Abstract
Objective
To correlate the planned dose to the nausea center (NC) - area postrema (AP) and dorsal vagal complex (DVC) - with nausea and vomiting symptoms in OPC patients treated with IMRT without chemotherapy. We also investigated whether it was possible to reduce doses to the NC without significant degradation of the clinically accepted treatment plan.
Methods
From 11/04 to 4/09, 37 OPC patients were treated with definitive or adjuvant IMRT without chemotherapy. Of these, only 23 patients had restorable plans and were included in this analysis. We contoured the NC with the assistance of an expert board-certified neuroradiologist. We searched for correlation between the delivered dose to the NC and patient-reported nausea and vomiting during IMRT. We used one-paired t-test: two-sample assuming equal variances to compare differences in dose to NC between symptomatic and asymptomatic patients. We then replanned each case to determine if reduced dose to the NC could be achieved without compromising coverage to target volumes, increasing unwarranted hotspots or increasing dose to surrounding critical normal tissues.
Results
Acute symptoms of nausea were as follows: Grade 0 (n=6), Grade 1 (n=13), Grade 2 (n=3), and Grade 3 (n=1). Patients with no complaints of nausea had a median dose to the DVC of 34.2 Gy (range 4.6-46.6 Gy) and AP of 32.6 Gy (range 7.0-41.4Gy); whereas those with any complaints of nausea had a median DVC dose of 40.4 Gy (range 19.3-49.4 Gy) and AP dose of 38.7 Gy (range 16.7-46.8 Gy) (p=0.04). Acute vomiting was as follows: Grade 0 (n=17), Grade 1 (n=4), Grade 2 (n=1), and Grade 3 (n=1). There was no significant difference in DVC or AP dose among those with and without vomiting symptoms (p=0.28).
Upon replanning of each case to minimize dose to the NC, we were, on average, able to reduce the radiation dose to AP by 18% and DVC by 17%; while the average dose variations to the PTV coverage, brainstem, cord, temporal lobes, and cochlea were never greater than 3%. Hotspots increased by 2% for 3 patients while hotspots for remaining patients were less than 2% variation.
Conclusion
For OPC cancer patients treated with IMRT without chemotherapy, dose to AP and DVC may be associated with development of nausea. We were able to show that reducing doses substantially to the NC is achievable without significant alteration of the clinically accepted plan and may reduce the incidence and grade of nausea. As symptoms of nausea can be devastating to patients, one can consider routine contouring and constraining of the NC to minimize chances of having this complication.
Keywords: Radiation-induced nausea and vomiting, intensity modulated radiation therapy, area postrema, dorsal vagal complex, head and neck
Introduction
Head and neck cancer patients receiving radiation therapy commonly suffer nausea and vomiting, a symptom that if severe enough may interrupt or delay radiotherapy treatment course. In a recent study from Italy, it was found that radiation-induced emesis occurred in 30% of head and neck patients treated with conventional radiation techniques (1). This study reported their results with concurrent use of chemotherapy and it was unclear whether intensity-modulated radiation therapy (IMRT) was performed, which has now become routinely used for radiotherapy in patients with head and neck cancer (2). IMRT improves dose conformality to the target volume but results in higher integral doses of radiation to surrounding normal tissue structures (3). For head and neck patients, IMRT techniques generally deliver a potentially significant dose to the area postrema (AP) and the dorsal vagal complex (DVC), even though care is taken to constrain dose to the brainstem. The AP and the DVC have been considered to be areas that regulate nausea and vomiting (4, 5). Whether radiation dose-response to these structures exists in relation to nausea and vomiting remains unclear.
Two studies examined IMRT doses to these structures and its relationship to nausea and vomiting (6, 7). In one retrospective study, it was found that radiation dose to the dorsal vagal complex may play a role in the development of nausea during head and neck IMRT (7). Patients with an acute nausea of grade 0 had a median dorsal vagal complex dose of 6.5 Gy while patients with grades 1 to 2 had a median dorsal vagal complex dose of 26.9 Gy. However, 23 of 43 patients (53%) received chemotherapy and 15 of these patients (35%) received multiagent chemotherapy. On multivariate analysis, only amifostine use and chemotherapy appeared to showed significance for nausea.
