Abstract
Purpose
A splice variant of the androgen receptor, AR-V7, confers resistance to AR-targeted therapies (ATTs) but not taxane chemotherapies in patients with metastatic castration-resistant prostate cancer. Since August 2015, a clinical-grade assay to detect AR-V7 messenger RNA expression in circulating tumors cells (CTCs) has been available to providers through a Clinical Laboratory Improvement Amendments–certified laboratory at Johns Hopkins University.
Methods
We contacted ordering providers of the first 150 consecutive tests by using a questionnaire-based survey to determine how the results of AR-V7 testing were used to influence clinical practice.
Results
In all, 142 (95%) of 150 questionnaires were completed by 38 providers from 29 sites across the United States and Canada. AR-V7 test results were reported either as CTC– (28%), CTC+/AR-V7– (30%), or CTC+/AR-V7+ (42%). Prevalence of AR-V7 detection increased with prior exposure to ATTs (abiraterone and enzalutamide naïve, 22%; after abiraterone or enzalutamide, 35%; after abiraterone and enzalutamide, 43%). Overall, management was affected by AR-V7 testing in 53% of the patients and even more often with CTC+/AR-V7+ results. AR-V7+ patients were commonly switched from ATT to taxane chemotherapy (43%) or were offered a clinical trial (43%); management remained unchanged in only 14% of these patients. Overall, patients who had a change in management on the basis of AR-V7 testing were significantly more likely to achieve a physician-reported 50% decline in prostate-specific antigen response on next-line therapy than those who did not change treatment (54% v 31%; P = .015).
Conclusion
Providers used AR-V7 testing to influence clinical decision making more often than not. Physicians reported that men with AR-V7+ results had the most treatment changes, and such men were preferentially managed with taxane therapy or offered a clinical trial, which may have improved outcomes.
INTRODUCTION
Metastatic castration-resistant prostate cancer (mCRPC) remains dependent on androgen receptor (AR) signaling for survival in the presence of low testosterone levels.1 Two novel AR-targeted therapies (ATTs), abiraterone and enzalutamide, induced high objective response rates and improved overall survival in patients with mCRPC.2,3 Treatment with abiraterone or enzalutamide in the prechemotherapy setting led to a 50% decline in prostate-specific antigen (PSA50) response in 62% and 78% of these patients, respectively.2,3 However, sequential use of abiraterone and enzalutamide has resulted in much lower PSA response rates with the use of the second ATT agent (ie, abiraterone after enzalutamide, 10% to 15%; enzalutamide after abiraterone, 20% to 30%).4-6 These data underscore the importance of identifying a predictive biomarker of resistance to ATT to prevent the use of subsequent futile therapy.
Potential mechanisms of resistance to ATT include AR amplification and mutation, as well as the expression of AR splice variants.7 A well-characterized splice variant, AR-V7, is a truncated form of full-length AR (AR-FL) that lacks the ligand-binding domain but retains both the transactivation and DNA-binding domains, allowing for constitutive AR signaling in the absence of androgen.8-10 In patients with mCRPC, detection of AR-V7 in circulating tumors cells (CTCs) was shown to predict resistance to novel ATTs.11-13 Moreover, AR-V7+ patients had a significantly shorter overall survival, suggesting a prognostic value of AR-V7 in addition to its use as a predictive biomarker.11,13
Chemotherapy is an alternative to ATT for AR-V7+ patients with mCRPC. Detection of AR-V7 has been shown not to preclude response to taxane-based chemotherapy.14,15 Further prospective investigation found a significant survival benefit with the use of taxanes versus ATTs in patients with AR-V7+ disease.16 Interestingly, the presence of this splice variant is a dynamic feature with possible conversion from AR-V7+ to AR-V7– status after chemotherapy with taxanes.14,17 These data suggest that serial AR-V7 testing may guide the clinical treatment of patients with mCRPC. Those patients with AR-V7– prostate cancer may continue to benefit from ATT, whereas chemotherapy may be more effective in patients with detectable AR-V7 transcript.18
If the clinical utility of AR-V7 testing can be confirmed, it may also have an economic benefit. In a recent study, we modeled the cost of treating all patients with mCRPC with abiraterone or enzalutamide versus using AR-V7 testing to direct treatment.19 By using a clinical scenario in which AR-V7+ patients were changed from treatment with abiraterone and/or enzalutamide to chemotherapy thus avoiding the cost of futile ATT therapy, AR-V7 testing resulted in a theoretical cost savings to the health care system of $150 million per year. To this end, since August 2015, clinical-grade AR-V7 testing performed in a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory at Johns Hopkins University has been available to health care providers for clinical use.20 However, despite the commercial availability of this AR-V7 test, its clinical utility is unknown. Here, we have retrospectively compiled questionnaire-based data on how ordering providers are applying the results of AR-V7 testing in their clinical practice to influence decision making.
