR cochrane OR embase OR cohort* OR observational* OR retrospective* OR non‐random* OR "before and after") #10 (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb])) #11 #8 AND #9 AND #10
Appendix 6. Transfusion Evidence Library search strategy
Subject Area: Plasma/FFP Search Box: spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR epidural OR lumbar OR intralumbar OR theca OR intrathecal OR subarachnoid OR peridural OR caudal OR myelograph OR myelography
Appendix 7. LILACS search strategy
tw:(((spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR lumbar* OR intralumbar* OR theca* OR intrathecal OR subarachnoid* OR peridural* OR caudal*) AND (punctur* OR inject* OR infus* OR anesth* OR anaesth* OR needle* OR tap OR taps OR catheter* OR block* OR drug* OR administ*)) OR ((intrathecal OR theca*) AND (treatment* OR chemotherapy OR antibiotic* OR therapy OR therapeutic* OR inject*)) OR epidural OR myelogra*) AND ((plasma AND (transfus* OR prophyla* OR fresh* OR frozen OR freez* OR prefrozen OR prefreez* OR thaw* OR prethaw* OR infus* OR treatment* OR therapy OR therapeutic* OR therapies OR administ* OR donor* OR donat* OR autologous OR allogen* OR allo‐gen* OR homolog* OR pathogen inactivated OR pathogen reduced OR universal OR donor* OR pasteurized OR pasteurised OR methylene OR solvent OR detergent OR cryoprecipitate OR supernatant OR cryosupernatant OR thawed)) OR (FFP OR SDFFP OR MBFFP OR uniplas* OR octaplas* OR FP24 OR frischplasma OR "clinical plasma")) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Appendix 8. IndMed search strategy
(spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR epidural OR lumbar OR intralumbar OR thecal OR intrathecal OR subarachnoid OR peridural OR caudal OR myelograph OR myelography) AND (FFP OR SDFFP OR MBFFP OR uniplas OR octaplas OR FP24 OR frischplasma OR plasma) AND (randomized OR randomised OR randomly OR blind OR blinded OR trial OR control group OR cohort OR cohorts OR groups OR observational OR retrospective OR retrospectively)
Appendix 9. KoreaMed search strategy
plasma [ALL] AND "Randomized Controlled Trial" [PT] plasma [ALL] AND "Clinical Trial"[PT] plasma [ALL] AND "Comparative Study"[PT] plasma [ALL] AND transfus*[ALL] AND retrospective* [ALL] plasma [ALL] AND transfus*[ALL] AND observational [ALL] plasma [ALL] AND transfus*[ALL] AND cohort* [ALL] plasma [ALL] AND transfus*[ALL] AND randomi* [ALL]
Appendix 10. PakMediNet search strategy
(plasma OR FFP) AND (randomised OR randomized OR observational OR retrospective OR cohort OR cohorts)
Appendix 11. Web of Science CPCI‐S search strategy
#1 TS=(((spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR lumbar* OR intralumbar* OR theca* OR intrathecal OR subarachnoid* OR peridural* OR caudal*) AND (punctur* OR inject* OR infus* OR anesth* OR anaesth* OR needle* OR tap OR taps OR catheter* OR block* OR drug* OR administ*)) OR ((intrathecal OR theca*) AND (treatment* OR chemotherapy OR antibiotic* OR therapy OR therapeutic* OR inject*)) OR epidural OR myelogra*) #2 TS=((plasma AND (transfus* OR prophyla* OR fresh* OR frozen OR freez* OR prefrozen OR prefreez* OR thaw* OR prethaw* OR infus* OR treatment* OR therapy OR therapeutic* OR therapies OR administ* OR donor* OR donat* OR autologous OR allogen* OR allo‐gen* OR homolog* OR pathogen inactivated OR pathogen reduced OR universal OR donor* OR pasteurized OR pasteurised OR methylene OR solvent OR detergent OR cryoprecipitate OR supernatant OR cryosupernatant OR thawed)) OR (FFP OR SDFFP OR MBFFP OR uniplas* OR octaplas* OR FP24 OR frischplasma OR "clinical plasma")) #3 TS= (systematic* OR random* OR blind* OR trial* OR control* OR groups OR cohort* OR observational* OR retrospective* OR cross‐sectional* OR "before and after") #4 #1 AND #2 AND #3
Appendix 12. ClinicalTrials.gov search strategy
Search Terms: spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR epidural OR lumbar OR intralumbar OR thecal OR intrathecal OR subarachnoid OR peridural OR caudal OR myelography Intervention: plasma OR FFP OR SDFFP OR MBFFP OR uniplas OR octaplas OR FP24 Study Type: All studies
Appendix 13. WHO ICTRP search strategy
Title/Intervention: spine OR spines OR spinal OR intraspinal OR dura OR dural OR intradural OR extradural OR epidural OR lumbar OR intralumbar OR thecal OR intrathecal OR subarachnoid OR peridural OR caudal OR myelograph OR myelography Title/Intervention: plasma OR FFP OR SDFFP OR MBFFP OR uniplas OR octaplas OR FP24 Recruitment Status: All
Appendix 14. ROBINS‐I (A Cochrane Risk Of Bias Assessment Tool: for Non‐randomised Studies of Interventions)
ROBINS‐I tool (Stage I)
Specify the review question
| Participants | People with abnormal coagulation (as defined by the included studies) requiring insertion of a lumbar puncture needle or epidural catheter. We will not include people with haemophilia as they should be treated with the appropriate factor concentrate. We will not include people on warfarin as guidelines recommend the use of prothrombin complex concentrate for emergency reversal of warfarin. |
| Experimental intervention 1 | Plasma transfusion when: INR 1.5 or above; INR 2 or above; INR 3 or above; or other study specified threshold; or thromboelastography guided |
| Control intervention 1 | No plasma transfusion |
| Experimental intervention 2 | Plasma transfusion when INR is at a higher threshold (for example INR 2 or above or INR 3 or above) or thromboelastography guided |
| Control intervention 2 | Plasma transfusion when INR 1.5 or above |
| Outcomes |
Primary outcomes
Secondary outcomes
|
List the confounding areas relevant to all or most studies
We have pre‐specified the main potential confounding factors.
