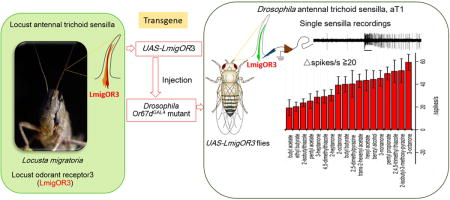
Abstract
Insects have evolved sophisticated olfactory reception systems to sense exogenous chemical signals. Odorant receptors (ORs) on the membrane of chemosensory neurons are believed to be key molecules in sensing exogenous chemical cues. ORs in different species of insects are diverse and should tune a species to its own specific semiochemicals relevant to their survival. The orthopteran insect, locust (Locusta migratoria), is a model hemimetabolous insect. There is very limited knowledge on the functions of locust ORs although many locust OR genes have been identified in genomic sequencing experiments. In this paper, a locust OR, LmigOR3 was localized to neurons housed in trichoid sensilla by in situ hybridization. LmigOR3 was expressed as a transgene in Drosophila trichoid olfactory neurons (aT1) lacking the endogenous receptor Or67d and the olfactory tuning curve and dose-response curves were established for this locust receptor. The results show that LmigOR3 sensitizes neurons to ketones, esters and heterocyclic compounds, indicating that LmigOR3 is a broadly tuned receptor. LmigOR3 is the first odorant receptor from Orthoptera that has been functionally analyzed in the Drosophila aT1 system. This work demonstrates the utility of the Drosophila aT1 system for functional analysis of locust odorant receptors and suggests that LmigOR3 may be involved in detecting food odorants, or perhaps locust body volatiles that may help us to develop new control methods for locusts.
Keywords: odorant receptor, localization, locust, trichoid sensilla, transgenic Drosophila aT1 system, odorants tuning
Graphical abstract
An odorant receptor, LmigOR3 is expressed in trichoid sensilla of locust (Locusta migratoria) and broadly tunes to odorants of C7–C10 ketones, C5–C8 esters and C5–C9 heterocyclics in antennal trichoid sensilla (aT1) of transgenic Drosophila.
1 Introduction
Insects have evolved sophisticated olfactory reception systems to sense exogenous chemical signals. Antennae are the most important chemosensory organs on which there are many hair-like structures, called sensilla innervated by olfactory receptor neurons (ORNs). On the membrane of insect ORNs, odorant receptors (ORs), a type of seven transmembrane domain protein are expressed with a co-receptor (ORco) to form a heteromer which functions as an odorant-gated ion channel (Sato et al., 2008; Smart et al., 2008; Wicher et al., 2008). Different odorant receptors in insects are tuned to different combinations of chemical compounds (Carey et al., 2010; Hallem et al., 2004; Wang et al., 2010).
The orthopteran insect, locust (Locusta migratoria), a model hemimetabolous insect, mainly relies on olfactory cues emitted from con-specifics, food plants or oviposition sites to guide and trigger important behaviors, such as aggregation, feeding, mating and oviposition (Hassanali et al., 2005). There is very limited knowledge on molecular mechanisms of olfaction which mediates locust behaviors though many locust OR genes have been identified (Xu et al., 2013; Wang et al., 2015). Understanding the molecular olfaction mechanisms that underlie these behaviors will be helpful to develop new locust control methods based on the functions of odorant receptors.
In insect species studied to date, odorant receptors expressed in the neurons located in basiconica sensilla are, generally, broadly tuned receptors that detect odors from plants and fruits, whereas those expressed in the neurons located in trichoid sensilla are often narrowly tuned receptors that detect important cues for social behaviors in most insects (van der Goes van Naters and Carlson, 2007). In Drosophila melanogaster, Or67d is expressed in aT1 sensilla neurons and specifically detects the male-specific pheromone, 11-cis-vaccenyl acetate (cVA) (Ha and Smith, 2006). cVA has sexually dimorphic effects in the two sexes: inhibiting mating behavior between males but promoting mating behavior in females (Kurtovic et al., 2007; Ronderos et al., 2010). Or47b and Or88a expressed in aT4 sensilla in D. melanogaster can sense the fly-produced odorants methyl laurate, methyl myristate and methyl palmitate (Dweck et al., 2015). BmorOr1 expressed in the trichoid sensilla neurons in Bombyx mori can sense bombykol, the first identified pheromone, which can help male moth to find suitable mate over large distances (Butenandt et al., 1959; Nakagawa et al., 2005; Sakurai et al., 2004). Mis-expression of the bombykol receptor in Drosophila trichoid neurons confers bombykol sensitivity (Syed et al., 2006).