Similarly, a study by Ciura et al. examined 100 patients treated for oropharyngeal cancer with IMRT to relate brainstem dose to nausea and vomiting symptoms (6). All patients were treated using a 9-beam IMRT arrangement. Post hoc analysis demonstrated that chemoradiation cases exhibited a trend towards dose response relationship with area postrema mean dose and brainstem mean dose. Fifty one percent of patients in this study received chemotherapy and the authors stated that the limited sample size made it hard to elucidate the effect of concurrent chemotherapy.
It is clear that many chemotherapy agents are emetogenic and contributes to these symptoms with concurrent radiation therapy. However, we found no studies that examined specifically whether radiation therapy alone, in the absence of chemotherapy, to the AP and DVC is correlated with nausea and vomiting. Therefore, we sought to retrospectively examine the relation of nausea and vomiting with doses to the AP and DVC for head and neck patients treated with definitive or adjuvant radiation therapy in the absence of chemotherapy. We also sought to determine whether it was possible to reduce doses to the NC without compromising a clinically accepted treatment plan.
Methods
Study design
From November 1, 2002 to April 2, 2009, 439 consecutive patients with newly diagnosed squamous cell carcinoma of the oropharynx treated with definitive or adjuvant radiotherapy at Memorial Sloan-Kettering Cancer Center or one of its satellites were reviewed. Patients were then excluded for any of the following reasons: induction chemotherapy, concurrent chemotherapy, or adjuvant chemotherapy (n = 402). Of the 37 patients who were eligible for our study, 23 patients had restorable plans that could be included in this analysis. Every effort was exhausted to obtain the treatment planning and those with irrestorable plans we found utilized an older Alpha system. Patient and treatment characteristics are listed in Table 1.
Table 1. Patient characteristics.
| Characteristic | [n (%)] |
|---|---|
| Sex | |
| Male | 15 (65%) |
| Female | 8 (35%) |
| Age | |
| Range | 34-84 |
| Median | 57 |
| Primary Site | |
| Base of tongue | 6 (26%) |
| Tonsil | 17 (74%) |
| Treatment type | |
| Definitive IMRT | 15 (65%) |
| Adjuvant IMRT | 8 (35%) |
| T stage | |
| T1 | 10 (43%) |
| T2 | 12 (52%) |
| T3 | 1 (4%) |
| T4 | 0 (0%) |
| N stage | |
| N0 | 17 (74%) |
| N1 | 2 (9%) |
| N2 | 4 (17%) |
| N3 | 0 |
The original clinical treatment plan for each patient was imported into the research database. The areas of interest were the brainstem, AP, and DVC (Figure 1). One radiation oncologist (T.J.W.) contoured the AP and DVC which were then reviewed and approved by an expert board-certified radiologist holding a certificate of added qualification in neuroradiology (R.J.Y.). These contours were then reviewed again by an expert head and neck radiation oncologist (N.Y.L.). Dose-volume histograms were generated to include the additional contours. From there, the hottest 5% (D05), maximum (Dmax) and mean radiation doses (Dmean) to these areas were computed and recorded. Additionally, each case was replanned (S.F. and P.M.) to attempt dose reduction to the NC without compromising coverage to critical organs. We analyzed the doses given to the surrounding critical organs including the cord, brainstem, cochlea, and temporal lobes.
Fig. 1.