METHODS
The analytical validation and test characteristics of our CLIA-grade AR-V7 assay have been described previously.20 Our molecular pathology database was queried for all AR-V7 tests ordered by internal and external providers for clinical purposes. We identified patients by name, date of birth, date of testing, AR-V7 status, and the ordering provider of each test. Clinical results of this test were reported as CTC–, CTC+/AR-V7–, or CTC+/AR-V7+, because each of these categories is associated with different outcomes.12 A clinical utility questionnaire (Data Supplement) was generated for each AR-V7 test ordered and was mailed or e-mailed to each ordering provider. We defined a biomarker-based change in treatment as a confirmation of treatment choice or a change from one therapy to another after AR-V7 testing. The institutional review board at Johns Hopkins University approved this study and granted a waiver of consent to contact the provider of each AR-V7 test ordered because that was considered a clinical audit. The ordering provider was then contacted for participation and asked to complete a questionnaire pertaining to treatment decisions that were made on the basis of results of that specific AR-V7 test. Participation in this study was voluntary. If a provider did not wish to participate or the questionnaire was not returned after two attempts to contact the provider, data for that patient were not included in the analysis.
One hundred fifty consecutive AR-V7 clinical test results were obtained between August 31, 2015, and August 31, 2016, representing the first 150 tests ordered. From these, 142 questionnaires (95%) were completed and returned by 38 providers across 29 sites (28 in the United States [in 22 states] and one in Canada). Statistical analyses for this project were largely descriptive. In specified cases, a two-tailed Fisher’s exact test was used to compare proportions between two or more groups. The significance level was set at P < .05, and corrections were not performed for multiple comparisons.
RESULTS
Information from 142 of 150 questionnaires sent (95% participant response rate) were included in this analysis. All 142 AR-V7 tests were ordered for patients with mCRPC. The number of lines of additional systemic therapies for mCRPC among these patients was reported as follows: 24% (n = 33) had received no other lines, 27% (n = 39) had received one line, 27% (n = 39) had received two lines, 13% (n = 18) had received three lines, and 9% (n = 13) had received four or more lines of systemic therapy before testing. Eighteen percent of men (n = 26) had previously received bicalutamide, 46% (n = 66) had received abiraterone, 49% (n = 70) had received enzalutamide, 48% (n = 69) had received docetaxel, and 13% (n = 18) had received cabazitaxel.
Overall, the prevalence of a CTC– result was 28%, the prevalence of a CTC+/AR-V7– result was 30%, and the prevalence of a CTC+/AR-V7+ result was 42%. We then subdivided test results according to physician-reported prior treatment with a novel ATT (Table 1). The majority of patients without detectable CTCs were naïve to both abiraterone and enzalutamide (53%). Patients who were treated with abiraterone and/or enzalutamide resulted in a higher prevalence of AR-V7 detection compared with patients who were not treated with an ATT: 22% of treatment-naïve patients were AR-V7+; after treatment with abiraterone or enzalutamide, 35% of patients were AR-V7+; and after treatment with abiraterone and enzalutamide, 43% of patients were AR-V7+.
Table 1.