The locust has evolved a complicated olfactory system that is anatomically distinct from flies or moths. In this insect olfactory system, there are three identified types of olfactory sensilla, including basiconica, trichoid and coeloconica sensilla (Ochieng and Hansson, 1999; Ochieng et al., 1998). However, the antennal lobes in locusts contain thousands of microglomeruli compared to roughly 60 in the fly. These microglomeruli are innervated by highly branched OSNs and PNs (Hansson and Stensmyr, 2011), indicating that the locust’s chemosensory system is distinct from that of the fly or moth. The functional significance of this complicated anatomy is unclear. The locust trichoid sensilla house 1–3 ORNs (Ochieng et al., 1998). Some types of ORNs in locust trichoid sensilla were identified with single sensilla recording technology (Cui et al., 2011; Ochieng and Hansson, 1999). Recently, locust odorant receptors, LmigOR1 and LmigOR2 have been demonstrated to be expressed in different neurons housed in basiconic sensilla (Xu et al., 2013). To date, no locust ORs have been localized in trichoid sensilla and functionally studied so far.
To explore the functional properties of locust odorant receptors, we localized LmigOR3 to trichoid sensilla by in situ hybridization. We successfully transformed LmigOR3 into Drosophila aT1 neurons and the chemical specificity of LmigOR3 in aT1 neurons was evaluated by single unit electrophysiology methods. The results indicate that LmigOR3 is a broadly tuned receptor which is expressed in the neurons of trichoid sensilla on locust antenna.
2 Materials and methods
2.1 Insects
Adult locusts (L. migratoria) in this study were obtained and raised as previously described (Xu et al., 2013). Intact antennae were dissected using forceps and stored at −80°C until further processing for in situ hybridization or to recover mRNA.
Drosophila stocks. The Or67dGAL4 mutants were described previously (Kurtovic et al., 2007). We injected receptor cDNAs regulated by UAS directly into the Or67dGAL4 mutant background to generate transgenic UAS-LmigOR3 flies.
2.2 Probe preparation and in situ hybridization
Plasmids containing the complete LmigOR3 gene were used as DNA template to prepare probes.
The following gene-specific primers were selected for 374 bp fragment:
LmigOR3-probe-F: 5′- TGCTTCTCCGTGTTCAACTG -3′ and
LmigOR3-probe-R: 5′- TGCACAAACCTGCAAACTTC -3′.
Digoxigenin (DIG)-labeled LmigOR3 antisense and sense probes were generated from linearized recombinant pGem-T Easy plasmids using the T7/SP6 RNA transcription system (Roche, Basel, Switzerland) following recommended protocols, as described previously, except that the probes were not fragmented by incubation in carbonate buffer (Xu et al., 2013). RNA in situ hybridization and analysis was also performed as described previously (Xu et al., 2013).
2.3 Single sensillum electrophysiology
Extracellular electrophysiological recordings of aT1 sensilla were performed according to Ha and Smith (2006). All of the chemicals were diluted in paraffin oil to different dilution (10%, 1%, 0.1%, 0.01%, 0.001% vol/vol or wt/vol). 185 chemicals presented as stimuli were of the highest purity available and responses to these compounds were recorded and analyzed (Table S1). Graphical summaries represent mean ± SEM.
3 Results
3.1 LmigOR3 is localized in chemosensory neuron of locust trichoid sensillum
Previous PCR experiment results showed that LmigOR3 was specifically expressed in the antennae of locust from nymph to adult at high levels (Xu et al., 2013). To localize LmigOR3 expression to a type of sensillum, we performed in situ hybridization experiments with DIG-labeled LmigOR3 sense and antisense probes. As expected only a small subset of distinct ORN cell bodies in the section were labeled by the LmigOR3 antisense probes. Figure 1A shows an example of a positive neuron localized to a trichoid sensillum. In contrast, we did not observe any labeled neurons in basiconica, coeloconica, or chaetica sensilla using the LmigOR3 antisense probes. No labeling was detected in olfactory neurons under the same conditions using sense probes of LmigOR3 (Figure 1B).
