Abstract
Purpose of review
To present an overview on the main agents (i.e., biomolecules and nanocompounds) and/or strategies currently available to amplify or stabilize resin-dentin bonding.
Recent findings
According to studies retrieved for full text reading (2014–2017), there are currently six major strategies available to overcome resin-dentin bond degradation: (i) use of collagen crosslinking agents, which may form stable covalent bonds with collagen fibrils, thus strengthening the hybrid layer; (ii) use of antioxidants, which may allow further polymerization reactions over time; (iii) use of protease inhibitors, which may inhibit or inactivate metalloproteinases; (iv) modification of the bonding procedure, which may be performed by using the ethanol wet-bonding technique or by applying an additional adhesive (hydrophobic) coating, thereby strengthening the hybrid layer; (v) laser treatment of the substrate prior to bonding, which may cause specific topographic changes in the surface of dental substrates, increasing bonding efficacy; and (vi) reinforcement of the resin matrix with inorganic fillers and/or remineralizing agents, which may positively enhance physico-mechanical properties of the hybrid layer.
Summary
With the present review, we contributed to the better understanding of adhesion concepts and mechanisms of resin-dentin bond degradation, showing the current prospects available to solve that problematic. Also, adhesively-bonded restorations may be benefited by the use of some biomolecules, nanocompounds or alternative bonding strategies in order to minimize bond strength degradation.
Keywords: Matrix Metalloproteinase Inhibitors, Collagen, Resins, Crosslinking Agents, Dentin-Bonding, Dentin
Introduction
Success in adhesive dentistry means long-lasting restorations. Notwithstanding, the loss of adhesion or retention of a resin composite restoration is a frequent problem observed by dental practitioners, especially due to bond strength degradation (1). In fact, this may occur based on two distinct series of events, one that starts naturally during hybridization/bonding procedures (collagen degradation), and the second that occurs after polymerization of the adhesive resin (adhesive degradation). Both events are responsible for compromising the integrity of the hybrid layer and reducing resin-dentin bond over time (2, 3).
Adhesion to dental substrates starts with an acid-etching step, which means that enamel or dentin would undergo surface demineralization. For enamel, the prisms that form its highly-mineralized structure would be selectively removed, leaving a porous/micro-retentive surface favorable to resin infiltration, which, in turn, results in stable resin-enamel bonds (4). On the other hand, dentin possesses a more complex composition (organic/inorganic content) and a heterogeneous morphology when compared to enamel, so that acid-etching would not only produce micro-retentions if applied to dentin, but would also result in collagen fibrils’ exposure (2, 4). This event activates bound matrix metalloproteinases−2, −3, −8, −9, and −20 (MMPs), and cysteine cathepsins (CCs), which will slowly degrade the collagen fibrils (5–9), making it difficult to achieve stable resin-dentin bonds.
Several studies have investigated the degradation mechanism of dental adhesive interfaces (2–4). Collectively, it seems that, to eliminate collagen degradation, any denuded collagen fibril that originated during acid-etching should be completely protected with resin monomers, thus preventing activation of MMPs and CCs. Even for self-etching adhesives in which surface demineralization and resin infiltration occur simultaneously, collagen degradation would be an unavoidable process due to incomplete collagen protection by monomers. Worth mentioning, bonding with most of the adhesive systems available today usually results in partially-infiltrated collagen fibrils, thus favoring degradation.
Generally speaking, dental adhesives are comprised of methacrylate-based monomers that present hydrolytically susceptible groups (e.g., ester, urethane, hydroxyl, carboxyl, phosphate) (10, 11), thereby compromising integrity of the adhesive layer. Currently, there are some chemicals/agents or bonding protocols available that increase or stabilize bond strength between resin-based materials and dental substrates, which will hereafter be discussed. Indeed, the literature was reviewed aiming to present the most recent advances in adhesive dentistry as a manner of improving the durability of adhesively-bonded resin composite restorations. With the present review, we expect to contribute to the better understanding of adhesion concepts and mechanisms of resin-dentin bond degradation, as well provide for future prospects in the field of adhesive dentistry.
Literature Search
The present review was performed in order to verify current strategies available to increase resin-dentin/enamel bonds or prevent bond strength reduction/degradation over time. The search was performed using distinct electronic databases (e.g., PubMed, ISI Web of Knowledge, and Scopus) using the following keywords: dental, adhesive, system, bond, and strength. The articles were screened according to their year of publication (2014 to March 2017). This criterion was chosen to report solely the relevant findings published over the past three years. Any study that reported on increased bond strength or stable resin-dentin bond over time was retrieved in full text. Studies that did not evaluate adhesion by means of bond strength tests were not included for data extraction, but they were analyzed for further discussion. Studies that reviewed the literature were also considered and used to collect relevant information for the search. Studies found during the screening process that presented negative results (e.g., contradictory findings to other included studies) were not used to collect data/information, but they were discussed throughout the text for critical consideration of the review topic.
Division of Studies
Each retrieved study was separated into two main groups, according to the major mechanism used to increase bond strength or prevent degradation of resin-dentin bond over time. The former group (Group 1) allocated the studies that investigated the effects of collagen crosslinking agents, antioxidants, or protease inhibitors on the increase/stabilization of resin- dentin bonds. The latter group (Group 2) allocated the studies that improved resin- dentin/enamel bonds by reinforcing the resin matrix with inorganic fillers/remineralizing agents, by applying laser on the substrate prior to bonding, or by modifying the bonding technique.
Data Extraction
For each study included in Groups 1 and 2, the data was recorded using a standardized form in Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). Data/information collected with studies in Group 1 were as follows: active agent used to increase/stabilize resin-dentin bonds, moment that the active agent was applied to the substrate, and main mechanism(s) of action. Data/information collected with studies in Group 2 were as follows: what was done to achieve increased bond strength or stability of resin-dentin/enamel bonds, and what were the main findings of the study.
Main Outcomes
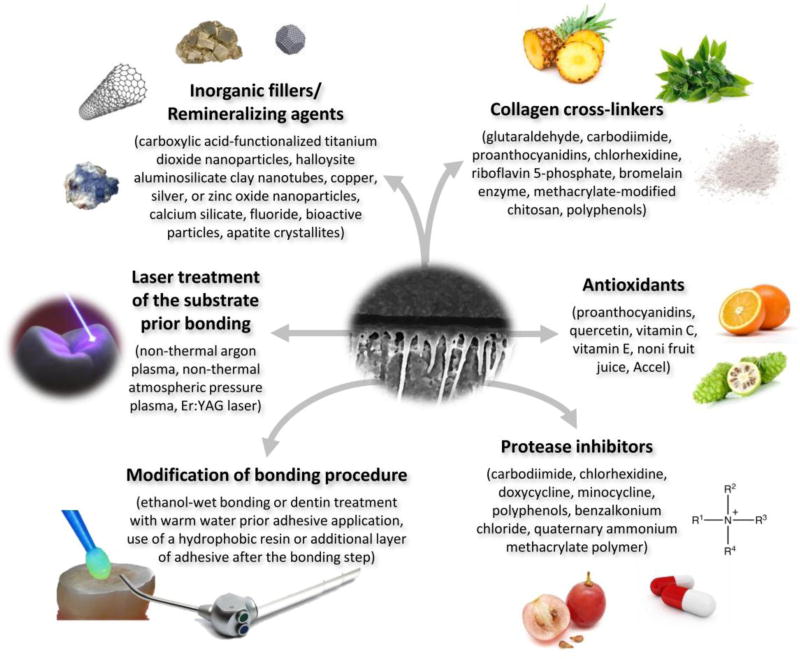
Over the past three years, several studies were published related to possible approaches available to overcome bond strength deterioration of adhesively-bonded restorations. According to the present review, there are currently six main strategies available (Figure): use of collagen crosslinking agents, use of antioxidants, use of protease inhibitors, modification of the bonding procedure, laser treatment of the substrate prior to bonding, and reinforcement of the resin matrix with inorganic fillers and/or remineralizing agents. Distinct agents/chemicals/approaches exist within each identified strategy, which may play specific roles in increased bond strength stability. Each strategy will be hereafter presented and discussed.