Primary diagnosis of patient (e.g. liver disease; critical illness; pregnancy)
Age: variability in the age of patients included, e.g. paediatric (less than 16 years) versus adult (> 16 years) versus older adult (> 60 years)
Gender: male to female ratio
Previous severe bleeding (e.g. WHO grade 3 or 4 or equivalent)
List the possible co‐interventions that could be different between intervention groups and could have an impact on outcomes
We have pre‐specified the possible co‐interventions that could be different between intervention groups and could have an impact on outcomes.
Use of antifibrinolytics or other procoagulant drugs
Use of anticoagulants or antiplatelet agents
The ROBINS‐I tool (Stage II): For each study
Specify a target trial specific to the study.
| Design | Individually‐randomised/cluster‐randomised/matched |
| Participants | |
| Experimental intervention | |
| Control intervention |
Is your aim for this study...?
□ To assess the effect of initiating intervention (as in an intention‐to‐treat analysis)
□ To assess the effect of initiating and adhering to intervention (as in a per‐protocol analysis)
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the 'Summary of findings' table). Specify whether this is a proposed benefit or harm of intervention.
Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR = 1.52 (95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
Preliminary consideration of confounders
Complete a row for each important confounding area
(i) listed in the review protocol; and
(ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.“Important” confounding areas are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. “Validity” refers to whether the confounding variable or variables fully measure the area, while “reliability” refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding areas listed in the review protocol | ||||
| Confounding area | Measured Variable (s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding area measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is adjusting for this variable (alone) expected to favour the experimental or the control group? |
| Yes / No / No information | Favour intervention / Favour control / No information | |||
| (ii) Additional confounding areas relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding area | Measured Variable (s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding area measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is adjusting for this variable (alone) expected to favour the experimental or the control group? |
| Yes / No / No information | Favour intervention / Favour control / No information | |||
* In the context of a particular study, variables can be demonstrated not to be confounders and so not included in the analysis: (a) if they are not predictive of the outcome; (b) if they are not predictive of intervention; or (c) because adjustment makes no or minimal difference to the estimated effect of the primary parameter. Note that “no statistically significant association” is not the same as “not predictive”.
Preliminary consideration of co‐interventions
Complete a row for each important co‐intervention (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
“Important” co‐interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co‐interventions listed in the review protocol | ||
| Co‐intervention | Is there evidence that controlling for this co‐intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co‐intervention likely to favour outcomes in the experimental or the control group |
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| (ii) Additional co‐interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
| Co‐intervention | Is there evidence that controlling for this co‐intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co‐intervention likely to favour outcomes in the experimental or the control group |
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
| Favour experimental / Favour comparator / No information | ||
'Risk of bias' assessment (cohort‐type studies)
| Bias domain | Signalling questions | Elaboration | Response options |
| Bias due to confounding | 1.1 Is there potential for confounding of the effect of intervention in this study? If N or PN to 1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
In rare situations, such as when studying harms that are very unlikely to be related to factors that influence treatment decisions, no confounding is expected and the study can be considered to be at low risk of bias due to confounding, equivalent to a fully randomised trial. There is no NI (No information) option for this signalling question. |
Y / PY / PN / N |
| If Y or PY to 1.1:determine whether there is a need to assess time‐varying confounding: | |||
| 1.2. Was the analysis based on splitting participants’ follow‐up time according to intervention received? If N or PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y or PY, proceed to question 1.3. |
If participants could switch between intervention groups then associations between intervention and outcome may be biased by time‐varying confounding. This occurs when prognostic factors influence switches between intended interventions. | NA / Y / PY / PN / N / NI | |
| 1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N or PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y or PY, answer questions relating to both baseline and time‐varying confounding (1.7 and 1.8) |
If intervention switches are unrelated to the outcome, for example when the outcome is an unexpected harm, then time‐varying confounding will not be present and only control for baseline confounding is required. | NA / Y / PY / PN / N / NI | |
| Questions relating to baseline confounding only | |||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding areas? | Appropriate methods to control for measured confounders include stratification, regression, matching, standardisation, and inverse probability weighting. They may control for individual variables or for the estimated propensity score. Inverse probability weighting is based on a function of the propensity score. Each method depends on the assumption that there is no unmeasured or residual confounding. | NA / Y / PY / PN / N / NI | |