Figure 1.
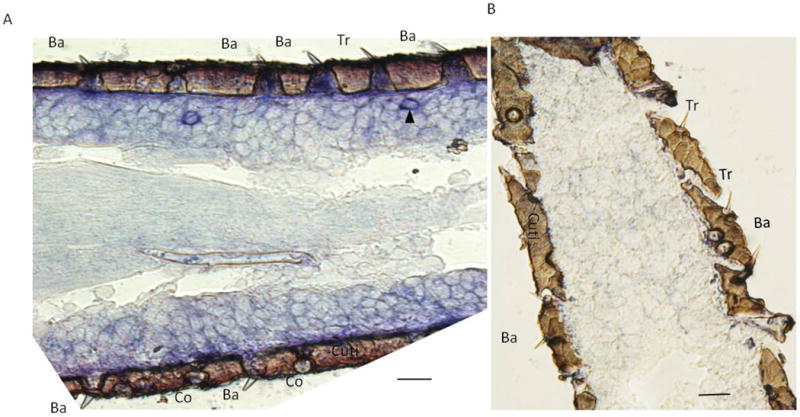
LmigOR3-expressing ORNs were associated with antennal trichoid sensilla of locust. RNA in situ hybridization experiments were performed using the Dig-labeled LmigOR3 antisense and sense probes. A. Neuron labeled by the LmigOR3 antisense probes housed to trichoid sensilla; B. No neurons were labeled by the LmigOR3 sense probes.
Arrow head marks LmigOR3-expressing neurons. Tr, trichoid sensillum; Ba, basiconic sensillum; Co, coeloconica sensillum; Ch, chaetica sensillum; Cuti, cuticle. Scale bars: A, B, 20 μm.
3.2 LmigOR3 broadly tunes to odorants in aT1 system of transgenic Drosophila
To reveal the function of LmigOR3 in olfaction, a LmigOR3 cDNA under control of the UAS promoter was transformed into Drosophila homozygous for Or67dGAL4. In these flies the Or67d receptor, that normally mediates cVA sensitivity, was replaced by the coding sequence for the yeast GAL4 transcription factor that drives expression from UAS promoters (Kurtovic et al., 2007). Thus, flies homozygous for both Or67dGAL4 and UAS-LmigOR3 express LmigOR3 in place of Or67d. Single sensillum electrophysiological recordings were used to record the responses of LmigOR3-expressing ORNs in trichoid neurons to a large and diverse panel of odorants as stimuli. 55 of 185 chemicals we tested can elicit excitatory responses from LmigOR3 at the concentration of 10% (v/v, or w/v) (Figure 2A). The strongest responses were elicited by ketones with 7–10 carbon atoms (C7–C10), esters with 5–8 carbon atoms (C5–C8) and heterocyclics with 5–9 carbon atoms (C5–C9) (Table S2). For ketones, 3-octanone (C8), 2-octanone (C8) and 3-nonanone (C9) can elicit stronger responses than 3-heptanone (C7), 2-heptanone (C7) and 2-decanone (C10). For esters, trans-2-hexenyl acetate (C7) and pentyl propionate (C8), hexyl acetate (C8), butyl butyrate (C8) can elicit stronger responses than other esters. These results showed that LmigOR3 is mainly sensitive to C7–C9 lengths of ketones and esters. For heterocyclic compounds, 2, 5-dimethylpyrazine, 2, 4, 5-trimethyl thiazole, C6-odorants, and 2-isobutyl-3-methoxy-pyrazine, C9-odorant can elicit stronger responses than other heterocyclics. In addition, benzyl alcohol (C7) also can elicit a strong excitatory response. 18 chemicals can produce responses of 20 spikes/s or greater (Figure 2B). Of the odorants we tested, 3-octanone is the strongest activator for LmigOR3 (△spikes/s=59.32±7.06) in this system. These results show that LmigOR3 is mainly activated by chemicals with 5–10 carbon atoms and is a broadly tuned odorant receptor.
Figure 2.
