Abstract
Purpose of review
This paper focuses on the sleep disorders in patients with spinal cord injury (SCI/D), particularly mechanism of sleep disordered breathing (SDB) and challenges in diagnosis and management. Based on a review of recent literatures and studies the paper summarizes some main challenges with respect to management of SDB in patients with SCI; and what are the responsible mechanisms of disease? What are the barriers in diagnosing and treating SDB using standard treatment such as positive airway pressure (CPAP)?.
Recent findings
Previous studies have shown that most SCI/D patients have SDB with heterogeneity in prevalence mainly related to using different definition or methods of diagnosing SDB, while recent studies using new definition of SDB based on recommended criteria from the American Academy of Sleep Medicine (AASM) and also include the data on effect of SCI/D level on prevalence and describe different type of SDB. Furthermore, recent data describes simplified method of diagnosing SDB by using a combination of home sleep apnea testing and transcutaneous CO2 monitoring. Finally, emerging data has been pointing at strong relationship between SDB and cardiovascular disease including nocturnal hypertension in patients with SCI/D.
Summary
The findings indicate that early testing for SDB and associated cardiovascular disease in patients with SCI is recommended and could be beneficial in reduced the high morbidity and mortality in this group of patients with disability. In addition, studies on treatment of other sleep disorders in SCI/D are not available to inform clinical decision making. Understanding the pathophysiology of sleep disorders in SCI/D is critical for the development of new effective therapies. This review provides evidence for best practices; highlights new discoveries for the diagnosis and management of sleep disorders in SCI/D, and discuss challenges and future directions.
Keywords: sleep-disordered breathing, sleep disturbances, periodic legs movements, spinal cord injury, tetraplegia
Introduction
Spinal cord injury/disorder (SCI/D) is the second leading cause for paralysis in the United States after stroke (41). It is estimated that 1,275,000 persons in the United States are living with SCI/D (1) with approximately 12,400 new SCI/Ds a year (2). Most SCI/D occurs during the second or third decade of life, leading to a major impact on young individuals in the community. The life expectancy for those who survive after the first year approaches the normal population and as a result, chronic illnesses impose significant health consequences and increased cost for many years. SCI/D alone costs roughly $40.5 billion annually (a study sponsored by the Christopher and Dana Reeve Foundation- date accessed is on 8/8/2016) (47), and managing comorbid disorders, including sleep disorders, in this patient population can be challenging.
Overview of sleep disturbances in individuals with spinal cord injury (SCI)
The majority of SCI individuals report poor sleep quality and increased rates of sleep disturbances compared to the general population, including patients with other neurological disorders that share similar morbidity (3). The mechanisms underlying sleep disturbances in SCI are multi-factorial and may include pain, bladder dysfunction, depression, circadian dysfunction and medication use. Furthermore, sleep disturbances may exacerbate chronic complaints such as depressed mood and impaired cognitive performance. Therefore, the relationship between sleep disturbance and co-morbid conditions is bidirectional and requires a comprehensive approach that addresses sleep and chronic conditions simultaneously.
Insomnia is a common sleep disorder, occurring in 10% of healthy adults has not been well-studied among SCI/D patients(4). One study found that 22% of individuals with multiple sclerosis met diagnostic criteria for insomnia disorder;(5) however, rates in patients with spinal cord injuries are not known. Some studies suggest that insomnia may contribute to depression and poor quality of life (6). Also, studies show that insomnia is common among other patient populations after injury (e.g., traumatic brain injury)(7), and there is some evidence that these patients can benefit from adapted versions of standard treatment of insomnia(8).
Abnormal leg movements, including periodic legs movements (PLM) and restless legs syndrome (RLS), contribute to disrupted sleep. The prevalence in the general population is estimated to be 4% and 5.5% for PLM and RLS respectively (9). Emerging data suggest that the prevalence of PLMs is higher in patients with SCI relative to the general population (10, 11). In a recent study, Peters et al. (12) found that more than 40 % of patients with tetraplegia had PLMs, which were evident during REM and NREM sleep as well as wakefulness. Interestingly, one study reported that physical activity improved both PLM and RLS severity in a group of volunteers with low thoracic complete SCI (T7-11), which was thought to be due to increased release of endorphins after physical activity (10). Overall, it appears that PLMs are common in patients with SCI, independent of OSA, and may contribute to poor nocturnal sleep.