Summary of AR-V7 Test Results According to the Number of Novel ATTs Previously Received
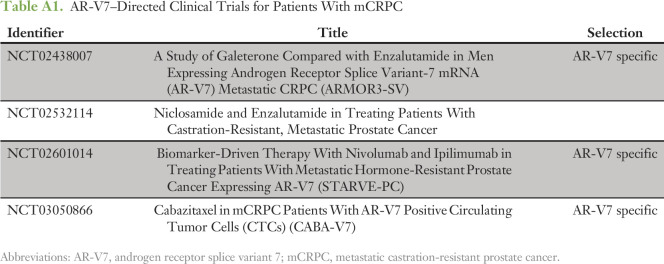
To assess the clinical utility of AR-V7 testing, providers were asked whether the AR-V7 status influenced their decision making. The majority of AR-V7– tests (CTC– or CTC+/AR-V7–) did not change the clinical practice of the providers (Table 2). However, almost two thirds (62%) of AR-V7+ tests resulted in a change in management. In patients for whom treatment was changed, providers were then asked to specify the type of therapy selected on the basis of the test result. We also stratified those responses by AR-V7 test result (Table 3). Patients with an AR-V7– result (CTC– or CTC+/AR-V7–) were preferentially treated with an ATT agent (confirmed AR treatment, or changed from taxane to AR therapy). A smaller subset of AR-V7– patients were treated on a clinical trial or changed to chemotherapy. Conversely, after an AR-V7+ result, most patients were changed from an ATT agent to taxane chemotherapy (43%) or were enrolled in a clinical trial (43%). A list of these AR-V7–directed clinical trials is provided in Appendix Table A1. Providers were next asked to self-report whether each patient achieved a PSA50 response on next-line systemic therapy (ie, the subsequent therapy selected after the AR-V7 test result (Table 4). The physician-reported PSA50 response rate (PSA50 RR) was significantly higher among patients in whom management was changed on the basis of AR-V7 testing compared with those in whom treatment was not altered (54% v 31%; P = .015). PSA50 RR data were missing from 16% (n = 12) and 22% (n = 15) of questionnaires in which management was changed or not changed, respectively.
Table 2.
Clinical Utility of AR-V7 Testing in Patients With mCRPC
Table 3.
Change in Management on the Basis of AR-V7 Testing in Patients with mCRPC
Table 4.
PSA50 Response Rate to Next-Line Therapy Based on Change in Clinical Practice After AR-V7 Testing

We also investigated which systemic therapy was used in patients with mCRPC after progression on both abiraterone and enzalutamide, according to AR-V7 status (Table 5). For patients with an AR-V7– result, most (44%) were offered standard taxane-based chemotherapy, and 19% enrolled on a clinical trial. By contrast, AR-V7+ patients who had already received abiraterone and enzalutamide were more often treated on a clinical trial (54%) compared with treatment using chemotherapy (19%). For patients who had received abiraterone and enzalutamide, we investigated the prevalence of AR-V7 positivity after physician-reported treatment with docetaxel (Appendix Table A2). No significant difference in prior docetaxel treatment was observed between AR-V7– (56% [nine of 16]) and AR-V7+ (73% [19 of 26]; P = .32) patients. In the chemotherapy-naïve group, no numerical difference was noted in the reported clinical trial enrollment based on AR-V7 status. In the patients who were treated with docetaxel, those who were AR-V7– were commonly treated with standard chemotherapy (ie, cabazitaxel; 67% [six of nine]) whereas AR-V7+ patients were more frequently placed on a clinical trial (58% [11 of 19]).
Table 5.
Next-Line Systemic Therapy in Patients After Treatment With Abiraterone and Enzalutamide on the Basis of AR-V7 Status
Finally, we examined provider treatment preferences for AR-V7+ patients irrespective of the prior therapies received (Table 6). These patients were most commonly treated on either a clinical trial (35%) or with taxane chemotherapy (32%), similar to those patients who had previously exhausted all ATT options. A minority of patients (7%) received either enzalutamide or abiraterone despite an AR-V7+ result, whereas all AR-V7+ patients managed with observation (10%) enrolled in hospice shortly thereafter.
Table 6.