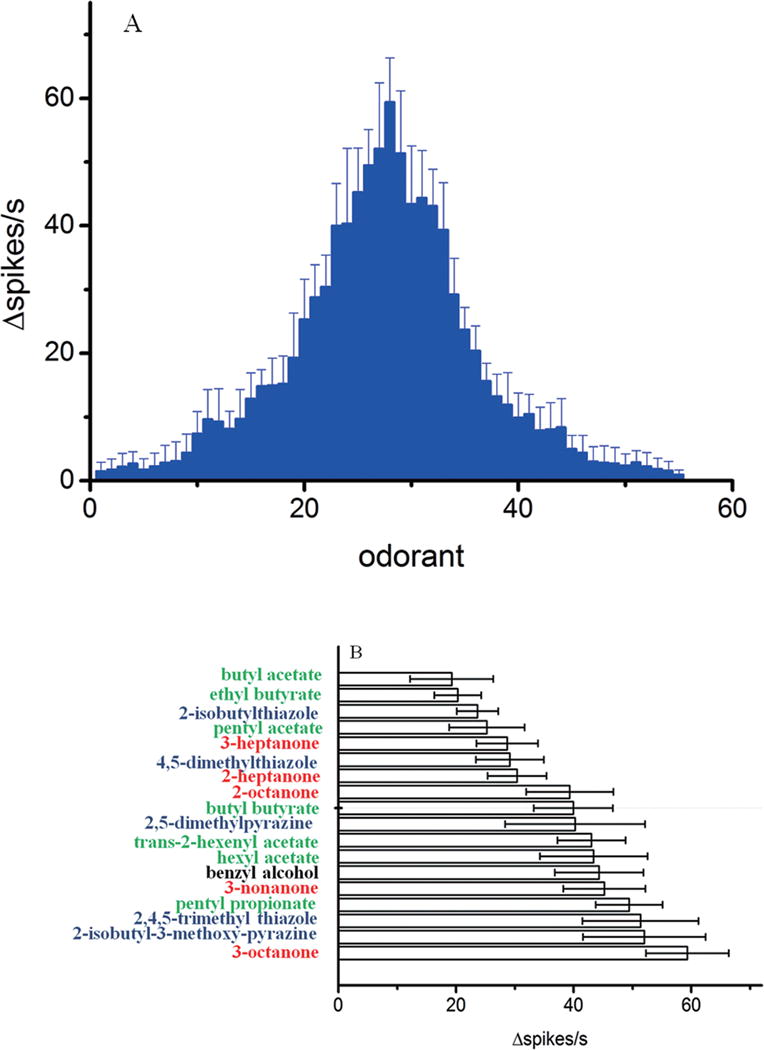
The electrophysiological responses of LmigOR3 to odorants in transgenic Drosophila aT1 system.
A. LmigOR3 was broadly tuned by 55 chemicals; B. 18 chemicals can result in responses of ≧20 △spikes/s. 185 chemicals were tested using single sensillum recordings. All the chemicals’ concentration used in this study is 10% (vol/vol). n = 6~7. Error bars = SEM.
3.3 LmigOR3 dose-dependently tunes to odorants in aT1 system of transgenic Drosophila
Next, we determined the dose/response relationships for the best activators of LmigOR3 in aT1 system. 14 chemicals that elicited high excitatory responses in the previous experiment were selected as stimuli to explore the dose-dependence responses of LmigOR3. The chemicals were serially diluted (10%, 1%, 0.1%, 0.01%, 0.001%; vol/vol) in paraffin oil. These dilutions were used to stimulate the aT1 sensilla from the transgenic flies. For heterocyclics, 2-isobutyl-3-methoxy-pyrazine can elicit stronger response than 2, 4, 5-trimethyl thiazole at the concentration of 10%, but the latter can elicit stronger response than the former at the lower concentration of 1% (Figure 3). For esters, pentyl propionate can elicit stronger responses than other tested esters at the concentration of 10% and no other tested esters can elicit strong responses at the lower concentration of 1% (Figure 4). For ketones, 3-octanone can elicit stronger response than other tested ketones at the concentration of 10% and no any ketone tested can elicit strong responses at the lower concentration of 1% (Figure 5). Benzyl alcohol also cannot elicit strong responses at the lower concentration of 1% (Figure 5). This means that the transgenic Drosophila aT1 system is suitable for analysis of locust odorant receptors. Besides, of all tested chemicals 2, 4, 5-trimethyl thiazole can elicit the strongest response at the concentration of 1%, which indicates it may be an effective activator for LmigOR3.