| 1.5. If Y or PY to 1.4: Were confounding areas that were controlled for measured validly and reliably by the variables available in this study? | Appropriate control of confounding requires that the variables adjusted for are valid and reliable measures of the confounding domains. For some topics, a list of valid and reliable measures of confounding domains will be specified in the review protocol but for others such a list may not be available. Study authors may cite references to support the use of a particular measure. If authors control for confounding variables with no indication of their validity or reliability pay attention to the subjectivity of the measure. Subjective measures (e.g. based on self‐report) may have lower validity and reliability than objective measures such as lab findings. | NA / Y / PY / PN / N / NI | |
| 1.6. Did the authors control for any post‐intervention variables? | Controlling for post‐intervention variables is not appropriate. Controlling for mediating variables estimates the direct effect of intervention and may introduce confounding. Controlling for common effects of intervention and outcome causes bias. | NA / Y / PY / PN / N / NI | |
| Questions relating to baseline and time‐varying confounding | |||
| 1.7. Did the authors use an appropriate analysis method that adjusted for all the important confounding areas and for time‐varying confounding? | Adjustment for time‐varying confounding is necessary to estimate per‐protocol effects in both randomised trials and NRSI. Appropriate methods include those based on inverse‐probability weighting. Standard regression models that include time‐updated confounders may be problematic if time‐varying confounding is present. | NA / Y / PY / PN / N / NI | |
| 1.8. If Y or PY to 1.7: Were confounding areas that were adjusted for measured validly and reliably by the variables available in this study? | See 1.5 above. | NA / Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ No confounding expected. | Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ Confounding expected, all known important confounding domains appropriately measured and controlled for; and Reliability and validity of measurement of important domains were sufficient, such that we do not expect serious residual confounding. | |||
|
Serious ‐ At least one known important domain was not appropriately measured, or not controlled for; or Reliability or validity of measurement of a important domain was low enough that we expect serious residual confounding. | |||
| Critical ‐ Confounding inherently not controllable, or the use of negative controls strongly suggests unmeasured confounding. | |||
| Optional: What is the predicted direction of bias due to confounding? | Can the true effect estimate be predicted to be greater or less than the estimated effect in the study because one or more of the important confounding domains was not controlled for? Answering this question will be based on expert knowledge and results in other studies and therefore can only be completed after all of the studies in the body of evidence have been reviewed. Consider the potential effect of each of the unmeasured domains and whether all important confounding domains not controlled for in the analysis would be likely to change the estimate in the same direction, or if one important confounding domain that was not controlled for in the analysis is likely to have a dominant impact. | Favours experimental / Favours comparator / Unpredictable | |
| Bias in selection of participants into the study | 2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? | This domain is concerned only with selection into the study based on participant characteristics observed after the start of intervention. Selection based on characteristics observed before the start of intervention can be addressed by controlling for imbalances between intervention and control groups in baseline characteristics that are prognostic for the outcome (baseline confounding). | Y / PY / PN / N / NI |
| If N or PN to 2.1: go to 2.4 | |||
| 2.2. If Y or PY to 2.1: Were the post‐intervention variables that influenced selection likely to be associated with intervention | Selection bias occurs when selection is related to an effect of either intervention or a cause of intervention and an effect of either the outcome or a cause of the outcome. Therefore, the result is at risk of selection bias if selection into the study is related to both the intervention and the outcome. | NA / Y / PY / PN / N / NI | |
| 2.3 If Y or PY to 2.2: Were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? | NA / Y / PY / PN / N / NI | ||
| 2.4. Do start of follow‐up and start of intervention coincide for most participants? | If participants are not followed from the start of the intervention then a period of follow‐up has been excluded, and individuals who experienced the outcome soon after intervention will be missing from analyses. This problem may occur when prevalent, rather than new (incident), users of the intervention are included in analyses. | Y / PY / PN / N / NI | |
| 2.5.If Y or PY to 2.2 and 2.3, or N or PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | It is in principle possible to correct for selection biases, for example by using inverse probability weights to create a pseudo‐population in which the selection bias has been removed, or by modelling the distributions of the missing participants or follow‐up times and outcome events and including them using missing data methodology. However such methods are rarely used and the answer to this question will usually be “No” | NA / Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ All participants who would have been eligible for the target trial were included in the study and start of follow‐up and start of intervention coincide for all participants. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ Selection into the study may have been related to intervention and outcome, but the authors used appropriate methods to adjust for the selection bias; or Start of follow‐up and start of intervention do not coincide for all participants, but (a) the proportion of participants for which this was the case was too low to induce important bias; (b) the authors used appropriate methods to adjust for the selection bias; or (c) the review authors are confident that the rate (hazard) ratio for the effect of intervention remains constant over time. | |||