The mechanisms of sleep disturbances may also vary by the level of injury. Specifically, nocturnal melatonin secretion is diminished, or absent, in patients with complete cervical SCI (13), owing to the interruption of sympathetic innervation to the pineal gland. This may therefore suggest circadian disruption as a contributory factor to poor overall sleep. However, the lack of a therapeutic benefit following the administration of melatonin or melatonin receptor agonists in patients with tetraplegia underscores the multi-factorial etiology of sleep disturbances in this population and the need to address the full continuum of factors that may disturb sleep(14).
Sleep-disordered breathing (SDB) is a common cause of sleep disturbances in individuals living with SCI/D. The prevalence of SDB is two to fourfold higher than the general population (15–18), likely making it the most common sleep disorder in this patient population. There is evidence that SDB develops within a few months following the injury (15), indicating that SCI/D may be an independent risk factor for the development of SDB. However, SDB remains woefully under recognized and under-diagnosed in patients with SCI/D despite the recognized adverse consequences of SDB and the high frequency of sleep disturbances in SCI/D patients (19). One reason that SDB is critically important in these patients is that cardiopulmonary complications are major causes of morbidity and mortality in SCI/D patients because of decreased lung volume, ineffective cough, mucus retention and atelectasis (20, 21). In fact, cardiovascular conditions have superseded respiratory causes of death in cervical SCI/D patients and have become the most common cause for mortality in this disabled population (22). On the basis of the extensive literature linking untreated SDB to cardiovascular mortality, SDB may represent a treatable risk factor among SCI/D patients. The following discussion will focus on the etiology, manifestations and consequences of SDB in this population.
Epidemiology of SDB in patients with SCI/D
The prevalence of SDB in sub-acute and chronic SCI population depends on the threshold of diagnosis based on an index called apnea-hypopnea index (AHI). This index consists of two types of events: apneas and hypopneas. Apneas are complete cessation of airflow, and could be further classified into obstructive apneas caused by complete recurrent upper airway obstruction, despite persistent respiratory (can’t breathe), and central apneas caused by periodic cessation of respiratory effort (won’t breathe). These two types of apnea often co-exist in individual patients. The other types of respiratory events are hypopneas, which represent decrease flow and hence decreased alveolar ventilation. While hypopneas can also be classified as obstructive or central; the distinction is often difficult on a clinical polysomnogram (PSG). The apnea/hypopnea index (AHI) is a composite index including the total number of apnea and hypopneas per hour of sleep. Importantly, patients with SCI/D often experience a combination of these respiratory events.
Several epidemiological studies have found a high prevalence of SDB in sub-acute and chronic SCI patients (ranging between 27% and 82%) (15–18, 23, 24). The heterogeneity in the estimated prevalence is related to multiple factors, including testing patents with different levels of SCI, using different modes of testing from nocturnal oximetry, portable monitoring or in-laboratory polysomnography, and using different thresholds of apnea-hypopnea index and different definition of respiratory events (especially hypopnea events). Use of limited recordings may prevent recognition of respiratory events that can only be identified using PSG.
The type of SDB and prevalence may depend also on the level of injury. For example patients with cervical SCI have higher prevalence of SDB than thoracic SCI (16). In a study by Berlowitz et al, (2005) (15) it was found that the prevalence of SDB in an Australian cohort of cervical SCI was 62% in the four weeks immediately post-injury and remained 60% after one-year follow-up. Respiratory complications are the major causes of morbidity and mortality in patients with SCI particularly at the cervical level (25). Our group has been studying SDB among SCI patients, and, we have found that SDB, defined by the AHI ⩾5 events per hour, was present in 77% of chronic SCI patients, with rates higher among those with cervical compared with thoracic injuries (93% vs 55%, respectively, P<0.05) (16). We also found that one in four cervical SCI patients had a Cheyne–Stokes respiration pattern during their overnight in-laboratory sleep study.