Figure 3.
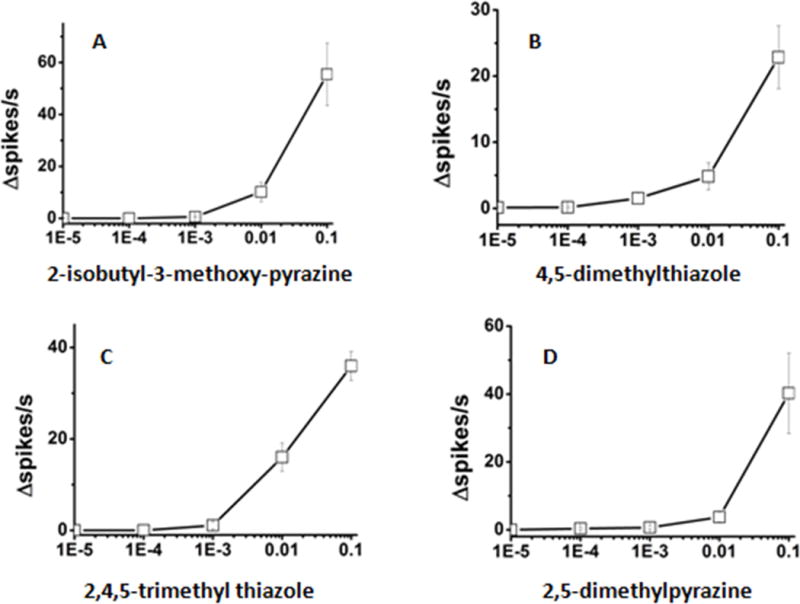
The dose-dependent electrophysiological responses of LmigOR3 to some heterocycles in transgenic Drosophila aT1 system.
(A) 2-isobutyl-3-methoxy-pyrazine; (B) 4, 5-dimethylthiazole; (C) 2, 4, 5-trimethyl thiazole; (D) 2, 5-dimethylpyrazine. 4 heterocycles were tested using single sensillum recordings. Every chemical here we used has five dilutions [10%, 1%, 0.1%, 0.01%, 0.001% (vol/vol)]. n = 6~11. Error bars = SEM.
Figure 4.
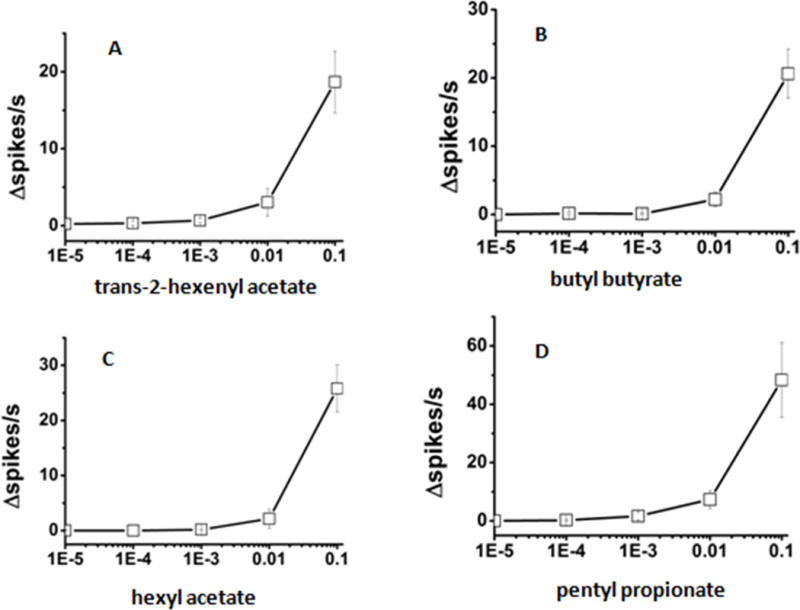
The dose-dependent electrophysiological responses of LmigOR3 to some esters in transgenic Drosophila aT1 system.