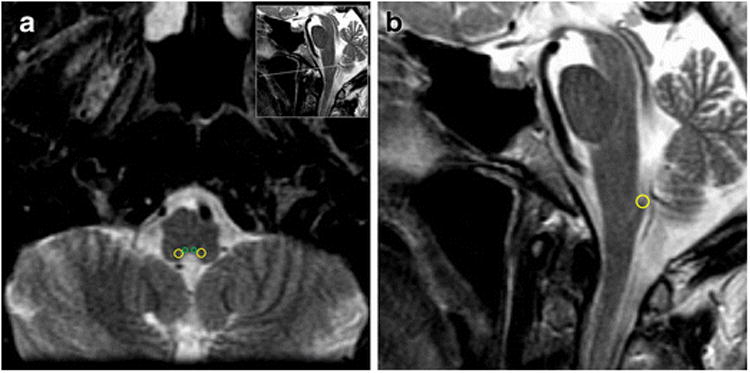
(A) T2 MRI axial contour of the area postrema (green) and dorsal vagal complex (yellow) (B) T2 MRI sagittal contour of the dorsal vagal complex (yellow).
Treatment
All patients received dental care followed by intensity-modulated radiation therapy (IMRT) using two, three, or four dose painting levels. For definitive treatment, we delivered 70 Gy at 2.12 Gy per fraction to the planning target volume (PTV) associated with the gross tumor volume (GTV), 59.4 Gy or 56.0 Gy at 1.8 Gy or 1.7 Gy per fraction to the PTV associated with the high-risk clinical target volume (CTV), and 54 Gy at 1.64 Gy per fraction to the PTV associated with the low-risk CTV. For adjuvant treatment, we delivered 66 Gy at 2 Gy per fraction to the PTV of the high-risk CTV, 60 Gy at 1.81 Gy per fraction to the PTV of intermediate-risk CTV, and 54 Gy at 1.64 Gy per fraction to the PTV of the low-risk CTV. The GTVs and CTVs were each expanded 3 to 5 mm to generate their respective PTVs.
Toxicity
Patients were assessed for acute complications by chart review using the Common Terminology Criteria for Adverse Events v4.0 (CTCAE). The maximum weekly nausea and vomiting grade during the entire radiotherapy course was recorded for each patient (Table 2). Additionally, weekly on-treatment evaluation notes were assessed to determine toxicity.
Table 2.
Acute Nausea and Vomiting Grade, CTCAE v3.0
| Acute nausea grade | |
|---|---|
| Grade 0 | None |
| Grade 1 | Loss of appetite without alteration in eating habits |
| Grade 2 | Oral intake decreased without significant weight loss, dehydration or malnutrition |
| Grade 3 | Inadequate oral caloric or fluid intake; tube feeding, TPN, or hospitalization indicated |
| Acute vomiting grade | |
| Grade 0 | None |
| Grade 1 | 1 to 2 episodes in 24 hrs |
| Grade 2 | 3 - 5 episodes in 24 hrs |
| Grade 3 | ≥ 6 episodes in 24 hrs; tube feeding, TPN or hospitalization indicated |
| Grade 4 | Life-threatening consequences; urgent intervention indicated |
| Grade 5 | Death |
Statistical Analysis
We investigated the presence of nausea or vomiting of any grade. We correlated nausea and vomiting to the dose that the area postrema and dorsal vagal complex received. One-paired t-test: two-sample assuming equal variances to compare differences in dose to NC between symptomatic and asymptomatic patients was performed.
Results
Seventeen patients (74%) reported acute nausea symptoms (Table 3). Fourteen patients (57%) developed grade 1 nausea, 3 patients (13%) developed grade 2 nausea, and 1 patient (4%) developed grade 3 nausea. Six patients (26%) reported no acute nausea symptoms. Four patients (17%) developed grade 1 vomiting, 1 patient (4%) developed grade 2 vomiting, and 1 patient (4%) developed grade 3 vomiting. Seventeen patients (74%) reported no acute vomiting symptoms.