|
Serious ‐ Selection into the study was related to intervention and outcome; or Start of follow‐up and start of intervention do not coincide, and a potentially important amount of follow‐up time is missing from analyses, and the rate ratio is not constant over time. | |||
|
Critical ‐ Selection into the study was strongly related to intervention and outcome; or A substantial amount of follow‐up time is likely to be missing from analyses, and the rate ratio is not constant over time. | |||
| Optional: What is the predicted direction of bias due to selection of participants into the study? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in classification of interventions | 3.1 Were intervention groups clearly defined? | A pre‐requisite for an appropriate comparison of interventions is that the interventions are well defined. Ambiguity in the definition may lead to bias in the classification of participants. For individual‐level interventions, criteria for considering individuals to have received each intervention should be clear and explicit, covering issues such as type, setting, dose, frequency, intensity and/or timing of intervention. For population‐level interventions (e.g. measures to control air pollution), the question relates to whether the population is clearly defined, and the answer is likely to be ‘Yes’. | Y / PY / PN / N / NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | In general, if information about interventions received is available from sources that could not have been affected by subsequent outcomes, then differential misclassification of intervention status is unlikely. Collection of the information at the time of the intervention makes it easier to avoid such misclassification. For population‐level interventions (e.g. measures to control air pollution), the answer to this question is likely to be ‘Yes’. | Y / PY / PN / N / NI | |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Collection of the information at the time of the intervention may not be sufficient to avoid bias. The way in which the data are collected for the purposes of the NRSI should also avoid misclassification. | Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ Intervention status is well defined and based solely on information collected at the time of intervention. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ Intervention status is well defined but some aspects of the assignments of intervention status were determined retrospectively | |||
| Serious ‐ Intervention status is not well defined, or major aspects of the assignments of intervention status were determined in a way that could have been affected by knowledge of the outcome. | |||
| Critical ‐ (Unusual) An extremely high amount of misclassification of intervention status, e.g. because of unusually strong recall biases. | |||
| Optional: What is the predicted direction of bias due to measurement of outcomes or interventions? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias due to departures from intended interventions | 4.1. Was the intervention implemented successfully for most participants? | Consider the success of implementation of the intervention in the context of its complexity. Was recommended practice followed by those administering the intervention? | Y / PY / PN / N / NI |
| If your aim for this study is to assess the effect of initiating and adhering to intervention (as in a per‐protocol analysis), answer questions 4.2 to 4.4 | |||
| 4.2. Did study participants adhere to the assigned intervention regimen? | Lack of adherence to assigned intervention includes cessation of intervention, cross‐overs to the comparator intervention and switches to another active intervention. We distinguish between analyses where: (1) intervention switches led to follow‐up time being assigned to the new intervention, and (2) intervention switches (including cessation of intervention) where follow‐up time remained allocated to the original intervention. (1) is addressed under time‐varying confounding, and should not be considered further here. Consider available information on the proportion of study participants who continued with their assigned intervention throughout follow‐up. Was lack of adherence sufficient to impact the intervention effect estimate? |
NA/ Y / PY / PN / N / NI | |
| 4.3. Were important co‐interventions balanced across intervention groups? | Consider the co‐interventions that are likely to affect the outcome and to have been administered in the context of this study, based on the preliminary consideration of co‐interventions and available literature. Consider whether these co‐interventions are balanced between intervention groups. | NA/ Y / PY / PN / N / NI | |
| 4.4. If N or PN to 4.1, 4.2 or 4.3: Were adjustment techniques used that are likely to correct for these issues? | Such adjustment techniques include inverse‐probability weighting to adjust for censoring at deviation from intended intervention, or inverse probability weighting of marginal structural models to adjust for time‐varying confounding. Specialist advice may be needed to assess studies that used these approaches. | NA / Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ No bias due to deviation from the intended intervention is expected, for example if both the intervention and comparator are implemented over a short time period, and subsequent interventions are part of routine medical care, or if the specified comparison relates to initiation of intervention regardless of whether it is continued. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ Bias due to deviation from the intended intervention is expected, and switches, co‐interventions, and some problems with intervention fidelity are appropriately measured and adjusted for in the analyses. Alternatively, most (but not all) deviations from intended intervention reflect the natural course of events after initiation of intervention. | |||
| Serious ‐ Switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | |||
| Critical ‐ Substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | |||