(A) trans-2-hexenyl acetate; (B) butyl butyrate; (C) hexyl acetate; (D) pentyl propionate. 4 esters were tested using single sensillum recordings. Every chemical here we used has five dilutions [10%, 1%, 0.1%, 0.01%, 0.001% (vol/vol)]. n = 6~11. Error bars = SEM.
Figure 5.
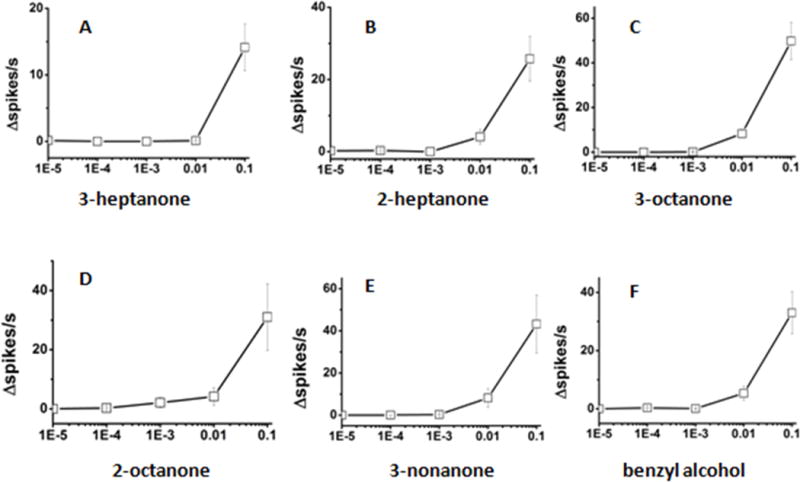
The dose-dependent electrophysiological responses of LmigOR3 to some ketones and benzyl alcohol in transgenic Drosophila aT1 system.
(A) 3-heptanone; (B) 2-heptanone; (C) 3-octanone; (D) 2-octanone; (E) 3-nonanone; (F) benzyl alcohol. 5 ketones and benzyl alcohol were tested using single sensillum recordings. Every chemical here we used has five dilutions [10%, 1%, 0.1%, 0.01%, 0.001% (vol/vol)]. n = 6~11. Error bars = SEM.
4 Discussion
LmigOR3 from locust was demonstrated previously to be specifically expressed in the antennae at high levels at all developmental stages (Xu et al., 2013). In this study we localized LmigOR3 in neurons of trichoid sensilla, making this the first odorant receptor identified that is expressed in the locust trichoid senilla neurons. Our in situ hybridization experiments showed that neurons expressing LmigOR3 are rare in sections which were abundantly positive for two other receptors, LmigOR1 and LmigOR2, expressed in basiconic sensilla (Xu et al., 2013).
The properties of a receptor repertoire in Drosophila adult and larvae were systematically investigated by ectopically expressing receptors in ab3A neurons (Hallem et al., 2004; Hallem and Carlson, 2006; Kreher et al., 2005). Combinatorial coding, that is one OR can sense multiple chemicals and one chemical cue can be detected by multiple ORs was an important property in insects as well as mammals (Carey and Carlson, 2011). Odorant receptors in neurons of basiconica sensilla are, generally, broadly tuned receptors that detect odors from plants and fruits, whereas those expressed in neurons of trichoid sensilla are narrowly tuned receptors that detect important cues for social behaviors in most insects (van der Goes van Naters and Carlson, 2007). Or67d receptors of D. melanogaster in aT1 sensilla neurons specifically detected the male-specific pheromone, 11-cis-vaccenyl acetate (cVA) (Ha and Smith, 2006). Similarly, BmorOr1 expressed in the trichoid sensilla neurons in Bombyx mori specifically detected bombykol (Nakagawa et al., 2005; Sakurai et al., 2004; Syed et al., 2006; Syed et al., 2010). Our results showed that expression of LmigOR3 in the transgenic Drosophila aT1 system confers broad odorant sensitivity, with strong responses to at least 18 chemicals, including ketones, esters, heterocyclics and benzyl alcohol.