Table 3. Acute nausea and vomiting results.
| Nausea (percent) | Vomiting (percent) | |
|---|---|---|
| Grade 0 | 6 (26%) | 17 (74%) |
| Grade 1 | 13 (57%) | 4 (17%) |
| Grade 2 | 3 (13%) | 1 (4%) |
| Grade 3 | 1 (4%) | 1 (4%) |
The median dose to the DVC and AP was significantly higher in patients with grade 1 to 3 nausea (Table 4). Patients without nausea symptoms had a median maximum dose to the DVC of 34.2 Gy (range 4.6-46.6 Gy) and AP of 32.6 Gy (range 7.0-41.4 Gy); while those with a nausea grade of 1 to 3 had a median DVC dose of 40.4 Gy (range 19.3-49.4 Gy) and AP dose of 38.7 Gy (range 16.7-46.8 Gy) (p=0.04). The median maximum dose to the DVC and AP was not different in patients with or without vomiting symptoms. Patients without vomiting symptoms had a median maximum dose to the DVC of 39.1 Gy (range 4.7-46.6 Gy) and AP dose of 35.6 Gy (range 4.3-46.8 Gy); while those with a vomiting grade of 1 to 3 had a median DVC dose of 44.7 Gy (range 19.4-49.4 Gy) and AP dose of 39.5 Gy (16.7-48.8 Gy) (p=0.28).
Table 4.
Average and median dose delivered to asymptomatic and symptomatic patients.
| Grade 0 nausea patients (n=6) | Grades 1-3 nausea patients (n=17) | Grade 0 vomiting patients (n=17) | Grades 1-3 vomiting patients (n=6) | |||||
|---|---|---|---|---|---|---|---|---|
| Average (cGy) | Median (cGy) | Average (cGy) | Median (cGy) | Average (cGy) | Median (cGy) | Average (cGy) | Median cGy) | |
| Area Postrema | ||||||||
| Dmax | 2956 | 3261 | 3716 | 3871 | 3434 | 3558 | 3765 | 3949 |
| Dmean | 2518 | 2943 | 3277 | 3311 | 2905 | 2960 | 3571 | 3673 |
| D05 | 2816 | 3143 | 3600 | 3669 | 3269 | 3349 | 3755 | 3958 |
| Dorsal Vagal Complex | ||||||||
| Dmax | 2901 | 3420 | 3937 | 4041 | 3544 | 3910 | 4028 | 4469 |
| Dmean | 2845 | 3177 | 3472 | 3564 | 3186 | 3363 | 3654 | 3834 |
| D05 | 3156 | 3412 | 3744 | 3812 | 3525 | 3742 | 3777 | 4063 |
| Tempora l Lobe | ||||||||
| Dmax | 1602 | 754 | 2492 | 571 | 2140 | 1600 | 3199 | 3664 |
| Dmean | 290 | 195 | 346 | 136 | 330 | 308 | 644 | 411 |
| D05 | 723 | 352 | 826 | 301 | 860 | 635 | 1245 | 1017 |
| Cochlea | ||||||||
| Dmax | 2616 | 2576 | 3614 | 810 | 3113 | 3062 | 3747 | 3832 |
| Dmean | 1561 | 1174 | 2227 | 326 | 1978 | 1839 | 2545 | 2407 |
| D05 | 2194 | 1972 | 3074 | 635 | 2702 | 2334 | 3183 | 3007 |
All 23 patients' treatment plans were replanned to minimize dose to the NC. On average, we were able to reduce the mean dose to the AP by 18% and DVC by 17% (Table 5). The average reduction of D05 to the AP was 18% and DVC by 17%. The average dose variations to the PTV coverage, brainstem, cord, temporal lobes, and cochlea were less than 3%. Hotspots increased by 2% for 3 patients while hotspots for remaining patients varied by less than 3%.