Mechanisms of SDB in SCI- the perfect storm
The complex mechanisms responsible for increased prevalence of SDB in patients with SCI include anatomic and neuromuscular factors, as well as factors related to the injury per se, such as the level and time since the injury. The development of SDB can be directly attributed to SCI, as it can occur as early as 2 weeks following acute cervical spinal injury. Several factors may play an important role in the pathogenesis of SDB in SCI including, level and completeness of injury (26, 27), concomitant neuromuscular weakness (25), large neck size(15), increased upper airway collapsibility (28), exposure to chronic intermittent hypoxia (29, 30), presence of sleep-related hypoventilation (26), and the use of medications that affect respiration such muscle relaxants, sedatives and opioids (17).
To understand the etiology of SDB in patients with SCI, it is important to recognize that sleep is a physiologic challenge, rather than relief, for the respiratory system. Several physiologic changes increase the propensity to develop apnea or hypopneas during sleep, and this propensity may be higher in SCI/D patients than in adults without SCI/D.
First, reduced activity of upper airway dilators is associated with upper airway narrowing and increased upper airway resistance. Snoring occurs when upper airway resistance increases significantly, leading to “fluttering” of the soft palate due to turbulent flow. In extreme cases of upper airway narrowing, complete closure may occur, leading to obstructive sleep apnea. Second, the sleep state (specifically non-rapid eye movement or NREM sleep) removes the wakefulness “drive to breathe”, rendering respiration critically dependent on PaCO2 (arterial CO2). Specifically, NREM sleep unmasks the hypocapnic apneic threshold; thus, central apnea occurs if arterial PaCO2 falls a highly sensitive “apneic threshold”. Overall, the sleep-related upper airway narrowing and unmasking of the apneic threshold are critical sleep effects that predisposes to the development of obstructive and central apnea respectively.
The physiologic effects of sleep may be well tolerated in healthy individuals; however, patients with SCI - especially cervical SCI - suffer from the full continuum of derangements that impair the ability of the ventilatory system to compensate for physiologic challenges of sleep, including neuromuscular weakness, small lung volume, abnormal chest wall mechanics, frequent use of CNS suppressants and an unopposed parasympathetic system promoting airway narrowing. This may explain the very high prevalence of SDB in this population.
Sleep related hypoventilation is a universal physiologic phenomenon, but is more pronounced in patients with cervical SCI (26), given diminished ventilatory motor neural output to respiratory muscles, the presence of restrictive ventilatory defect, loss of sympathetic innervations and impaired respiratory mechanics. The aforementioned factors, combined with impaired load compensation and the use of analgesics/hypnotics result in worsening alveolar hypoventilation and ventilatory de-compensation. Thus, it is not surprising that SDB is common in sub-acute and chronic SCI patients.
Role of ventilatory control
Ventilatory control during sleep operates as a negative feedback closed-loop cycle, often described in using the engineering concept of “loop gain” as a framework for breathing instability (31). The propensity to central apnea during NREM sleep is determined by an interaction between the response of the brain and chemoreceptors to changing PETCO2, representing the controller, and the effectiveness of the lung/respiratory system in lowering PETCO2 in response to hyperventilation (the plant).
The occurrence of central apnea may initiate several processes that promote further instability including inertia of the ventilatory control system, hypoxia and transient arousal, with ensuing ventilatory overshoot, hypocapnia and recurrent central apnea. A less recognized phenomenon is that central apnea may also influence the development of obstructive sleep apnea (OSA). There is evidence that patients with unfavorable upper airway anatomy are dependent on ventilatory motor output to preserve upper airway patency, with pharyngeal narrowing and/or occlusion occurring during central apnea (32).