Figure.
Main agents or strategies available to increase/stabilize resin-dentin/enamel bonds.
Collagen Crosslinking Agents
Covalent crosslinks produced with external crosslinkers may be very stable over time, thus inactivating the active sites of dentin proteases by reducing the molecular mobility or by changing negatively-charged ionized carboxyl groups into positively charged amides (12). This approach aims to reinforce collagen fibrils through intermolecular crosslinking (13–15). Table 1 lists collagen crosslinkers found in this review.
Table 1.
Main findings collected from research studies that investigated the effects of collagen cross-linking agents, antioxidants, or MMP inhibitors on the increase/stabilization of resin-dentin bonds.
| Active Agent | Moment of Application | Main Mechanism(s) |
|---|---|---|
|
| ||
| Glutaraldehyde | After acid-etching.[16–17] | Collagen cross-linking: the amount of collagen solubilized from dental matrices in the hybrid layer may be reduced, and the cohesive strength of collagen fibrils may be enhanced, thus increasing resin-dentin bonds. |
|
| ||
| Carbodiimide | After acid-etching.[23] | Collagen cross-linking: this agent may form stable covalent bonds with collagen fibrils, thus strengthening the hybrid layer. |
|
| ||
| Proanthocyanidin | After acid-etching.[23,27] | Collagen cross-linking: this agent may interact non-covalently to collagen fibrils and may increase the mechanical properties of dentin, thus enhancing the quality of the hybrid layer. |
|
| ||
| After sodium hypochlorite (NaOCl) treatment; prior to bonding steps.[38] | Antioxidant effect: this agent possesses radical- scavenging properties, reversing the oxidizing effect of NaOCl, and restoring the redox potential of the oxidized dentin, thus allowing for proper bonding. | |
|
| ||
| After acid-etching[27] | Protease inhibition: this agent may reduce collagenolytic activity, thus improving the quality of the hybrid layer. | |
| During the priming step (experimental primer).[55,69] | ||
|
| ||
| Chlorhexidine digluconate | After acid-etching.[50–51] | Collagen cross-linking: this agent may present cationic properties with a strong affinity for organic (collagen) and inorganic (hydroxyapatite) structures in dentin, thus strengthening the hybrid layer. |
| Prior to the priming step.[17] | ||
| During the priming step as an experimental primer.[55] | ||
| Before the luting step with etch-and-rinse resin-based cements.[33] | Protease inhibition: may prevent binding of ions, such as Zn+2 and Ca+2 to the MMPs structure, thus inhibiting its catalytic activity. | |
|
| ||
| Chlorhexidine-methacrylate | After acid-etching as a therapeutic primer.[32] | Collagen cross-linking: this monomer may copolymerize with monomers of adhesive resin and with collagen fibrils, thus improving stability of the hybrid-layer. |
| Protease inhibition: this monomer may inhibit matrix proteases, as well as can chlorhexidine digluconate. | ||
|
| ||
| Riboflavin 5-phosphate | After acid-etching.[23] | Collagen cross-linking: this agent may produce free radicals through photo-oxidation (UVA), resulting in improved collagen matrix stiffness and effective adhesive resin penetration. |
|
| ||
| Bromelain enzyme | After acid-etching.[34] | Collagen cross-linking: this agent may remove unsupported collagen fibrils of acid-etched dentin and improve the surface energy of dentin, thus enhancing resin infiltration and hybrid layer formation. |
|
| ||
| Methacrylate-modified chitosan | During the priming step, as a modified primer.[35] | Collagen cross-linking: this agent may induce electrostatic interactions (physical binding) of collagen fibrils, thus increasing stability of the resin-dentin bond. |
|
| ||
| Polyphenols(grape seed extract, green tea extract, genipin, epigallocatechin gallate) | After acid-etching.[36] | Collagen cross-linking: these agents may produce stable covalent bonds with collagen fibrils. |
| During the priming step as a modified primer.[69] | Protease inhibition: these agents may present anticollagenolytic effects, thus preventing degradation of dentin collagen within the hybrid layer. | |
|
| ||
| Noni fruit juice
|
After sodium hypochlorite (NaOCl) treatment; prior to bonding steps.[38] | Antioxidant effect: these agents possess radical- scavenging properties, reversing the oxidizing effect of NaOCl and restoring the redox potential of the oxidized dentin, thus allowing proper bonding. |
| Accel | ||
|
| ||
| L-ascorbic acid (Vitamin C)
|
During the priming or bonding steps as modified primer/resin adhesives.[39] | Antioxidant effect: these agents may strengthen the hybrid layer and be available for further reaction with products of degradation, leading to a late polymerization process, and consequently, to improvement of resin-dentin bonds over time. |
| α-tocopherol (Vitamin E)
| ||
| Quercetin | ||
|
| ||
| Minocycline | After acid-etching.[51, 52] | Protease inhibition: these agents may prevent the binding of ions, such as Zn+2 and Ca+2, to the MMPs structure, thus inhibiting its catalytic activity. |
|
| ||
| Doxycycline | During the priming step as an experimental primer.[55] | |
| During the bonding step as a modified resin adhesive.[68] | ||
|
| ||
| Benzalkonium chloride | During the bonding step as a modified one-step adhesive.[71–72] | Protease inhibition: this agent may inhibit endogenous dentin proteases, thus preserving resin-dentin bonds. |
|
| ||
| Quaternary ammonium methacrylate polymer | During the priming step as a modified primer.[70] | Protease inhibition: this agent may copolymerize within the hybrid layer, thus prolonging its MMP inhibition effect, which, in turn, results in stable resin-dentin bonds over time. |
Table presents data/information solely from studies published between 2014 and 2017.
Regarding glutaraldehyde-containing agents, studies have shown that these agents can reduce the amount of collagen solubilized from dental matrices in the hybrid layer (16) and enhance the cohesive strength of collagen fibrils (17), thus contributing to the preservation of adhesive interfaces. Indeed, glutaraldehyde possesses the property of acting as a true crosslinking agent, making the hybrid layer stronger. According to the studies included in this article, these agents were applied after acid-etching, so that the denuded collagen fibrils could interact with each other by forming strong covalent bonds, thus positively influencing on the stiffness of the substrate (18). However, and despite these positive effects, it has been suggested that glutaraldehyde may be strongly toxic (19), thus limiting its clinical application.
With regard to the use of carbodiimide (1-ethyl-3-[3-dimethylamino-propyl] carbodiimide; EDC), several studies have shown promising results with clinically acceptable application times (20–22). In addition, EDC has very low cytotoxicity (19), showing great biocompatibility than other crosslinkers. Usually, carbodiimide has been applied to dentin after acid-etching, resulting in strengthening the hybrid layer due to the formation of stable covalent bonds with collagen fibrils (23). Some studies have also shown that EDC possesses protease inhibition properties (24, 25), although at present, it is difficult to ascertain whether collagen crosslinking ability or the protease inhibition properties of EDC play an equally important role or whether one factor predominates over the other in increasing resin-dentin bond durability (26).