Table 5. Overall percent average increase and decrease in critical organ doses after replanning.
| Average change in dose after replanning (percent) | |
|---|---|
| Area Postrema | |
| Dmax | -18% |
| Dmean | -17% |
| D05 | -18% |
| Dorsal Vagal Complex | |
| Dmax | -16% |
| Dmean | -17% |
| D05 | -17% |
| Brainstem | -2% |
| Cord | -1% |
| Temporal Lobe | |
| Dmax | -3% |
| Dmean | -1% |
| D05 | -2% |
| Cochlea | |
| Dmax | -1% |
| Dmean | -1% |
| D05 | 0% |
Discussion
IMRT can result in higher integral dose to regions outside of the target volume, and is directly proportional to the number of beams used (8). Many studies have reported dose constraints to organs at risk, particularly the parotid gland, cord, brainstem, pharyngeal constrictors, larynx, optic nerves, and optic chiasm (8-13). It is reasonable to assume that sparing of functional anatomical sites may be achieved with current technologies. It is not always clear, however, whether sparing organs at risk function will compromise PTV coverage and degrade plan quality. In our study, we were able to achieve an average dose reduction of 16% to 18% to the NC after replanning without compromising coverage to target volumes or increasing dose to surrounding normal tissues. Interestingly, the median dose of the NC in symptomatic patients after replanning would be similar to, if not less, than the median dose of asymptomatic patients. However, it is unclear if intentional sparing of the NC will result in reduced toxicity. Our findings suggest that radiation dose alone, in the absence of chemotherapy, to the AP and DVC may be associated with nausea.
Monroe et al. suggested that radiation dose to the dorsal vagal complex was associated in the development of nausea during IMRT. They reported a median dose of 26.9 Gy to the DVC for grade 1 to 2 nausea and 6.5 Gy for grade 0 which is lower than our results of 40.4 Gy and 34.2 Gy, respectively. One possible explanation for this finding is that the majority of their patients concurrently received chemotherapy, which may have required a smaller radiation dose to induce symptoms. These results were also consistent with Ciura et al. who suggested that 15-25 Gy dose to the nausea center correlated with toxicity where majority of patients received chemotherapy (6).
We acknowledge this study is retrospective with small patient numbers, and thus selection bias and sample size have to be taken to account. Furthermore, although we excluded chemotherapy in our study, medications other than chemotherapy may also induce nausea and confound our results. Narcotics are well known to have emetogenic properties. In a study from Taiwan, 23.9% and 16.6% of patients reported vomiting and nausea, respectively, when transdermal fentanyl was given to head and neck cancer patients treated with radiotherapy (14). We also did not assess whether patients received anti-nausea medications which could have influenced the data. Additionally, higher doses to the AP and DVC may also have been a result of more extensive dose distributions which may have given rise to nausea for entirely unrelated reasons. For example, more extensive xerostomia and mucositis generally leads to more problems with ropy mucous, which can lead to nausea and vomiting. Our numbers were too small to determine if correlation between nausea and vomiting and dose to the AP were independent to mucositis and/or xerostomia. Our data is suggestive and that perhaps cooperative efforts are needed among institutions so that we can have adequate statistical power to describe an effect due to radiation.
We acknowledge that the AP and DVC are not easily identified (13). There are no clear guidelines on contouring these structures as there are for other critical head and neck normal structures (15-17). In our study, we relied on the expertise from a board-certified neuroradiologist to help review the AP and DVC (18). Many radiation oncology facilities are unlikely to have this resource available. Still, contouring the DVC or AP precisely may not be absolutely necessary as long as the posterior medulla oblongata is spared as much dose as possible.
Historically, many head and neck cancer patients received radiation therapy using two-dimensional radiotherapy. Significant portions of the brainstem likely received doses greater than IMRT techniques. A retrospective study by Rosenthal et al. showed that nausea and vomiting was associated with a brainstem mean dose of ≥ 36 Gy (8). In their study, they reported 76% and 38% of patients treated with IMRT without chemotherapy had nausea and vomiting, respectively. These rates are similar to our small series of 74% and 26% that developed nausea and vomiting, respectively. One of the major differences compared to our study was rather than the entire brainstem dose, we focused on the AP and DVC which has been well known to regulate nausea and vomiting.
Avoidance of dose to the AP and DVC may reduce nausea and vomiting symptoms. Whether this is true will require a prospective study of how IMRT dose affects nausea and vomiting.