We recently found that more than 90% cervical SCI patients demonstrated SDB, with the majority demonstrating central SDB, not explained by daytime hypoventilation, cardiac dysfunction or use of narcotics(16). The SDB events in cervical SCI patients were predominantly central while events in the thoracic SCI group were obstructive (16). This unique observation may have significant implications regarding the mechanism of SDB in patients with SCI. Recent data in animal SCI models showed that cervical SCI predisposes to alterations in ventilatory motor output suggesting an important role for breathing instability in the development of SDB in SCI patients (33). To determine the susceptibility to central apnea in cervical and thoracic SCI compared to able-bodied controls we induced hyperventilation using non-invasive ventilation in chronic SCI patients and measured the CO2 reserve (a marker of susceptibility to central apnea), plant grain and ventilation during sleep (34). We found that the CO2 reserve was narrower in cervical SCI patients compared to thoracic SCI patients and able-bodied comparison subjects, and plant gain was increased in cervical SCI compared to the thoracic and able-bodied subjects. Specifically, we have found that chronic cervical SCI patients demonstrate a central periodic breathing pattern with spontaneously occurring central apnea, that is, a very close proximity between eupneic PETCO2 (end-tidal CO2) and the hypocapnic apneic threshold. Despite this, many cervical SCI patients in the supine position can maintain minute ventilation with a normal respiratory rate and tidal volume (16). In contrast, these patients will often have significantly decreased tidal volume when they transition from wake to sleep (26).
Upper airway collapsibility
Increased upper airway (UA) collapsibility plays an important role in the pathogenesis of SDB in able-bodied individuals (35, 36). Available evidence suggests that individuals living with thoracic or lower SCI are at increased risk of SDB, mostly obstructive sleep apnea, due to higher prevalence of the same risk factors of OSA in the general population, such as obesity and male gender (15). In contrast, the etiology of SDB in patients with cervical SCI is particularly complex, as these individuals have risk factors for UA obstruction as well as increased propensity to recurrent central apnea during sleep. We have recently demonstrated that the SCI individuals exhibit elevated passive critical closing pressure (Pcrit), indicative of increased upper airway collapsibility (28). However, upper airway resistance was similar between the three groups. However, similar increase in the inspiratory duty cycle was noted in chronic SCI and was independent of the level of injury. Furthermore, it was reported recently that nasal resistance is elevated in people with cervical SCI which is believed to be due to unopposed parasympathetic activity (37). But the mechanistic contribution of increased nasal resistance to UA collapsibility is unclear.
Neuromuscular output
While high cervical SCI leads to severe impairment of the respiratory muscles including the diaphragm, mid and low cervical lesions spare most respiratory muscles (38). Specifically, decreased ventilatory neuromuscular output caused by intercostal muscle paralysis play an important role in the development of sleep apnea shortly post-injury to the cervical spine, while increased chemoreflex sensitivity and control of breathing mechanisms rostral to the injury level may contribute to the residual neuromuscular impairment and reduced lung volume in chronic tetraplegia when ventilation is more dependent on O2 and CO2 levels (39). As illustrated in figure 1, pronounced sleep-related hypoventilation occurs in cervical SCI patients (26), secondary to loss of intercostal muscle activity. Hypoventilation, combined with enhanced chemoreflex sensitivity may contribute to respiratory instability by increasing plant gain and controller gain respectively.
Figure 1.