Some of chemicals that can elicit strong responses from LmigOR3, include 2, 5-dimethylpyrazine and 2-heptanone. 2-heptanone is the component of maize leaf volatiles (Buttery and Ling, 1984) as well as that of locust body volatiles (Li and Zhang, 2011). 2, 5-dimethylpyrazine is the component of locust body volatiles (Cui et al., 2011; Yu et al., 2007). Previously, 16 types of neurons in 7 subtypes of trichoid sensilla were identified by electrophysiology (at2-1, at2-2, at2-3, at2-4, at2-5, at3-1, at3-2) in locust antennae in response to 9 odors (octanal, hexanal, 2, 5-dimethylpyrazine, 2, 6, 6-trimethyl-2-cyclohexene-1, 4-dione, trans-2-hexenal, cyclohexanol, 2-heptanone, guaiacol, benzaldehyde) present in fecal volatiles in the locust (Cui et al., 2011). Interestingly, at3-1B strongly responded to 2-heptanone and 2, 5-dimethylpyrazine but did not respond to cyclohexanol. This response pattern in the locust is similar to that of LmigOR3 expressed in Drosophila (Figure S1). However, we cannot definitely confirm whether LmigOR3 is expressed in at3-1B or if there is another receptor in at3-1B with similar tuning characteristics. It would be interesting to test the additional odorants we identified that activate LmigOR3 using Drosophila on at3-1B neurons. Additional confirmation needs to be performed to test the activities of these same odorants that are active in LmigOR3 transgenic Drosophila aT1 system on locust antennal trichoid sensilla, because additional families of proteins, such as odorant-binding proteins (OBPs) (Pelosi et al., 2006), and sensory neuron membrane proteins (SNMPs) (Jin et al., 2008), are believed to be involved in odorant recognition. For instance, benzaldehyde elicited inhibitory responses for all trichoid sensilla in locusts (Cui et al., 2011), but we observed weak excitatory responses from LmigOR3 expressed in transgenic flies. It is possible LmigOR3 is expressed in trichoid sensilla that have escaped previous characterization, or that the response in vivo relies on additional factors, such as OBPs (Grosse-Wilde et al., 2007; Laughin et al., 2008; Leal, 2013). It would be interesting to utilize RNAi to knockdown LmigOR3 gene in locust to further explore its function, in combination with behavioral experiments in the future.
In the transgenic system, aT1 neurons expressing LmigOR3 responded to 3-octanone which was the most active odorant (△spikes/s=59.32±7.06) at the concentration of 10%. When BmorOR1 was transformed into the Drosophila ab3A empty neuron system, bombykol could only elicit low activity (△spikes/s≤60), unlike food odorant receptors expressed in these neurons that could elicit much higher activity (up to 250△spikes/s) (Syed et al., 2006). Similarly, low activity (△spikes/s≤60) was observed when the sex pheromone cVA was applied to ab3A neurons expressing Or67d receptors, but much stronger activity is produced when these receptors are expressed in trichoid neurons (Ha and Smith, 2006; Kurtovic et al., 2007). LmigOR3 locust receptors are functional in the Drosophila aT1 system, and this system will be useful to analyze the odorant specificities of additional orphan receptors expressed in this agricultural pest.
In conclusion, we describe the first functional analysis of an odorant receptor from any member of the Orthoptera using the transgenic Drosophila aT1 system. We showed that LmigOR3 is localized to neurons localized in trichoid sensilla of the locust antenna and that it is a broadly tuned odorant receptor. Future studies will evaluate the biological role of this receptor in vivo.
Supplementary Material
Highlights.
LmigOR3, an odorant receptor identified from locust, is localized in chemosensory neurons of trichoid sensilla.
LmigOR3 is successfully transferred and expressed in the chemosensory neuron (aT1) in Drosophila antennal trichoid sensillum.
In the Drosophila aT1 system LmigOR3 is strongly activated by some ketones, esters and heterocyclics, exhibiting a broad-tuning feature.
Drosophila aT1 system is suitable for functional analysis of locust odorant receptors.
Acknowledgments
We would like to thank reviewers for their helpful comments and suggestions. This work is supported by the grants from National Natural Science Foundation of China (No.31472037 and No.31471762) and NIH R01DC011751 (USA).