Conclusion
For head and neck cancer patients treated with IMRT without chemotherapy, dose to AP and DVC may be associated with development of nausea. Our data shows significant difference in the NC doses for asymptomatic versus symptomatic patients. We were also able to show that reducing doses substantially to the NC is achievable without significant alteration of the clinically accepted plan and may reduce the incidence of nausea. As symptoms of nausea can be devastating to patients, one can consider routine contouring and constraining of the NC to minimize chances of having this complication.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. David P. Horowitz for the review of this manuscript.
Footnotes
This article does not contain any studies with human subjected performed by any of the authors.
Presented in part in abstract form at the 54th Annual Meeting of the American Society of Radiation Oncology (ASTRO), Boston, MA, Oct 28-31, 2012.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Maranzano E, De Angelis V, Pergolizzi S, et al. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol. 2010;94:36–41. doi: 10.1016/j.radonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Guadagnolo BA, Liu CC, Cormier JN, et al. Evaluation of trends in the use of intensity-modulated radiotherapy for head and neck cancer from 2000 through 2005: socioeconomic disparity and geographic variation in a large population-based cohort. Cancer. 116:3505–3512. doi: 10.1002/cncr.25205. [DOI] [PubMed] [Google Scholar]
- 3.Purdy JA. Dose to normal tissues outside the radiation therapy patient's treated volume: a review of different radiation therapy techniques. Health Phys. 2008;95:666–676. doi: 10.1097/01.HP.0000326342.47348.06. [DOI] [PubMed] [Google Scholar]
- 4.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15:301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- 5.Urba S. Radiation-induced nausea and vomiting. J Natl Compr Canc Netw. 2007;5:60–65. doi: 10.6004/jnccn.2007.0008. [DOI] [PubMed] [Google Scholar]
- 6.Ciura K, McBurney M, Nguyen B, et al. Effect of brain stem and dorsal vagus complex dosimetry on nausea and vomiting in head and neck intensity-modulated radiation therapy. Med Dosim. 2011;36:41–45. doi: 10.1016/j.meddos.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroe AT, Reddy SC, Gibbs GL, et al. Factors associated with radiation-induced nausea and vomiting in head and neck cancer patients treated with intensity modulated radiation therapy. Radiother Oncol. 2008;87:188–194. doi: 10.1016/j.radonc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750–757. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Taylor JM, Ten Haken RK, et al. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:660–669. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martel MK, Sandler HM, Cornblath WT, et al. Dose-volume complication analysis for visual pathway structures of patients with advanced paranasal sinus tumors. Int J Radiat Oncol Biol Phys. 1997;38:273–284. doi: 10.1016/s0360-3016(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 13.Voordeckers M, Farrag A, Everaert H, et al. Parotid Gland Sparing With Helical Tomotherapy in Head-and-Neck Cancer. Int J Radiat Oncol Biol Phys. 2012;84:443–448. doi: 10.1016/j.ijrobp.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Chang JT, Lin CY, Lin JC, et al. Transdermal fentanyl for pain caused by radiotherapy in head and neck cancer patients treated in an outpatient setting: a multicenter trial in Taiwan. Jpn J Clin Oncol. 2010;40:307–312. doi: 10.1093/jjco/hyp166. [DOI] [PubMed] [Google Scholar]
- 15.Commowick O, Gregoire V, Malandain G. Atlas-based delineation of lymph node levels in head and neck computed tomography images. Radiother Oncol. 2008;87:281–289. doi: 10.1016/j.radonc.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire V, Eisbruch A, Hamoir M, et al. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Hall WH, Guiou M, Lee NY, et al. Development and validation of a standardized method for contouring the brachial plexus: preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:1362–1367. doi: 10.1016/j.ijrobp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Lee NY, Young RJ. An unusual cause of isolated vomiting. Neurology. 2012;78:72–73. doi: 10.1212/WNL.0b013e3182420613. [DOI] [PubMed] [Google Scholar]