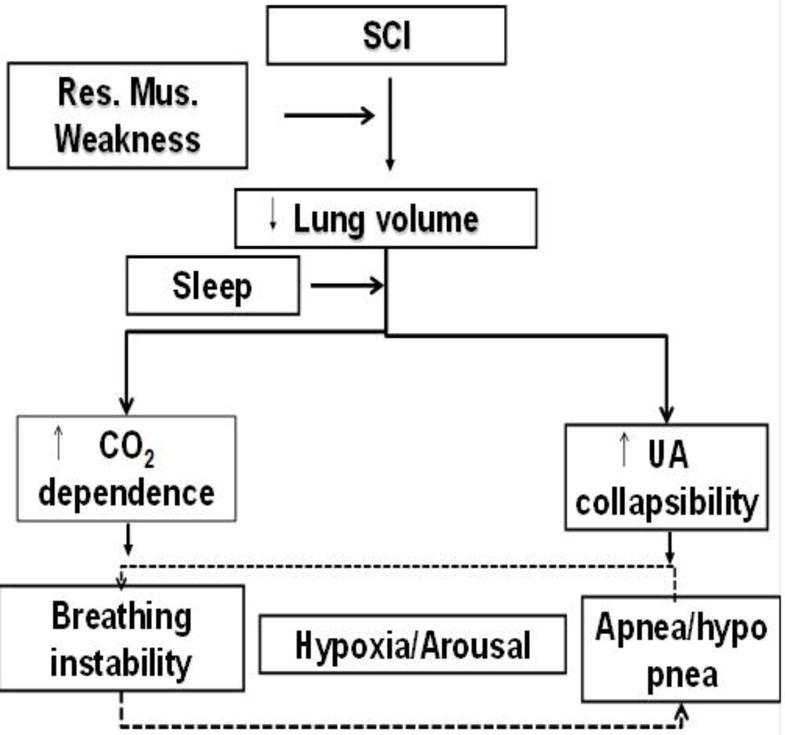
A diagram that illustrates the contributing mechanisms of sleep disordered breathing in patients with spinal cord injury (SCI).
Clinical implications of SDB in SCI patients
There is a strong relationship between SDB and cardiovascular disease in SCI. One in five of persons with SCI has hypertension or cardiac disease (19). Furthermore, cardiac medication use has been found to be higher in tetraplegic patients who have SDB which may implicate a link between SDB and cardiovascular morbidities in SCI patients (23). In a retrospective study of SCI patients, those with cervical injuries were found to have more reversed dipping (i.e., higher blood pressure at night rather than during the day) and nocturnal hypertension compared to those with thoracic injuries (40). The strong relationship between untreated SDB and nocturnal hypertension had been repeatedly confirmed in able-bodied individuals with SDB (41, 42). Ambulatory BP monitoring allows accurate assessment of circadian BP changes, which can identify a blunted nocturnal decline in BP and indicate the possible secondary cause of hypertension, such as sleep apnea. Specifically, the non-dipping BP phenomenon measured by ambulatory BP monitoring was found in to be predominant in patients with acute cervical SCI (43) and in untreated able bodied patients with SDB and was associated with poor outcome. Importantly, this phenomenon can be improved with CPAP treatment (44). However, long term outcome data on the effect of untreated SDB in individuals with SCI are lacking, in part due to challenges in use of PAP for patients with SCI.
Challenges in the Diagnosis and Treatment of SDB among SCI Patients
We recently reviewed the medical records of 168 Veterans with SCI (19) and found that only 37 patients (22%) had been evaluated for SDB of whom 34 (20%) had SDB diagnosis confirmed by an overnight sleep study. In other words, a clinical evaluation for possible sleep disorders was conducted in only one out of 5 patients, despite the overwhelming prevalence of sleep disorders in this population. One reason that SDB remains underdiagnosed in patients with SCI, especially cervical SCI, partly due to limited access to in-lab testing (45). Many sleep labs are simply not equipped to handle patients with limited mobility or those who cannot independently transfer in and out of bed.
However, the challenges in diagnosing SDB are also coupled with limited use of standard PAP therapy in this patient group. In the same study, we also found that of the 34 patients with diagnosed SDB, only 6 patients (18%) were using positive airway pressure (PAP) therapy. The treatment of choice for SDB is positive airway pressure (PAP) therapy. Adherence to PAP therapy, however, remains a challenge despite education, follow-up and support (46). Particularly since patients with SCI may be less adherent to PAP therapy than able-bodied individuals, alternative therapies beyond PAP are needed. Some factors that lead to discontinuation of therapy are related to weakness and mobility impairment to the upper extremities, mask claustrophobia, increased awakenings, nasal congestion, lack of education and inconvenience. Many of these can be mitigated through avoidance of large full-face masks and use nasal interface and PAP education.
Challenges in diagnosing a treating SDB may need to be addressed by educating both patients and providers. Given the well-recognized increased prevalence of SDB in SCI patients and its impact on quality of life and poor health outcomes including heart disease, stroke, hypertension and poor cognition. It is also important to address PAP adherence specifically in SCI/D patients and to develop and test novel treatments, other than PAP, for patients who are unable to tolerate this standard approach.