| Optional: What is the predicted direction of bias due to departures from the intended interventions? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterised either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias due to missing data | 5.1 Were there missing outcome data? | This aims to elicit whether the proportion of missing observations is likely to result in missing information that could substantially impact our ability to answer the question being addressed. Guidance will be needed on what is meant by ‘reasonably complete’. One aspect of this is that review authors would ideally try and locate an analysis plan for the study. | Y / PY / PN / N / NI |
| 5.2 Were participants excluded due to missing data on intervention status? | Missing intervention status may be a problem. This requires that the intended study sample is clear, which it may not be in practice. | Y / PY / PN / N / NI | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | This question relates particularly to participants excluded from the analysis because of missing information on confounders that were controlled for in the analysis. | Y / PY / PN / N / NI | |
| 5.4 If Y or PY to 5.1, 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | This aims to elicit whether either (i) differential proportion of missing observations or (ii) differences in reasons for missing observations could substantially impact on our ability to answer the question being addressed. | NA / Y / PY / PN / N / NI | |
| 5.5 If Y or PY to 5.1, 5.2 or 5.3: Were appropriate statistical methods used to account for missing data? | It is important to assess whether assumptions employed in analyses are clear and plausible. Both content knowledge and statistical expertise will often be required for this. For instance, use of a statistical method such as multiple imputation does not guarantee an appropriate answer. Review authors should seek naïve (complete‐case) analyses for comparison, and clear differences between complete‐case and multiple imputation‐based findings should lead to careful assessment of the validity of the methods used. | NA / Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ Data were reasonably complete; or Proportions of and reasons for missing participants were similar across intervention groups; or Analyses that addressed missing data are likely to have removed any risk of bias. | Low / Moderate / Serious / Critical / NI | |
| Moderate ‐ Proportions of missing participants differ across interventions; or Reasons for missingness differ minimally across interventions; and Missing data were not addressed in the analysis. | |||
| Serious ‐ Proportions of missing participants differ substantially across interventions; or Reasons for missingness differ substantially across interventions; and Missing data were addressed inappropriately in the analysis; or The nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. | |||
| Critical ‐ (Unusual) There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. | |||
| Optional: What is the predicted direction of bias due to missing data? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterised either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in measurement of outcomes | 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Some outcome measures involve negligible assessor judgment, e.g. all‐cause mortality or non‐repeatable automated laboratory assessments. Risk of bias due to measurement of these outcomes would be expected to be low. | Y / PY / PN / N / NI |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | If outcome assessors were blinded to intervention status, the answer to this question would be ‘No’. In other situations, outcome assessors may be unaware of the interventions being received by participants despite there being no active blinding by the study investigators; the answer this question would then also be ‘No’. In studies where participants report their outcomes themselves, for example in a questionnaire, the outcome assessor is the study participant. In an observational study, the answer to this question will usually be ‘Yes’ when the participants report their outcomes themselves. | Y / PY / PN / N / NI | |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Comparable assessment methods (i.e. data collection) would involve the same outcome detection methods and thresholds, same time point, same definition, and same measurements | Y / PY / PN / N / NI | |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | This question refers to differential misclassification of outcomes. Systematic errors in measuring the outcome, if present, could cause bias if they are related to intervention or to a confounder of the intervention‐outcome relationship. This will usually be due either to outcome assessors being aware of the intervention received or to non‐comparability of outcome assessment methods, but there are examples of differential misclassification arising despite these controls being in place. | Y / PY / PN / N / NI | |
| 'Risk of bias' judgement |
Low ‐ The methods of outcome assessment were comparable across intervention groups; and The outcome measure was unlikely to be influenced by knowledge of the intervention received by study participants (i.e. is objective) or the outcome assessors were unaware of the intervention received by study participants; and Any error in measuring the outcome is unrelated to intervention status. |
Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ The methods of outcome assessment were comparable across intervention groups; and The outcome measure is only minimally influenced by knowledge of the intervention received by study participants; and Any error in measuring the outcome is only minimally related to intervention status. | |||
|
Serious ‐ The methods of outcome assessment were not comparable across intervention groups; or The outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or Error in measuring the outcome was related to intervention status. | |||
| Critical ‐ The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | |||
| Optional: What is the predicted direction of bias due to measurement of outcomes? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterized either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Bias in selection of the reported result | Is the reported effect estimate unlikely to be selected, on the basis of the results, from... | ||