Proanthocyanidins are natural crosslinkers derived from polyphenols, which are able to enhance the mechanical properties of dentin, thus improving the quality of the hybrid layer (23, 27, 28). The incorporation of proanthocyanidin to an experimental adhesive did not show adverse effects on the immediate resin-dentin bond strength when its concentration was less than or equal to 2% (29). Moreover, proanthocyanidin was shown to be extraordinarily efficient in stabilizing demineralized dentin collagen against enzymatic activity in a clinically relevant setting, probably due to the non-covalent nature of its interaction with collagen molecules, i.e., collagen’s crosslinking effect (30).
Chlorhexidine, which is an antimicrobial agent broadly used in dentistry, was also revealed to present collagen crosslinking ability (31–33). As shown by studies retrieved for full-text reading, two distinct forms of chlorhexidine have been tested: chlorhexidine- methacrylate (32) and chlorhexidine digluconate (33). The former was tested by adding it into an experimental primer (therapeutic primer) and, according to the authors of that study, the monomer could co-polymerize with other monomers, thus improving stability of the hybrid layer. Conversely, the latter was applied isolated and before luting to an etch-and-rinse resin cement; the authors demonstrated that the hybrid layer was stronger than the control, resulting in improved resin-dentin bonds.
The present review also identified other collagen crosslinking agents; namely, riboflavin 5-phosphate, bromelain enzyme, methacrylate-modified chitosan, and other polyphenols. Riboflavin 5-phosphate is a crosslinker that produces free radicals through photo-oxidation (UVA), resulting in improved collagen matrix stiffness and effective adhesive resin penetration (23). Bromelain enzyme acts by removing unsupported collagen fibrils of acid- etched dentin and by improving the surface energy of dentin, thus enhancing resin infiltration and hybrid layer formation (34). The methacrylate-modified chitosan, on the other hand, was revealed to induce electrostatic interactions (physical binding) of collagen fibrils, thus increasing resin-dentin bonds (35). Concerning polyphenols, which can be obtained from grape seed or green tea extracts (e.g., genipin, epigallocatechin gallate, among others), their effectiveness as crosslinkers may be attributed to the increased possibility in forming stable covalent bonds with collagen fibrils, thus improving the immediate dentin bond strength (18, 37).
Antioxidants
There are some clinical procedures that may generate and retain oxidizing species in dental substrates, thereby hampering polymerization of resin-based materials (e.g., adhesives, restoratives). Among these clinical circumstances, sodium hypochlorite (NaOCl) treatment or bleaching with hydrogen peroxide-based agents are the most frequently oxidizing substances used in dentistry. In order to eliminate any oxidizing specie from the dental structure, the use of antioxidants has been suggested as an effective strategy. According to the present review (Table 1), several agents are available for that purpose; namely, proanthocyanidins, noni fruit juice, Accel, vitamin C, vitamin E, and quercetin (38, 39). Concerning the three former agents, they were applied in NaOCl-treated dentin prior to bonding steps and, according to the authors, they presented radical-scavenging properties, reversing the oxidizing effect of NaOCl and restoring the redox potential of the oxidized dentin, thus allowing proper bonding (38). Meanwhile, the latter three agents were first incorporated into primer/resin adhesives and then applied during the priming/bonding step; as suggested, these agents strengthened the hybrid layer and became available for further reaction with products of degradation, leading to a late polymerization process, and consequently, to improvement of the resin-dentin bonds over time (39). It seems that the use of antioxidants holds importance for increasing bond strength between the adhesively-bonded restorations and substrates that underwent an oxidizing process, with the possibility of preventing their resin-dentin bonds over time.
Protease Inhibitors
MMPs and CCs are both proteases. MMPs are a class of zinc- and calcium-dependent endopeptidases that show intrinsic activity in many biological and pathological processes associated with the degradation of most extracellular matrix components (40). Among all MMPs, the dentin matrix contains at least the following: stromelysin-1 (MMP-3) (41, 42), collagenase (MMP-8) (43), and gelatinases A and B (MMP-2 and MMP-9, respectively) (44, 45). CCs are intracellular proteases that mediate housekeeping functions in the cell (46), and most are expressed in native pulp tissue and in odontoblasts. Several studies have recommended the use of protease inhibitors to prolong dental bond durability, which will be presented as follows.
Carbodiimides (EDC), which also possess collagen crosslinking ability (as discussed earlier), have been shown to present MMP inhibition effects. It seems that EDC may produce covalent bonds to MMPs, thereby inactivating their structure. According to Mazzoni et al. (24), the pre-treatment of dentin with 0.3M EDC prior to bonding procedures following the etch-and-rinse approach resulted in nearly complete inactivation of MMP-2 and −9; whereas, the control groups that did not receive EDC pre-treatment resulted in activation of dentinal gelatinases. In another study (25), when mixed with the adhesive component, 0.5M EDC significantly reduced MMP activity. Comparing the findings of the foregoing studies, we can suggest that the concentration of EDC should be higher if the agent is applied during the priming or bonding steps; however, lower amounts of EDC should be sufficient to properly inhibit MMP activity if applied after acid-etching and prior to bonding procedures. This should be investigated by future studies, i.e., in terms of which would be the best moment for using carbodiimides in order to achieve higher and more durable resin-dentin bonds.
Chlorhexidine (CHX) is undoubtedly one of the most often used antimicrobial agents for the inhibition of MMPs (41, 47). Among its several benefits, CHX possesses substantivity by binding to mineralized dentin for at least 12 weeks (48) or longer to demineralized dentin (49), thus contributing to preserving resin-dentin bonds. CHX may be applied after acid-etching (5–7, 17, 50–52) or as therapeutic solutions if incorporated into an acid conditioner (53, 54), primer solution (55), or adhesive (56). Regarding CHX concentration, low levels of CHX (from 0.5 to 5 wt.%) were shown to preserve dentin bonding (5, 52, 57–59); however, concentrations lower than 0.1 wt.% were found to be not as effective (59, 60).
Tetracycline, doxycycline, and minocycline are a family of broad-spectrum antibiotics with cationic chelating properties that may also serve as MMPs inhibitors (61–63). Li et al. (51) showed a positive effect of applying minocycline after acid-etching, thus obtaining improved immediate bond strength and enhanced dentin bonding stability. Loguercio et al. (52) demonstrated that a 2 wt.% solution of minocycline applied for 15 s after acid-etching was effective in preventing degradation of the resin-dentin interface over a 24-month period. The authors reasoned that, although the minocycline used is a chemically-modified tetracycline (CMT), it was able to chelate Ca+2 and Zn+2 (64, 65), which are two essential ions for MMPs to maintain their structure and functionally active sites (66), thus inactivating endogenous proteases. Similarly, doxycycline is another CMT that has also been shown to be a potent MMP inhibitor (55, 67). However, doxycycline incorporation into an adhesive solution has already been attempted, but due to phase separation reaction, the adhesive resulted in lower immediate bond strength to dentin (54). In light of this, doxycycline has been currently encapsulated into nanotubes and then incorporated into dentin adhesives in an attempt to offer inhibition of MMPs, as well as adhesive stability (68). Doxycycline encapsulation resulted in stable solutions without negative effects on monomers’ conversion and in immediate resin-dentin bonds; also, MMPs were effectively inhibited.
Finally, but no less important, polyphenols (e.g., proanthocyanidins, genipin, epigallocatechin gallate), benzalkonium chloride, and quaternary ammonium methacrylate polymer have all demonstrated effective protease inhibition potential (27, 69–72). It was suggested that these agents may reduce dentin biodegradability of host-derived MMPs, thus improving the quality of the hybrid layer. According to this review, these agents may be applied after acid-etching (27), during the priming step as modified primers (69, 70), or during the bonding step as modified resin adhesives (71, 72).