Conclusion
Sleep disturbances, particularly sleep disordered breathing, are common among individuals with SCI across different demographic and levels of spinal cord injuries. The mechanism(s) for increased prevalence of SDB after surviving SCI are not clear yet but evidence point toward complex pathways that include upper airway collapsibility, ventilatory instability, reduced lung volume, sleep related hypoventilation and neuromuscular weakness. Therefore, conventional therapies my not be fully accepted by these disabled patients with many chronic issues and individualized care is greatly needed. Further studies are required in human and pre-clinical models to elucidate the exact etiology of disease and identify therapeutic targets that are effective in improving symptoms and preventing resulting comorbidities.
Footnotes
Conflict of Interest
Abdulghani Sankari and Jennifer L. Martin each declare no potential conflicts of interest.
M. Safwan Badr is a section editor for Current Sleep Medicine Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Lasfargues J, Custis D, Morrone F, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Spinal Cord. 1995;33(2):62–8. doi: 10.1038/sc.1995.16. [DOI] [PubMed] [Google Scholar]
- 2.Devivo M. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal cord. 2012;50(5):365–72. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 3.Fogelberg D, Hughes A, Vitiello M, Hoffman J, Amtmann D. Comparison of Sleep Problems in Individuals with Spinal Cord Injury and Multiple Sclerosis. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2015;12(5):695–701. doi: 10.5664/jcsm.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X-L, Zheng X-Y, Yang J, Ye C-P, Chen Y-Y, Zhang Z-G, Xiao Z-J. A systematic review of studies on the prevalence of insomnia in university students. Public health. 2015;129(12):1579–84. doi: 10.1016/j.puhe.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Viana P, Rodrigues E, Fernandes C, Matas A, Barreto R, Mendonça M, Peralta R, Geraldes R. InMS: Chronic insomnia disorder in multiple sclerosis–a Portuguese multicentre study on prevalence, subtypes, associated factors and impact on quality of life. Multiple sclerosis and related disorders. 2015;4(5):477–83. doi: 10.1016/j.msard.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Williamson ML, Elliott TR. Major depressive disorder and factorial dimensions among individuals with recent-onset spinal cord injury. Rehabilitation psychology. 2013;58(1):10. doi: 10.1037/a0031265. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Mittal R, Sharma A, Sharma A, Gupta I. Study of insomnia and associated factors in traumatic brain injury. Asian journal of psychiatry. 2014;8:99–103. doi: 10.1016/j.ajp.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen S, McKay A, Wong D, Rajaratnam SM, Spitz G, Williams G, Mansfield D, Ponsford JL. Cognitive Behavior Therapy to Treat Sleep Disturbance and Fatigue After Traumatic Brain Injury: A Pilot Randomized Controlled Trial. Archives of physical medicine and rehabilitation. 2017;98(8):1508–17.e2. doi: 10.1016/j.apmr.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. Journal of Psychosomatic Research. 2002;53(1):547–54. doi: 10.1016/s0022-3999(02)00443-9. doi: http://dx.doi.org/10.1016/S0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 10.De Mello M, Lauro F, Silva A, Tufik S. Incidence of periodic leg movements and of the restless legs syndrome during sleep following acute physical activity in spinal cord injury subjects. Spinal Cord. 1996;34(5):294–6. doi: 10.1038/sc.1996.53. [DOI] [PubMed] [Google Scholar]
- 11.Yokota T, Hirose K, Tanabe H, Tsukagoshi H. Sleep-related periodic leg movements (nocturnal myoclonus) due to spinal cord lesion. Journal of the neurological sciences. 1991;104(1):13–8. doi: 10.1016/0022-510x(91)90210-x. [DOI] [PubMed] [Google Scholar]