| 7.1. ... multiple outcome measurements within the outcome domain? | For a specified outcome domain, it is possible to generate multiple effect estimates for different measurements. If multiple measurements were made, but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| 7.2 ... multiple analyses of the intervention‐outcome relationship? | Because of the limitations of using data from non‐randomised studies for analyses of effectiveness (need to control confounding, substantial missing data, etc), analysts may implement different analytic methods to address these limitations. Examples include unadjusted and adjusted models; use of final value vs change from baseline vs analysis of covariance; different transformations of variables; a continuously scaled outcome converted to categorical data with different cut‐points; different sets of co‐variates used for adjustment; and different analytic strategies for dealing with missing data. Application of such methods generates multiple effect estimates for a specific outcome metric. If the analyst does not pre‐specify the methods to be applied, and multiple estimates are generated but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| 7.3 ... different subgroups? | Particularly with large cohorts often available from routine data sources, it is possible to generate multiple effect estimates for different subgroups or simply to omit varying proportions of the original cohort. If multiple estimates are generated but only one or a subset is reported, there is a risk of selective reporting on the basis of results. | Y / PY / PN / N / NI | |
| 'Risk of bias' judgement | Low ‐ There is clear evidence (usually through examination of a pre‐registered protocol or statistical analysis plan) that all reported results correspond to all intended outcomes, analyses and sub‐cohorts. | Low / Moderate / Serious / Critical / NI | |
|
Moderate ‐ The outcome measurements and analyses are consistent with an a priori plan; or are clearly defined and both internally and externally consistent; and There is no indication of selection of the reported analysis from among multiple analyses; and There is no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. | |||
| Serious ‐ Outcome measurements or analyses are internally or externally inconsistent; or There is a high risk of selective reporting from among multiple analyses; or The cohort or subgroup is selected from a larger study for analysis and appears to be reported on the basis of the results. | |||
| Critical ‐ There is evidence or strong suspicion of selective reporting of results, and the unreported results are likely to be substantially different from the reported results. | |||
| Optional: What is the predicted direction of bias due to selection of the reported result? | If the likely direction of bias can be predicted, it is helpful to state this. The direction might be characterised either as being towards (or away from) the null, or as being in favour of one of the interventions. | Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | |
| Overall bias | 'Risk of bias' judgement | Low ‐ The study is judged to be at low risk of bias for all domains. | Low / Moderate / Serious / Critical / NI |
| Moderate ‐ The study is judged to be at low or moderate risk of bias for all domains. | |||
| Serious ‐ The study is judged to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. | |||
| Critical ‐ The study is judged to be at critical risk of bias in at least one domain. | |||
| No information ‐ There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in one or more key domains of bias (a judgement is required for this). | |||
| Optional: What is the overall predicted direction of bias for this outcome? |
Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable | ||
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bellini 2014 | Review |
| Friedman 1989 | Wrong study design (single‐centre retrospective observational study) |
| Lim 2006 | Wrong patient population |
| Tryba 1989 | Review |
Differences between protocol and review
There are several differences between the protocol (Estcourt 2016), and this review due to lack of data. The search found no completed studies and therefore we could not: • report on any of the primary or secondary outcomes of the review; • perform a ’Risk of bias’ assessment; • assess the quality of the evidence or produce a ’Summary of findings’ table; • assess publication bias; • perform any analyses or subgroup analyses.
Contributions of authors
Lise Estcourt: conceiving the review, protocol development, content expert.
Michael Desborough: protocol development, content expert
Carolyn Doree: protocol development and search specialist
Sally Hopewell: protocol development and methodological expert.
Marialena Trivella: protocol development and statistical expert.
Simon Stanworth: protocol development, content expert
Sources of support
Internal sources
-
NHS Blood and Transplant, Research and Development, UK.
To fund the work of the Systematic Review Initiative (SRI)
External sources
-
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
To provide funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Michael Desborough: none to declare.
Carolyn Doree: none to declare.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Simon Stanworth: none to declare.
New
References
References to studies excluded from this review
Bellini 2014 {published data only}
- Bellini M, Barbieri M. Coagulation management in epidural steroid injection. Anaesthesiology Intensive Therapy 2014;46(3):195‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Friedman 1989 {published data only}
- Friedman EW, Sussman II. Safety of invasive procedures in patients with the coagulopathy of liver disease. Clinical & Laboratory Haematology 1989;11(3):199‐204. [DOI] [PubMed] [Google Scholar]
Lim 2006 {published data only}
- Lim HJ, Koay CK, Lee LS. Postoperative coagulopathy after liver resection ‐ Implications for epidural analgesia. Anaesthesia and Intensive Care 2006;34(1):118‐9. [PubMed] [Google Scholar]
Tryba 1989 {published data only}
- Tryba M. [Hemostatic requirements for the performance of regional anesthesia. Workshop on hemostatic problems in regional anesthesia] [Hamostaseologische Voraussetzungen zur Durchfuhrung von Regionalanaesthesien. Workshop uber hamostaseologische Probleme bei Regionalanesthesien]. Regional Anaesthesie 1989;12(6):127‐31. [PubMed] [Google Scholar]