Resin Matrix Reinforcement with Fillers and/or Remineralizing Agents
Taking into consideration that hybrid layers may not only be degraded by enzymatic activity, but also by chemical degradation of adhesive components, any attempt to reinforce the strength of adhesives would probably contribute to hybrid layer resistance against degradation. To this end, the literature has shown the advantageous effects of distinct compounds that may be used to reinforce dental adhesives.
The loading of adhesive compositions with fillers and nanoparticles has led to a significant reinforcement effect of the adhesive. According to Table 2, carboxylic acid-functionalized titanium dioxide (73), copper (74), silver (75), and zinc oxide (76) nanoparticles have been used to reinforce the organic matrix of resin adhesives, thus improving physico-mechanical properties of the material, and consequently, bond strength between the restoratives and dental substrates. In another study, by Lohbauer et al. (77), zirconia nanoparticles were incorporated into the primer or adhesive of a commercial three-step etch-and-rinse adhesive system (SBMP, Scotchbond™ Multipurpose™, 3M ESPE, St. Paul, MN, USA), resulting in increased dentin bond strength. The formation of a strong adhesive interface is usually associated with a higher resistance to hydrolytic phenomenon, which may enhance bond durability. Once the hybrid layer is strong, water uptake is reduced, hydrolysis is diminished, and proteases activity is retarded, thereby reducing the rate of bond degradation over time (4, 19).
Table 2.
Main findings of in vitro research studies showing improvement of resin-dentin bonds by resin matrix reinforcement with fillers and/or remineralizing agents, laser treatment of the substrate prior to bonding, or modification of the bonding procedure.
| Main Method | What Was Done? | Main Findings |
|---|---|---|
| Resin matrix reinforcement with fillers and/or remineralizing agents | Carboxylic acid-functionalized titanium dioxide nanoparticles were incorporated (0.1 wt.%) into an experimental adhesive resin.[73] | The modified adhesive presented improved vinyl conversion of monomers and produced greater resin-dentin bonds than unmodified adhesives. |
| Copper nanoparticles were added (up to 1 wt.%) into a two-step etch-and-rinse adhesive system.[74] | Modified adhesives provided antimicrobial activity and preservation of resin-dentin bonds without reducing the adhesives’ mechanical properties. | |
| Silver nanoparticles were incorporated (535 ppm) into distinct adhesive systems.[75] | Modified adhesives gained in wettability, which resulted in improved resin-dentin bonds as compared to unmodified adhesives. | |
| Zinc oxide (ZnO) nanoparticles and zinc methacrylate (Zn-Mt) were incorporated into experimental resin adhesives.[76] | ZnO nanoparticles (1 wt.%) preserved the integrity of the hybrid layer and reduced cytotoxicity and polymerization shrinkage of the model dentin adhesive; whereas, the addition of Zn-Mt to the adhesive had no beneficial effects. | |
| Acid-etched dentin was treated with biomimetic primers containing analogs of phosphoproteins, followed by an ion- releasing resin adhesive containing calcium silicate (CaP).[83] | The combination of a 40 wt% CaP-filled adhesive with a primed dentin treated with agents, such as poly-L-aspartic acid and/or sodium trimetaphosphate, provided a suitable bonding approach for biomimetic remineralization of resin-dentin interfaces. | |
| Halloysite aluminosilicate clay nanotubes (HNTs) were added into adhesive resins and applied over acid-etched dentin for the etch-and-rinse adhesive or neat dentin for the self-etch adhesive.[79] | HNTs can infiltrate into the dentinal tubules, along with the resin tags, thus reinforcing the hybrid layer. Consequently, HNT-modified adhesives increased resin-dentin bonds, although with a threshold of up to 20% (w/v) in the two-step etch-and-rinse adhesive and up to 10% (w/v) in the one-step self-etch adhesive. | |
| Laser treatment of the substrate prior bonding | Enamel surface was etched with Er:YAG laser prior to bonding with an all-in-one self-adhesive agent.[91] | Er:YAG laser irradiation may cause specific topographic changes in the surface of dental substrates, such as removal of the smear layer, evaporation of water and organic content, as well as the creation of crater-like areas, all of which may contribute to increasing the surface area that is important for dental bonding. |
| Dentin exposed to NaOCl was irradiated with a non-thermal argon plasma laser.[93] | Application of the laser for 30 seconds was effective in increasing the resin-dentin bond, probably due to dentin etching and/or increased hydrogen bonding interactions of the collagen fibrils with the adhesive resin. | |
| Dentin was irradiated with a nonthermal atmospheric pressure plasma (NT-APP) laser prior to bonding.[92] | Application of the laser in a pulsed or conventional mode improved the immediate resin-dentin bonds and preserved them even after aging (i.e., thermocycling with 5,000 cycles), probably due to creation of grafting active species, such as carboxyl and carbonyl groups onto the dentin surface, thereby improving wetting and chemical interaction of the adhesive monomers. | |
| Dentin submitted to bleaching was irradiated with an Er:YAG laser prior to bonding.[90] | Application of the laser immediately after bleaching accelerated the release of free radicals originated during bleaching, thus restoring the dentin to a substrate that is better able to receive the adhesion procedures, which, in turn, contributed to improved resin-dentin bonds. | |
| Modification of the bonding procedure | Prior to bonding steps, dentin was dehydrated by application of 50% ethanol for 10 seconds (s) followed by 100% absolute ethanol for 10 s; excess ethanol was then blot-dried.[23] | The ethanol-wet bonding technique allowed for better resin infiltration due to early evaporation of water molecules from the interfibrillar spaces of collagen fibrils. Moreover, adhesive polymerization may be facilitated, thus improving the resin-dentin bonds. |
| Prior to the bonding steps, the dentin was rinsed with distilled water at warm (50±2°C), cold (5±2°C), or room temperature (22±2°C) for 20 seconds, and then blotted dry with a cotton pellet to reduce temperature fluctuations.[94] | At least for the etch-and-rinse approach, rinsing dentin with warm water prior to the bonding procedure may increase free energy of the surface, thus increasing wettability of the adhesive and resin infiltration. The polymerization reaction of monomers may also be improved, and any residual water eliminated from the hybrid layer, thus increasing the resin-dentin bonds. | |
| Intra-radicular dentin was treated with 100% ethanol prior to bonding procedures.[50] | Ethanol-wet bonding may reduce the amount of residual water into the root canal, as well as at the hybrid layer level, thus improving resin-dentin bonds. | |
| After bonding steps, a hydrophobic resin was applied in the substrate, followed by an air blower to achieve an optimally thin layer of adhesive.[95,97–98] | The extra layer of hydrophobic resin adds unsolvated hydrophobic monomers to the adhesive interface, thus decreasing the relative concentration of retained solvents and unreacted monomers in the adhesive layer. Consequently, the hybrid layer becomes more densely packed, more resistant to tensile forces, and less prone to degradation effects over time. The application of a hydrophobic resin coat may improve the degree of conversion in resin-dentin interfaces formed with either the self-etch or the etch-and-rinse strategy. | |
| The bonding step was performed by applying two consecutive layers of the one-step self-etch adhesive.[96] | Application of double coats of the adhesive resin produced longer resin tags than a single coat on both sound and demineralized dentin. Also, hydrolysis was probably lesser in the former situation, resulting in improved resin-dentin bonds. |
Table presents data/information solely from studies published between 2014 and 2017.