- 12.Peters AEJ, van Silfhout L, Graco M, Schembri R, Thijssen D, Berlowitz DJ. Periodic Limb Movements in Tetraplegia. The Journal of Spinal Cord Medicine. 2017:1–8. doi: 10.1080/10790268.2017.1320874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheer FAJL, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2005;44(2):78–81. doi: 10.1038/sj.sc.3101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeitzer JM, Ku B, Ota D, Kiratli BJ. Randomized controlled trial of pharmacological replacement of melatonin for sleep disruption in individuals with tetraplegia. The journal of spinal cord medicine. 2014;37(1):46–53. doi: 10.1179/2045772313Y.0000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Archives of physical medicine and rehabilitation. 2005;86(6):1193–9. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 16*.Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2014;10(1):65. doi: 10.5664/jcsm.3362. This is a recent article that describes sleep disordered breathing in two levels of spinal cord injuries using full in laboratory polysomnography and pharyngeal pressure catheter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Bauman KA, Kurili A, Schotland HM, Rodriguez GM, Chiodo AE, Sitrin RG. A Simplified Approach to Diagnosing Sleep Disordered Breathing and Nocturnal Hypercapnia in Individuals with Spinal Cord Injury. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2015.07.026. This is a recent article that describes a simplified method to diagnose sleep disordered breathing using home sleep apnea testing and transcutaneous CO2 monitoring. [DOI] [PubMed] [Google Scholar]
- 18.Chiodo AE, Sitrin RG, Bauman KA. Sleep disordered breathing in spinal cord injury: A systematic review. The journal of spinal cord medicine. 2016;39(4):374–82. doi: 10.1080/10790268.2015.1126449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankari A, Martin J, Badr M. A retrospective review of sleep-disordered breathing, hypertenstion and cardiovascular diseases in spinal cord injury patients. Spinal cord. 2015;53(6):496. doi: 10.1038/sc.2015.16. [DOI] [PubMed] [Google Scholar]
- 20.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. American journal of physical medicine & rehabilitation. 2007;86(2):142–52. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 21.DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Archives of physical medicine and rehabilitation. 1993;74:248. [PubMed] [Google Scholar]
- 22.Weaver LC, Fleming JC, Mathias CJ, Krassioukov AV. Disordered cardiovascular control after spinal cord injury. Handb Clin Neurol. 2012;109:213–33. doi: 10.1016/B978-0-444-52137-8.00013-9. [DOI] [PubMed] [Google Scholar]
- 23.Stockhammer E, Tobon A, Michel F, Eser P, Scheuler W, Bauer W, Baumberger M, Müller W, Kakebeeke T, Knecht H. Characteristics of sleep apnea syndrome in tetraplegic patients. Spinal Cord. 2002;40(6):286. doi: 10.1038/sj.sc.3101301. [DOI] [PubMed] [Google Scholar]
- 24.Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea–hypopnea syndrome after cervical cord injury. Archives of physical medicine and rehabilitation. 2007;88(3):333–7. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. The Cochrane Library. 2013 doi: 10.1002/14651858.CD008507.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bascom AT, Sankari A, Goshgarian HG, Badr MS. Sleep onset hypoventilation in chronic spinal cord injury. Physiological reports. 2015;3(8):e12490. doi: 10.14814/phy2.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berlowitz DJ, Spong J, Gordon I, Howard ME, Brown DJ. Relationships between objective sleep indices and symptoms in a community sample of people with tetraplegia. Archives of physical medicine and rehabilitation. 2012;93(7):1246–52. doi: 10.1016/j.apmr.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Sankari A, Bascom AT, Badr MS. Upper airway mechanics in chronic spinal cord injury during sleep. Journal of Applied Physiology. 2014;116(11):1390–5. doi: 10.1152/japplphysiol.00139.2014. [DOI] [PubMed] [Google Scholar]