Additional references
AAGBI 2013
- Association of Anaesthetists of Great Britain and Ireland, Obstetric Anaesthetists’Association and Regional Anaesthesia UK. Regional anaesthesia and patients with abnormalities of coagulation. Anaesthesia 2013;68:699‐72. [DOI] [PubMed] [Google Scholar]
Abdel‐Wahab 2006
- Abdel‐Wahab OI, Healy B, Dzik WH. Effect of fresh‐frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46(8):1279‐85. [DOI] [PubMed] [Google Scholar]
Amini 2015
- Amini N, Kim Y, Hyder O, Spolverato G, Wu CL, Page AJ, et al. A nationwide analysis of the use and outcomes of perioperative epidural analgesia in patients undergoing hepatic and pancreatic surgery. American Journal of Surgery 2015;210(3):483‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2012
- Benjamin R, McLaughlin L. Plasma components: properties, differences, and uses. Transfusion 2012;52 Suppl 1:9S‐19S. [DOI] [PubMed] [Google Scholar]
Cook 2009
- Cook TM, Counsell D, Wildsmith JAW. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. British Journal of Anaesthesia 2009;102:179‐90. [DOI] [PubMed] [Google Scholar]
Dara 2005
- Dara S, Rana R, Afessa B, Moore S, Gajic O. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Critical Care Medicine 2005;33(11):2667‐71. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Desborough 2012
- Desborough M, Stanworth S. Plasma transfusion for bedside, radiologically guided, and operating room invasive procedures. Transfusion 2012;52:20S‐9S. [DOI] [PubMed] [Google Scholar]
Doherty 2014
- Doherty CM, Forbes RB. Diagnostic lumbar puncture. Ulster Medical Journal 2014;83(2):93‐102. [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2015. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Erbay 2014
- Erbay RH, Senoglu N, Atalay H. Spinal or epidural haematoma. Topics in Spinal Anaesthesia. Rijeka, Croatia: In‐Tech, 2014. [DOI: 10.5772/58702] [DOI] [Google Scholar]
Hall 2014
- Hall D, Walsh T. FFP transfusion in the intensive care unit. In: Juffermans N, Walsh T editor(s). Transfusion in the Intensive Care Unit. 1st Edition. Springer, 2014:151‐9. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in Statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 2014
- Hunt B. Bleeding and coagulopathies in critical care. New England Journal of Medicine 2014;370(9):847‐59. [DOI] [PubMed] [Google Scholar]
Keeling 2011
- Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin – fourth edition. British Journal of Haematology 2011;154(3):311‐24. [DOI: 10.1111/j.1365-2141.2011.08753.x] [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan H, Belsher J, Yilmaz M, Afessa B, Winters J, Moore S, et al. Fresh‐frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007;131(5):1308‐14. [DOI] [PubMed] [Google Scholar]
Kinnaird 2013
- Kinard TN, Christie A, Greilich PE, Sarode R. Comparison of thromboelastography (TEG) with rotational thromboelastrometry (ROTEM) in surgical patients. Blood 2013;122:3659. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (authors) on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2010
- Li S‐L, Wang D‐X, Ma D. Epidural hematoma after neuraxial blockade: a retrospective report from China. Anesthesia & Analgesia 2010;111(5):1322‐4. [DOI] [PubMed] [Google Scholar]
Mendola 2009
- Mendola C, Ferrante D, Oldani E, Cammarota G, Cecci G, Vaschetto R, et al. Thoracic epidural analgesia in post‐thoracotomy patients: comparison of three different concentrations of levobupivacaine and sufentanil. British Journal of Anaesthesia 2009;102(3):418‐23. [DOI] [PubMed] [Google Scholar]
Moen 2004
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990‐1999. Anesthesiology 2004;101(4):950‐9. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic reviews and Meta‐Analyses:The PRISMA Statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Moiz 2006
- Moiz B, Arif FM, Hashmi KZ. Appropriate and inappropriate use of fresh frozen plasma. Journal of Pakistan Medical Association 2006;56(8):356‐9. [PubMed] [Google Scholar]
Murad 2010
- Murad M, Stubbs J, Gandhi M, Wang A, Paul A, Erwin P, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta‐analysis. Transfusion 2010;50(6):1370‐83. [DOI] [PubMed] [Google Scholar]
Narick 2011
- Narick C, Triulzi D, Yazer M. Transfusion‐associated circulatory overload after plasma transfusion. Transfusion 2011;52(1):160‐5. [DOI] [PubMed] [Google Scholar]
Ng 2004
- Ng KW, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003765.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Clinical Excellence. Blood transfusion NG24. www.nice.org.uk/guidance/ng24 2015; Vol. [Accessed 01 March 2016].
O'Shaughnessy 2004
- O'Shaughnessy D, Atterbury C, Bolton M, Murphy M, Thomas D, Yates S, et al. Guidelines for the use of fresh‐frozen plasma, cryoprecipitate and cryosupernatant. British Journal of Haematology 2004;126(1):11‐28. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Rana 2006
- Rana R, Fernández‐Pérez E, Khan S, Rana S, Winters J, Lesnick T, et al. Transfusion‐related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion 2006;46(9):1478‐83. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA (authors) on behalf of the Cochrane Non‐Randomised Studies Methods Group. Chapter 13: Including non‐randomised studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Riordan 2002
- Riordan FA, Cant AJ. When to do a lumbar puncture. Archives of Disease in Childhood 2002;87(3):235‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruppen 2006
- Ruppen W, Derry S, McQuay H, Moore RA. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology 2006;105(2):394‐9. [DOI] [PubMed] [Google Scholar]
Sanford 2015
- Sanford DE, Hawkins WG, Fields RC. Improved peri‐operative outcomes with epidural analgesia in patients undergoing a pancreatectomy: a nationwide analysis. HPB: the official journal of the International Hepato Pancreato Biliary Association (Oxford) 2015;17(6):551‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarani 2008
- Sarani B, Dunkman W, Dean L, Sonnad S, Rohrbach J, Gracias V. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Critical Care Medicine 2008;36(4):1114‐8. [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (authors) on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al (authors on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The CochraneCollaboration.