Nanotubes have also been used as fillers to reinforce the resin matrix of resin-based restorative materials. Nanotubes are a hexagonal network of carbon atoms rolled up to form cylindrical nanostructures that may be extremely strong and stiff, displaying both excellent thermal and electrical properties (78). It is worth mentioning that nanotubes were recently shown to reinforce adhesive resins and thus resin-dentin bonds (79–81). The incorporation of nanotubes up to 20 wt.% in etch-and-rinse and up to 10 wt.% in self-etch adhesives showed increased bond strength results compared to experimental controls (79). Of note, the most interesting characteristic of nanotubes is not their reinforcing ability, but the possibility of using their cylindrical hollow structure as a vehicle for the encapsulation of therapeutic molecules, minerals, and, no less important, protease inhibitors (68). In a study by Feitosa et al. (68), doxycycline, a potent inhibitor of MMPs (as discussed earlier), was encapsulated into halloysite nanotubes and then incorporated into an adhesive resin. The nanotube-modified adhesive was able to inhibit MMP-1 activity as compared to the control (unmodified adhesive) without compromising bond strength results. In the same fashion, nanotubes could, therefore, be used as a vehicle for encapsulation of several compounds that have been presented in this review in an attempt to prevent bond strength degradation due to the continuous release of MMP inhibitors, antioxidants, or collagen crosslinkers.
Currently, studies have reported on biomimetic “smart” materials that may possess remineralizing potential. Bioactive silicates, such as calcium/sodium phosphosilicate (Bioglass 45S5), have been combined with hydrophilic, biodegradable polymers in order to allow for lower degrees of collagen degradation as compared to neat resins (82). According to the study mentioned, these silicate-containing adhesives may show biological activity when exposed to biological fluids, thereby producing ionic dissolution products that will directly act at the hybrid layer level. However, this mechanism is controversial and must be further explored: on one hand, adhesive degradation may release the silicates, thus enhancing bioactivity, but on the other hand, degradation of adhesive components could lead to reduced mechanical properties, which would ultimately compromise bond durability (12). This field is fairly new in adhesive dentistry and needs further investigation.
In a study by Sauro and co-workers (83), acid-etched dentin was treated with biomimetic primers containing analogs of phosphoproteins, followed by an ion-releasing resin adhesive containing calcium silicate (CaP). It was demonstrated that the combination of a 40 wt.% CaP-filled adhesive with a primed dentin treated with agents such as poly-L-aspartic acid and/or sodium trimetaphosphate provided a suitable bonding approach for biomimetic remineralization of resin-dentin interfaces. Also, another important element that would contribute to tooth remineralization processes is fluorine (84). At least in theory, fluoride-containing adhesives would be an interesting choice for the preservation of resin-dentin bonds via hybrid layer remineralization, since the adhesives would be able to release fluoride continuously to the adhesive interface, thus contributing to strengthening of the hybrid layer and secondary caries prevention. However, the ability of fluoride to remineralize the collagen matrix via adhesive resin application has been questioned (85, 86), because remineralization of collagen requires seed apatite crystallites that determine the orientation of remineralized crystalline lattices, thus limiting remineralization of hybrid layers that contain little or no residual apatite (85). Of note, another study (87) showed that a fluoride-containing adhesive significantly increased bond strength to dentin after water storage, thus being able to create an acid inhibition zone in dentin. These results highlight the importance that fluoride incorporation into adhesives may produce in dental bonding, although this issue deserves further investigation (12, 19, 85).
Despite the beneficial effects that nanoparticles may have on adhesives, filler loading of low viscosity solutions, such as adhesive resins, is very susceptible to agglomeration or nonuniform dispersion of fillers in the resin phase, thus reducing strength and physical stability of the adhesive. Usually, there is a threshold for filler loading into adhesives, and it depends on the composition of the adhesive material and the type of filler (88). For instance, in a study by Wagner et al. (89), the addition of hydroxyapatite nanoparticles into SBMP resulted in a significant increase in resin-dentin bond strength, although this effect was observed only upon incorporation of up to 10 wt.% of nanoparticles. Therefore, reinforcing dental adhesives with fillers/nanoparticles may be an excellent strategy for improving physical properties and strengthening the hybrid layers, although there are some concerns regarding stability of the adhesive material.
Laser Treatment of the Substrate Prior to Bonding
Over the last few years, several studies have demonstrated that laser irradiation of enamel/dentin prior to bonding may result in bond strength enhancement. According to the studies retrieved for full text reading (Table 2), the two main laser sources used are the Erbium-doped Yttrium Aluminum Garnet (Er:YAG) (90, 91) or plasma-based lasers (92, 93).
Concerning the use of Er:YAG lasers, current findings have shown that laser irradiation may cause specific topographic changes in the surface of dental substrates, such as removal of the smear layer, evaporation of water and organic content, and the creation of crater-like that may contribute to increasing the surface area, key for successful dental bonding (91). Another study (92) irradiated dentin with a non-thermal atmospheric pressure plasma (NTAPP) laser prior to bonding, thus improving the immediate resin-dentin bonds and allowing for stable bonds after aging. The authors stated that application of the laser created grafting active species, such as carboxyl and carbonyl groups onto the dentin surface, thereby improving wetting and chemical interaction of the adhesive monomers.
Worth mentioning, laser irradiation was also revealed to result in positive effects when applied to dentin submitted to oxidizing agents. In the study by Curylofo et al. (90), Er:YAG laser irradiation of dentin was performed immediately after bleaching, resulting in greater resin-dentin bonds when compared to the control group. It was suggested that laser irradiation accelerated the release of free radicals originated during bleaching, thus restoring dentin to a substrate that was better able to receive the adhesion procedures. Similarly, but now considering NaOCl-treated dentin, the application of a non-thermal argon plasma laser for 30 s prior to bonding procedures was an effective approach for increasing the resin-dentin bond, probably due to dentin etching and/or increased hydrogen bonding interactions of the collagen fibrils with adhesive resin (93).
Modification of the Bonding Protocol
A few recent studies have also suggested that, by modifying the bonding protocol of adhesive systems, significant gains in the immediate and/or long-term resin-dentin bonds may be obtained (23, 50, 94–98). The most cited approach was the application of a hydrophobic resin after bonding steps, followed by an air blower to achieve an optimally thin adhesive layer (95, 97, 98). The authors suggested that the extra layer of hydrophobic resin would add unsolvated hydrophobic monomers to the adhesive interface, thus decreasing the relative concentration of retained solvents and unreacted monomers in the adhesive layer. Consequently, the hybrid layer would become more densely packed, more resistant to tensile forces, and less prone to long-term degradation (95, 98). Also, the application of a hydrophobic resin coating may improve the degree of conversion in resin-dentin interfaces formed with either the self-etch or the etch-and-rinse bonding strategies (97). Similarly, Oliveira et al. (96) demonstrated that application of double coats of the adhesive resin (one-step self-etch adhesive) produced longer resin tags than a single coat, at both sound and demineralized dentin; moreover, hydrolysis was probably lesser in the former situation, thus resulting in improved resin-dentin bonds.