Abbreviations
- LmigOR3
Locusta migratoria odorant receptor 3
- aT1
antenna trichoid sensilla 1
- cVA
11-cis-vaccenyl acetate
- ORN
olfactory receptor neuron
- C7
7 carbon atoms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Butenandt A, Beckmann R, Stamm D, Hecker E. Über den Sexuallockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z Naturforsch. 1959;14b:283–284. [Google Scholar]
- Buttery RG, Ling LC. Corn leaf volatiles: identification using Tenax trapping for possible insect attractants. J Agric Food Chem. 1984;32:1104–1106. [Google Scholar]
- Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proc Natl Acad Sci USA. 2011;108:12987–95. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wu C, Zhang L. Electrophysiological response patterns of 16 olfactory neurons from the trichoid sensilla to odorant from fecal volatiles in the locust, Locusta migratoria manilensis. Arch Insect Biochem Physiol. 2011;77:45–57. doi: 10.1002/arch.20420. [DOI] [PubMed] [Google Scholar]
- Dweck HK, Ebrahim SA, Thoma M, Mohamed AA, Keesey IW, Trona F, Lavista-Llanos S, Svatoš A, Sachse S, Knaden M, Hansson BS. Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci USA. 2015;112:E2829–35. doi: 10.1073/pnas.1504527112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde E, Gohl T, Bouché E, Breer H, Krieger J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur J Neurosci. 2007;25:2364–2373. doi: 10.1111/j.1460-9568.2007.05512.x. [DOI] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–33. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–60. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–79. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hansson BS, Stensmyr MC. Evolution of insect olfaction. Neuron. 2011;72:698–711. doi: 10.1016/j.neuron.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hassanali A, Njagi PG, Bashir MO. Chemical ecology of locusts and related acridids. Annu Rev Entomol. 2005;50:223–45. doi: 10.1146/annurev.ento.50.071803.130345. [DOI] [PubMed] [Google Scholar]
- Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105:10996–11001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–56. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–6. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DN, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang L. Indication of bioactive candidates among body volatiles of gregarious adult locusts Locusta migratoria manilensis by electroantennography (EAG) test. Afr J Biotechnol. 2011;10:9170–9176. [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–42. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- Ochieng SA, Hallberg E, Hansson BS. Fine structure and distribution of antennal sensilla of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae) Cell Tissue Res. 1998;291:525–36. doi: 10.1007/s004410051022. [DOI] [PubMed] [Google Scholar]
- Ochieng SA, Hansson BS. Responses of olfactory receptor neurones to behaviourally important odours in gregarious and solitarious desert locust, Schistocerca gregaria. Physiol Entomol. 1999;24:28–36. [Google Scholar]
- Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronderos DS, Smith DP. Activation of the T1 neuronal circuit is necessary and sufficient to induce sexually dimorphic mating behavior in Drosophila melanogaster. J Neurosci. 2010;30:2595–9. doi: 10.1523/JNEUROSCI.4819-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhara K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc Natl Acad Sci USA. 2004;101:16653–8. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–6. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–80. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Syed Z, Ishida Y, Taylor K, Kimbrell DA, Leal WS. Pheromone reception in fruit flies expressing a moth’s odorant receptor. Proc Natl Acad Sci USA. 2006;103:16538–43. doi: 10.1073/pnas.0607874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Kopp A, Kimbrell DA, Leal WS. Bombykol receptors in the silkworm moth and the fruit fly. Proc Natl Acad Sci USA. 2010;107:9436–9. doi: 10.1073/pnas.1003881107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–12. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:4418–23. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang P, Chen D, Jiang F, Li Y, Wang X, Kang L. Identification and functional analysis of olfactory receptor family reveal unusual characteristics of the olfactory system in the migratory locust. Cell Mol Life Sci. 2015;72:4429–43. doi: 10.1007/s00018-015-2009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–11. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Xu H, Guo M, Yang Y, You Y, Zhang L. Differential expression of two novel odorant receptors in the locust (Locusta migratoria) BMC Neurosci. 2013;14:50. doi: 10.1186/1471-2202-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Cui X, Jiang Q, Jin X, Guo Z, Zhao X, Bi Y, Zhang L. New isoforms of odorant-binding proteins and potential semiochemicals of locusts. Arch Insect Biochem Physiol. 2007;65:39–49. doi: 10.1002/arch.20176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