- 29.Bascom AT, Sankari A, Badr MS. Spinal cord injury is associated with enhanced peripheral chemoreflex sensitivity. Physiological Reports. 2016;4(17):e12948. doi: 10.14814/phy2.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Sankari A, Bascom AT, Riehani A, Badr MS. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. Journal of Applied Physiology. 2015;119(10):1183–93. doi: 10.1152/japplphysiol.00088.2015. This is a recent study that demonstrate the effect of intermittent hypoxia on ventilatory plasticity in three groups of individuals (cervical, thoracic SCI and able-bodied). This study demonstrates the highest plasticity ever described in humans following acute intermittent hypoxia during wakefulness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoo M, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. Journal of Applied Physiology. 1982;53(3):644–59. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 32.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. Journal of Applied Physiology. 1995;78(5):1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 33.Zimmer MB, Goshgarian HG. Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Experimental Neurology. 2007;206(1):137–45. doi: 10.1016/j.expneurol.2007.05.003. doi: http://dx.doi.org/10.1016/j.expneurol.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Sankari A, Bascom A, Chowdhuri S, Badr M. Tetraplegia is a risk factor for central sleep apnea (vol 116, pg 345, 2014) JOURNAL OF APPLIED PHYSIOLOGY. 2014;117(8):940. doi: 10.1152/japplphysiol.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. Journal of applied physiology. 2007;102(2):547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 36.Schneider H, Krishnan V, Pichard L, Patil S, Smith P, Schwartz A. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. European Respiratory Journal. 2009;33(5):1068–76. doi: 10.1183/09031936.00063008. [DOI] [PubMed] [Google Scholar]
- 37.Gainche L, Berlowitz DJ, LeGuen M, Ruehland WR, O’Donoghue FJ, Trinder J, Graco M, Schembri R, Eckert DJ, Rochford PD. Nasal resistance is elevated in people with tetraplegia and is reduced by topical sympathomimetic administration. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2016;12(11):1487. doi: 10.5664/jcsm.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. CHEST Journal. 1990;97(6):1446–52. doi: 10.1378/chest.97.6.1446. [DOI] [PubMed] [Google Scholar]
- 39.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respiratory care. 2006;51(8):853–70. [PMC free article] [PubMed] [Google Scholar]
- 40.Goh M, Wong E, Millard M, Brown DJ, O’Callaghan CJ. A retrospective review of the ambulatory blood pressure patterns and diurnal urine production in subgroups of spinal cord injured patients. Spinal cord. 2015;53(1):49–53. doi: 10.1038/sc.2014.192. [DOI] [PubMed] [Google Scholar]
- 41.Martínez-García M, Capote F, Campos-Rodríguez F, et al. Effect of cpap on blood pressure in patients with obstructive sleep apnea and resistant hypertension: The hiparco randomized clinical trial. JAMA. 2013;310(22):2407–15. doi: 10.1001/jama.2013.281250.. [DOI] [PubMed] [Google Scholar]
- 42.Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Current Respiratory Medicine Reviews. 2007;3(4):258–69. [Google Scholar]
- 43.Goh MY, Millard MS, Wong EC, Brown DJ, Frauman AG, O’Callaghan CJ. Diurnal blood pressure and urine production in acute spinal cord injury compared with controls. Spinal Cord. 2017;55(1):39–46. doi: 10.1038/sc.2016.100. Epub 2016/06/28. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-García MA, Gómez-Aldaraví R, Soler-Cataluña J-J, Martínez TG, Bernácer-Alpera B, Román-Sánchez P. Positive effect of CPAP treatment on the control of difficult-to-treat hypertension. European Respiratory Journal. 2007;29(5):951–7. doi: 10.1183/09031936.00048606. [DOI] [PubMed] [Google Scholar]
- 45.Sankari A, Martin J, Bascom A, Mitchell M, Badr M. Identification and treatment of sleep-disordered breathing in chronic spinal cord injury. Spinal cord. 2015;53(2):145–9. doi: 10.1038/sc.2014.216. [DOI] [PubMed] [Google Scholar]
- 46.Balachandran JS, Yu X, Wroblewski K, Mokhlesi B. A brief survey of patients’ first impression after CPAP titration predicts future CPAP adherence: a pilot study. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(3):199. doi: 10.5664/jcsm.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeve Foundation Paralysis Resource Center. Spinal Cord Injury Paralysis Resource Center. Last accessed January 12, 2017. http://www.christopherreeve.org/site/c.mtKZKgMWKwG/b.5184189/k.5587/Paralysis_Facts__Figures.htm.