The second approach identified with the present review was the ethanol-wet bonding (EWB) technique, which has been the purpose of investigation for several studies; however, only two studies (23, 50) were included, according to our literature search criteria. One of the studies tested the effects of the EWB technique on coronal dentin (23). Indeed, the dentin was dehydrated by application of 50% ethanol for 10 s, followed by 100% absolute ethanol for 10 s; ethanol excess was then blot-dried. The foregoing technique allowed for better resin infiltration due to the early evaporation of water molecules from the interfibrillar spaces of collagen fibrils, as well as facilitated adhesive polymerization, thus improving the resin-dentin bonds. The other study tested the effects of the EWB technique on intra-radicular dentin (50), which was treated with 100% ethanol prior to the bonding procedures. According to the authors’ main findings, the EWB technique may reduce the amount of residual water into the root canal as well as at the hybrid layer level, thus improving resin-dentin bonds.
Finally, the study by Malekipour and colleagues (94) tested a different approach as compared to the other studies: prior to the bonding steps, the dentin was rinsed with distilled water at warm (50 ± 2°C), cold (5 ± 2°C), or room temperature (22 ± 2°C) for 20 s, and then blot dried with a cotton pellet to reduce temperature fluctuations. In fact, and at least for the etch-and-rinse approach, dentin rinsing with warm water prior to the bonding procedure increased the free energy of the surface, thereby increasing wettability of the adhesive and resin infiltration. The polymerization reaction of the monomers was also improved, and any residual water was eliminated from the hybrid layer, thus increasing the resin-dentin bonds.
It seems that the foregoing approaches are quite simple to perform in a clinically-based setting, so they could be interestingly used in order to improve resin-dentin bonds or keep them stable over time. Further studies are of paramount importance on this topic.
Conclusions
Although immediate resin-dentin bonds are satisfactory, degradation of the adhesive interface is a common problem that reduces the longevity of restorations. However, and based on the current literature, recent improvements in the formulation of dental adhesives have contributed to the development of “smart” adhesives that may prevent bond strength degradation. Also, dental practitioners are directing efforts to propose new bonding techniques that would contribute to making resin-dentin bond stronger and more durable.
Regarding future prospects, there is a fairly new proof-of-concept strategy introduced to dentistry that proposes the idea of dental tissue regeneration. It is called biomimetic mineralization, which has been briefly discussed during this review. The use of novel nanoparticles or compounds at the atomic level would be important for endowing the adhesives with highly bioactive properties. Moreover, nanofibers have been used as a vehicle for the controlled release of drugs, growth factors, or bioactive compounds, so that nanofibers could also be used to improve the bioactivity of adhesives.
Despite all information that has been collected and discussed in the current review, it is of utmost importance to highlight that we used a list of keywords related to the topic as well as a criterion “year of publication”, so that the strategies and clinical approaches discussed may not comprise all of information available today. Indeed, it is urgent to note that knowledge and the science involving adhesive dentistry are constantly evolving, so we can expect several new trends and innovative products in the near future.
Acknowledgments
M.C.B. acknowledges start-up funds from the Indiana University School of Dentistry and the NIH/NIDCR (Grant#DE023552). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Tezvergil-Mutluay A, Mutluay M, Seseogullari-Dirihan R, Agee KA, Key WO, Scheffel DL, et al. Effect of phosphoric acid on the degradation of human dentin matrix. J Dent Res. 2013;92:87–91. doi: 10.1177/0022034512466264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. This article represents the state of the art of etch-and-rinse dental adhesives, explaining how bond strength degradation may occur under clinical conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL, et al. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. This article represents the state of the art of self-etch dental adhesives, showing important chemical explanations concerning bond strength degradation of resin-dentin adhesion. [DOI] [PubMed] [Google Scholar]
- 4**.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–32. doi: 10.1177/154405910508400204. This article is a classical study that critically reviewed the literature with regards to the durability of resin to dentin/enamel bonds. [DOI] [PubMed] [Google Scholar]
- 5.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32:107–11. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 6.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjaderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 7.Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 8.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Paakkonen V, Martins MT, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SC, Kern M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci. 2009;1:163–76. doi: 10.4248/IJOS.09044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. This article is an important source of knowledge for understanding the chemical and physical degradation of resin-based dental materials. [DOI] [PubMed] [Google Scholar]
- 11.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Tjaderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88:807–11. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellan CS, Pereira PN, Viana G, Chen SN, Pauli GF, Bedran-Russo AK. Solubility study of phytochemical cross-linking agents on dentin stiffness. J Dent. 2010;38:431–6. doi: 10.1016/j.jdent.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Sabatini C. Glutaraldehyde collagen cross-linking stabilizes resin-dentin interfaces and reduces bond degradation. Eur J Oral Sci. 2017;125:63–71. doi: 10.1111/eos.12317. [DOI] [PubMed] [Google Scholar]
- 17.Souza IM, Araujo CS, Soares CJ, Faria ESAL. Effect of dentin pretreatment on bond strength stability of self-etching and etch-and-rinse adhesives to intracoronally bleached dentin. J Adhes Dent. 2016;18:349–54. doi: 10.3290/j.jad.a36518. [DOI] [PubMed] [Google Scholar]
- 18*.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86:330–4. doi: 10.1002/jbm.b.31022. This article brings one of the first insights concerning dentin biomodification with collagen crosslinking agents. [DOI] [PubMed] [Google Scholar]
- 19.Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability--A literature review. Dent Mater. 2016;32:e41–53. doi: 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res B Appl Biomater. 2010;94:250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjaderhane L, Tezvergil-Mutluay A, et al. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater. 2013;29:1040–7. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, et al. Carbodiimide cross-linking inactivates soluble, matrix-bound MMPs in vitro. J Dent Res. 2012;91:192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venigalla BS, Jyothi P, Kamishetty S, Reddy S, Cherukupalli RC, Reddy DA. Resin bond strength to water versus ethanol-saturated human dentin pretreated with three different cross-linking agents. J Conserv Dent. 2016;19:555–9. doi: 10.4103/0972-0707.