Segal 2005
- Segal J, Dzik W, Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence‐based review. Transfusion 2005;45(9):1413‐25. [DOI] [PubMed] [Google Scholar]
Stanworth 2004
- Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. British Journal of Haematology 2004;126(1):139‐52. [DOI] [PubMed] [Google Scholar]
Stanworth 2007
- Stanworth S. The evidence‐based use of FFP and cryoprecipitate for abnormalities of coagulation tests and clinical coagulopathy. Hematology 2007;2007:179‐86. [DOI] [PubMed] [Google Scholar]
Stanworth 2010
- Stanworth S, Grant‐Casey J, Lowe D, Laffan M, New H, Murphy M, et al. The use of fresh‐frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 2010;51(1):62‐70. [DOI] [PubMed] [Google Scholar]
Stanworth 2011
- Stanworth S, Walsh T, Prescott R, Lee R, Watson D, Wyncoll D, et al. A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time. Critical Care 2011;15(2):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2014
- Sterne JAC, Higgins JPT, Reeves B, C on behalf of the development group for ACROBAT‐ NRSI. A Cochrane Risk of Bias Assessment Tool: for Non‐Randomized Studies of Interventions (ACROBAT‐ NRSI), Version 1.0.0. http://www.riskofbias.info. 2014 [Accessed 12 February 2016].
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; Vol. 8, issue 16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed]
Tran 2013
- Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Medical Journal of Australia 2013;198(4):198‐9. [DOI: 10.5694/mja12.10614] [DOI] [PubMed] [Google Scholar]
Vandermeulen 1994
- Vandermeulen EP, Aken H, Vermylen J. Anticoagulants and spinal‐epidural anesthesia. Anesthesia & Analgesia 1994;79:1165‐77. [DOI] [PubMed] [Google Scholar]
Vavricka 2003
- Vavricka SR, Walter RB, Irani S, Halter J, Schanz U. Safety of lumbar puncture for adults with acute leukaemia and restrictive prophylactic platelet transfusion. Annals of Hematology 2003;82(9):570‐3. [DOI] [PubMed] [Google Scholar]
Venn 2015
- Venn PJH. Key points on the provision of anaesthesia services 2015. www.rcoa.ac.uk/gpas2015 (accessed 26 November 2015).
Vlaar 2009
- Vlaar APJ, In Der Maur AL, Binnekade JM, Schultz MJ, Juffermans NP. A survey of physicians' reasons to transfuse plasma and platelets in the critically ill: a prospective single‐centre cohort study. Transfusion Medicine 2009;19:207‐12. [DOI] [PubMed] [Google Scholar]
Walsh 2010
- Walsh T, Stanworth S, Prescott R, Lee R, Watson D, Wyncoll D. Prevalence, management, and outcomes of critically ill patients with prothrombin time prolongation in United Kingdom intensive care units. Critical Care Medicine 2010;38(10):1939‐46. [DOI] [PubMed] [Google Scholar]
Watson 2009
- Watson G, Sperry J, Rosengart M, Minei J, Harbrecht B, Moore E, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. Journal of Trauma 2009;67(2):221‐8. [DOI] [PubMed] [Google Scholar]
Watson 2011
- Watson D, Stanworth S, Wyncoll D, McAuley D, Perkins G, Young D, et al. A national clinical scenario‐based survey of clinicians' attitudes towards fresh frozen plasma transfusion for critically ill patients. Transfusion Medicine 2011;21(2):124‐9. [DOI] [PubMed] [Google Scholar]
WFH 2012
- World Federation of Hemophilia. Haemophilia. 2nd Edition. Wiley, 2012. [DOI: 10.1111/j.1365-2516.2012.02909.x.] [DOI] [Google Scholar]
Whiting 2014
- Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. American Journal of Hematology 2014;89(2):228‐32. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams J, Lye DC, Umapathi T. Diagnostic lumbar puncture: minimising complications. Internal Medicine Journal 2008;38(7):587‐91. [DOI] [PubMed] [Google Scholar]
Yaddanapudi 2014
- Yaddanapudi S, Yaddanapudi LN. Indications for blood and blood product transfusion. Indian Journal of Anaesthesia 2014;58(5):538‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2012
- Yang L, Stanworth S, Hopewell S, Doree C, Murphy M. Is fresh‐frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 52;8:1673‐86. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Estcourt 2016
- Estcourt LJ, Desborough M, Doree C, Hopewell S, Trivella M, Stanworth SJ. Plasma transfusions prior to lumbar punctures and epidural catheters for people with abnormal coagulation. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012497] [DOI] [PMC free article] [PubMed] [Google Scholar]