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzoni A, Apolonio FM, Saboia VP, Santi S, Angeloni V, Checchi V, et al. Carbodiimide inactivation of MMPs and effect on dentin bonding. J Dent Res. 2014;93:263–8. doi: 10.1177/0022034513516465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffel DL, Hebling J, Scheffel RH, Agee K, Turco G, de Souza Costa CA, et al. Inactivation of matrix-bound matrix metalloproteinases by cross-linking agents in acid-etched dentin. Oper Dent. 2014;39:152–8. doi: 10.2341/12-425-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh P, Nagpal R, Singh UP, Manuja N. Effect of carbodiimide on the structural stability of resin/dentin interface. J Conserv Dent. 2016;19:501–9. doi: 10.4103/0972-0707.194020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho C, Fernandes FP, Freitas Vda P, Franca FM, Basting RT, Turssi CP, et al. Effect of green tea extract on bonding durability of an etch-and-rinse adhesive system to caries-affected dentin. J Appl Oral Sci. 2016;24:211–7. doi: 10.1590/1678-775720150518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu N, Li F, Chen YJ, Zhang L, Lu S, Kang JJ, et al. The inhibitory effect of a polymerisable cationic monomer on functional matrix metalloproteinases. J Dent. 2013;41:1101–8. doi: 10.1016/j.jdent.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Epasinghe DJ, Yiu CK, Burrow MF, Tay FR, King NM. Effect of proanthocyanidin incorporation into dental adhesive resin on resin-dentine bond strength. J Dent. 2012;40:173–80. doi: 10.1016/j.jdent.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Dusevich V, Wang Y. Proanthocyanidins rapidly stabilize the demineralized dentin layer. J Dent Res. 2013;92:746–52. doi: 10.1177/0022034513492769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Montagner AF, Sarkis-Onofre R, Pereira-Cenci T, Cenci MS. Inhibitors on dentin stability: a systematic review and meta-analysis. J Dent Res. 2014;93:733–43. doi: 10.1177/0022034514538046. This article represents a systematic review of studies that used MMP inhibitors to produce resin-dentin bonding stability over time, which demonstrates that self-etching and etch-and-rinse adhesives are benefited by the use of chlorhexidine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu Nawareg M, Elkassas D, Zidan A, Abuelenain D, Abu Haimed T, Hassan AH, et al. Is chlorhexidine-methacrylate as effective as chlorhexidine digluconate in preserving resin dentin interfaces? J Dent. 2016;45:7–13. doi: 10.1016/j.jdent.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Toman M, Toksavul S, Tamac E, Sarikanat M, Karagozoglu I. Effect of chlorhexidine on bond strength between glass-fiber post and root canal dentine after six month of water storage. Eur J Prosthodont Restor Dent. 2014;22:29–34. [PubMed] [Google Scholar]
- 34.Chauhan K, Basavanna RS, Shivanna V. Effect of bromelain enzyme for dentin deproteinization on bond strength of adhesive system. J Conserv Dent. 2015;18:360–3. doi: 10.4103/0972-0707.164029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diolosa M, Donati I, Turco G, Cadenaro M, Di Lenarda R, Breschi L, et al. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules. 2014;15:4606–13. doi: 10.1021/bm5014124. [DOI] [PubMed] [Google Scholar]
- 36.Cecchin D, Pin LC, Farina AP, Souza M, Vidal Cde M, Bello YD, et al. Bond Strength between Fiber Posts and Root Dentin Treated with Natural Cross-linkers. J Endod. 2015;41:1667–71. doi: 10.1016/j.joen.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J Dent. 2012;40:458–66. doi: 10.1016/j.jdent.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Dikmen B, Gurbuz O, Ozsoy A, Eren MM, Cilingir A, Yucel T. Effect of different antioxidants on the microtensile bond strength of an adhesive system to sodium hypochlorite-treated dentin. J Adhes Dent. 2015;17:499–504. doi: 10.3290/j.jad.a35257. [DOI] [PubMed] [Google Scholar]
- 39.Gotti VB, Feitosa VP, Sauro S, Correr-Sobrinho L, Leal FB, Stansbury JW, et al. Effect of antioxidants on the dentin interface bond stability of adhesives exposed to hydrolytic degradation. J Adhes Dent. 2015;17:35–44. doi: 10.3290/j.jad.a33515. [DOI] [PubMed] [Google Scholar]
- 40.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 41.Boukpessi T, Menashi S, Camoin L, Tencate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–73. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Mazzoni A, Papa V, Nato F, Carrilho M, Tjaderhane L, Ruggeri A, Jr, et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent. 2011;39:231–7. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Martin-De Las Heras A, Valenzuela S, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 45.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and −9 forms in human sound dentin. J Dent Res. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 46.Dickinson DP. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit Rev Oral Biol Med. 2002;13:238–75. doi: 10.1177/154411130201300304. [DOI] [PubMed] [Google Scholar]
- 47.Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res. 2012;91:420–5. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 48.Mohammadi Z, Abbott PV. Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J. 2009;35:131–9. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomes Franca FM, Vaneli RC, Conti Cde M, Basting RT, do Amaral FL, Turssi CP. Effect of chlorhexidine and ethanol application on long-term push-out bond strength of fiber posts to dentin. J Contemp Dent Pract. 2015;16:547–53. doi: 10.5005/jp-journals-10024-1720. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Li T, Li X, Zhang Z, Li P, Li Z. Morphological effects of MMPs inhibitors on the dentin bonding. Int J Clin Exp Med. 2015;8:10793–803. [PMC free article] [PubMed] [Google Scholar]
- 52.Loguercio AD, Stanislawczuk R, Malaquias P, Gutierrez MF, Bauer J, Reis A. Effect of minocycline on the durability of dentin bonding produced with etch-and-rinse adhesives. Oper Dent. 2016;41:511–9. doi: 10.2341/15-023-L. [DOI] [PubMed] [Google Scholar]
- 53.Loguercio AD, Hass V, Gutierrez MF, Luque-Martinez IV, Szezs A, Stanislawczuk R, et al. Five-year effects of chlorhexidine on the in vitro durability of resin/dentin interfaces. J Adhes Dent. 2016;18:35–42. doi: 10.3290/j.jad.a35514. [DOI] [PubMed] [Google Scholar]
- 54.Stanislawczuk R, Reis A, Loguercio AD. A 2-year in vitro evaluation of a chlorhexidine- containing acid on the durability of resin-dentin interfaces. J Dent. 2011;39:40–7. doi: 10.1016/j.jdent.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Silva Sousa AB, Vidal CM, Leme-Kraus AA, Pires-de-Souza FC, Bedran-Russo AK. Experimental primers containing synthetic and natural compounds reduce enzymatic activity at the dentin-adhesive interface under cyclic loading. Dent Mater. 2016;32:1248–55. doi: 10.1016/j.dental.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yiu CK, Hiraishi N, Tay FR, King NM. Effect of chlorhexidine incorporation into dental adhesive resin on durability of resin-dentin bond. J Adhes Dent. 2012;14:355–62. doi: 10.3290/j.jad.a25674. [DOI] [PubMed] [Google Scholar]
- 57.Mobarak EH. Effect of chlorhexidine pretreatment on bond strength durability of caries-affected dentin over 2-year aging in artificial saliva and under simulated intrapulpal pressure. Oper Dent. 2011;36:649–60. doi: 10.2341/11-018-L. [DOI] [PubMed] [Google Scholar]
- 58.Campos EA, Correr GM, Leonardi DP, Barato-Filho F, Gonzaga CC, Zielak JC. Chlorhexidine diminishes the loss of bond strength over time under simulated pulpal pressure and thermo-mechanical stressing. J Dent. 2009;37:108–14. doi: 10.1016/j.jdent.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Tan J, Chen L, Li D, Tan Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J Dent. 2009;37:807–12. doi: 10.1016/j.jdent.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 60.De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, et al. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci. 2010;118:494–501. doi: 10.1111/j.1600-0722.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 61.Sulkala M, Wahlgren J, Larmas M, Sorsa T, Teronen O, Salo T, et al. The effects of MMP inhibitors on human salivary MMP activity and caries progression in rats. J Dent Res. 2001;80:1545–9. doi: 10.1177/00220345010800061301. [DOI] [PubMed] [Google Scholar]
- 62.Lauhio A, Salo T, Tjaderhane L, Lahdevirta J, Golub LM, Sorsa T. Tetracyclines in treatment of rheumatoid arthritis. Lancet. 1995;346:645–6. doi: 10.1016/s0140-6736(95)91484-6. [DOI] [PubMed] [Google Scholar]
- 63.Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 64.Acharya MR, Venitz J, Figg WD, Sparreboom A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist Updat. 2004;7:195–208. doi: 10.1016/j.drup.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Ryan ME, Greenwald RA, Golub LM. Potential of tetracyclines to modify cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1996;8:238–47. doi: 10.1097/00002281-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley DH, Tay FR, et al. Zinc reduces collagen degradation in demineralized human dentin explants. J Dent. 2011;39:148–53. doi: 10.1016/j.jdent.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Feitosa SA, Palasuk J, Kamocki K, Geraldeli S, Gregory RL, Platt JA, et al. Doxycycline-encapsulated nanotube-modified dentin adhesives. J Dent Res. 2014;93:1270–6. doi: 10.1177/0022034514549997. This article shows how nanotubes can be used as a delivery system for the increase/stabilization of resin-dentin bonds, which may present an innovation in modern adhesive dentistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khamverdi Z, Rezaei-Soufi L, Rostamzadeh T. The effect of epigallocatechin gallate on the dentin bond durability of two self-etch adhesives. J Dent (Shiraz) 2015;16:68–74. [PMC free article] [PubMed] [Google Scholar]
- 70.Pupo YM, Farago PV, Nadal JM, Simao LC, Esmerino LA, Gomes OM, et al. Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int J Mol Sci. 2014;15:8998–9015. doi: 10.3390/ijms15058998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabatini C, Ortiz PA, Pashley DH. Preservation of resin-dentin interfaces treated with benzalkonium chloride adhesive blends. Eur J Oral Sci. 2015;123:108–15. doi: 10.1111/eos.12176. [DOI] [PubMed] [Google Scholar]
- 72.Sabatini C, Pashley DH. Aging of adhesive interfaces treated with benzalkonium chloride and benzalkonium methacrylate. Eur J Oral Sci. 2015;123:102–7. doi: 10.1111/eos.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun J, Petersen EJ, Watson SS, Sims CM, Kassman A, Frukhtbeyn S, et al. Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives. Acta Biomater. 2017;53:585–97. doi: 10.1016/j.actbio.2017.01.084. [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez MF, Malaquias P, Matos TP, Szesz A, Souza S, Bermudez J, et al. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent Mater. 2017;33:309–20. doi: 10.1016/j.dental.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 75.Torres-Mendez F, Martinez-Castanon GA, Torres-Gallegos I, Zavala-Alonso NV, Patino-Marin N, Nino-Martinez N, et al. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent Mater J. 2017 Feb 01; doi: 10.4012/dmj.2015-299. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.Barcellos DC, Fonseca BM, Pucci CR, Cavalcanti B, Persici Ede S, Goncalves SE. Zn-doped etch-and-rinse modeldentin adhesives: Dentin bond integrity, biocompatibility and properties. Dent Mater. 2016;32:940–50. doi: 10.1016/j.dental.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Lohbauer U, Wagner A, Belli R, Stoetzel C, Hilpert A, Kurland HD, et al. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010;6:4539–46. doi: 10.1016/j.actbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Cadek M, Coleman JN, Barron K, Hedicke K, Blau WJ. Morphological and mechanical properties of carbon-nanotube-reinforced semicrystalline and amorphous polymer composites. Appl Phys Letters. 2002;81:5123–5. [Google Scholar]
- 79.Alkatheeri MS, Palasuk J, Eckert GJ, Platt JA, Bottino MC. Halloysite nanotube incorporation into adhesive systems-effect on bond strength to human dentin. Clin Oral Investig. 2015;19:1905–12. doi: 10.1007/s00784-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 80.Bottino MC, Batarseh G, Palasuk J, Alkatheeri MS, Windsor LJ, Platt JA. Nanotube-modified dentin adhesive--physicochemical and dentin bonding characterizations. Dent Mater. 2013;29:1158–65. doi: 10.1016/j.dental.2013.08.211. [DOI] [PubMed] [Google Scholar]
- 81.Feitosa SA, Munchow EA, Al-Zain AO, Kamocki K, Platt JA, Bottino MC. Synthesis and characterization of novel halloysite-incorporated adhesive resins. J Dent. 2015;43:1316–22. doi: 10.1016/j.jdent.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 82.Osorio R, Yamauti M, Sauro S, Watson TF, Toledano M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J Endod. 2012;38:1227–32. doi: 10.1016/j.joen.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Sauro S, Osorio R, Watson TF, Toledano M. Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin-dentin interfaces created with ion-releasing resin-based systems. Dent Mater. 2015;31:759–77. doi: 10.1016/j.dental.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Kawai K, Torii M, Tsuchitani Y. [Inhibition of water insoluble glucan formation by eluate from amalgams] Shika Zairyo Kikai. 1989;8:890–5. [PubMed] [Google Scholar]
- 85*.Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. This article brings an overview regarding limitations in bonding to dentin and the possible strategies available to prevent bond degradation, which may include increasing conversion and esterase resistance of hydrophilic monomers, using MMP inhibitors or collagen cross-linkers, using the ethanol wet-bonding technique or biomimetic remineralization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res. 2005;84:355–9. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 87.Shinohara MS, De Goes MF, Schneider LF, Ferracane JL, Pereira PN, Di Hipolito V, et al. Fluoride-containing adhesive: durability on dentin bonding. Dent Mater. 2009;25:1383–91. doi: 10.1016/j.dental.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 88.Leitune VC, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SM. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013;41:321–7. doi: 10.1016/j.jdent.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Wagner A, Belli R, Stotzel C, Hilpert A, Muller FA, Lohbauer U. Biomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesives. J Adhes Dent. 2013;15:413–22. doi: 10.3290/j.jad.a29534. [DOI] [PubMed] [Google Scholar]
- 90.Curylofo FA, Messias DC, Silva-Sousa YT, Souza-Gabriel AE. Bond strength of restorative material to dentin submitted to bleaching and Er:YAG laser post-treatment. Photomed Laser Surg. 2014;32:495–9. doi: 10.1089/pho.2014.3721. [DOI] [PubMed] [Google Scholar]
- 91.Kasraei S, Yarmohammadi E, Ghazizadeh MV. Microshear bond strength of OptiBond All-in-One self-adhesive agent to Er:YAG laser treated enamel after thermocycling and water storage. J Lasers Med Sci. 2016;7:152–8. doi: 10.15171/jlms.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han GJ, Kim JH, Chung SN, Chun BH, Kim CK, Seo DG, et al. Effects of non-thermal atmospheric pressure pulsed plasma on the adhesion and durability of resin composite to dentin. Eur J Oral Sci. 2014;122:417–23. doi: 10.1111/eos.12153. [DOI] [PubMed] [Google Scholar]
- 93.Abreu JL, Prado M, Simao RA, Silva EM, Dias KR. Effect of non-thermal argon plasma on bond strength of a self-etch adhesive system to NaOCl-treated dentin. Braz Dent J. 2016;27:446–51. doi: 10.1590/0103-6440201600914. [DOI] [PubMed] [Google Scholar]
- 94.Malekipour MR, Shirani F, Ebrahimi M. The effect of washing water temperature on resin-dentin micro-shear bond strength. Dent Res J (Isfahan) 2016;13:174–80. doi: 10.4103/1735-3327.178208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munoz MA, Sezinando A, Luque-Martinez I, Szesz AL, Reis A, Loguercio AD, et al. Influence of a hydrophobic resin coating on the bonding efficacy of three universal adhesives. J Dent. 2014;42:595–602. doi: 10.1016/j.jdent.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira CA, Franca FM, Basting RT, Turssi CP, do Amaral FL. Effect of double coating of one-step self-etching adhesive on micromorphology and microtensile bond strength to sound vs demineralized dentin. J Contemp Dent Pract. 2014;15:385–91. doi: 10.5005/jp-journals-10024-1549. [DOI] [PubMed] [Google Scholar]
- 97.Perdigao J, Munoz MA, Sezinando A, Luque-Martinez IV, Staichak R, Reis A, et al. Immediate adhesive properties to dentin and enamel of a universal adhesive associated with a hydrophobic resin coat. Oper Dent. 2014;39:489–99. doi: 10.2341/13-203-LR. [DOI] [PubMed] [Google Scholar]
- 98.Sezinando A, Luque-Martinez I, Munoz MA, Reis A, Loguercio AD, Perdigao J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent Mater. 2015;31:e236–46. doi: 10.1016/j.dental.2015.07.002. [DOI] [PubMed] [Google Scholar]