Next-Line Systemic Therapy Selected by Treating Physicians for Patients With AR-V7+ mCRPC
To summarize the data compiled from providers’ real-world experience with AR-V7 testing, we propose a hypothetical treatment algorithm for making decisions regarding patients with mCRPC using AR-V7 as a potential treatment-selection biomarker (Fig 1). After first-line systemic therapy with abiraterone or enzalutamide, AR-V7+ patients would preferentially cross over to taxane-based therapy, whereas those who are AR-V7– may continue on a second ATT. Because of occasional conversions from AR-V7+ to AR-V7– status, men progressing on taxane treatment can be retested and could potentially consider treatment with an ATT if the AR-V7 status reverts to negative. Finally, even patients progressing after treatment with abiraterone and enzalutamide may be considered for AR-V7 testing if an AR-V7–directed clinical trial is available.
Fig 1.
Potential decision algorithm based on serial androgen receptor splice variant 7 (AR-V7) testing across the castration-resistant prostate cancer landscape. After each line of therapy, we propose an algorithm for consideration of AR-V7 testing. Patients with a positive AR-V7 test could be changed from an AR-targeted therapy (ATT) agent to taxane-based chemotherapy. After taxane treatment, repeat AR-V7 testing may be clinically helpful, and patients with a negative AR-V7 test can be considered for additional ATT. After abiraterone and enzalutamide treatment, patients should be subject to AR-V7 testing only if an AR-V7–directed clinical trial is available. (*) Indicates limited clinical data supporting the use of ATTs in AR-V7+ patients that subsequently convert to AR-V7–. (†) Denotes either docetaxel (first-line) or cabazitaxel (second-line), depending on prior treatment. (‡) Denotes a clinical trial that does not require a positive AR-V7 test for AR-V7– patients, whereas AR-V7+ patients should consider an AR-V7–directed trial, if available.
DISCUSSION
The optimal sequencing of therapeutic agents in patients with mCRPC is unknown and remains a major challenge. The recent discovery of CTC-based AR-V7 detection as a potential predictive biomarker of ATT resistance (but not taxane resistance) may aid in such treatment decisions. To this end, the National Comprehensive Cancer Network prostate cancer guidelines now suggest that AR-V7 testing can be considered and may play a role in guiding therapy selection in mCRPC, but at this time, these guidelines have not gone as far as recommending testing to determine treatment choice. In another recent consensus report, the majority of prostate cancer specialists polled (59%) stated that AR-V7 testing would be useful for some (majority or minority of) patients with mCRPC.21 Although further prospective validation of the predictive ability of AR-V7 is currently ongoing, here we investigated the clinical utility of AR-V7 testing in a real-world setting.
We asked providers whether the result of the AR-V7 test influenced their clinical practice for that specific patient. Overall, more than 50% of providers stated that the AR-V7 test changed their treatment decision. Providers were also asked to self-report whether patients achieved a PSA50 response on their next-line therapy. Importantly, we observed a significantly higher PSA50 RR in patients whose providers used the AR-V7 result to change their therapy. Although our findings are retrospective, they suggest that AR-V7 testing may possibly lead to improved clinical responses to treatment, at least in terms of PSA50 RR. We did not assess for radiographic progression-free or overall survival, which may not have correlated with PSA50 RR.
The statistically significant PSA50 RR difference between the change and no-change groups is largely driven by the AR-V7+ subgroup (described in Appendix Table A3). AR-V7+ patients for whom management did not change had a PSA50 RR of 5% (v 39% in AR-V7+ patients for whom treatment was changed). A clear limitation to our study is that we did not explicitly ask providers to list the specific next-line therapy for those patients who did not have a treatment change based on the AR-V7 test. Therefore we cannot directly compare between these groups. We hypothesize that AR-V7+ patients in the change group were more commonly treated with chemotherapy compared with the no-change group, resulting in improved outcomes. The no-change group may also have been less clinically fit for chemotherapy or did not meet eligibility criteria for enrolling on a clinical trial. We also acknowledge a high PSA50 RR in AR-V7+ patients treated with chemotherapy and AR-V7– patients treated with ATT (both in the change group). Another potential weakness is the subjective definition of changing clinical practice and including patients whose treatment choice was confirmed on the basis of AR-V7 testing. This may have inflated the perceived clinical utility of the biomarker. Nonetheless, these data suggest that further prospective investigation is warranted to study biomarker-driven clinical outcomes.
By using the treatment history captured by our questionnaires, we were able to observe an increasing prevalence of AR-V7 detection with prior exposure to ATTs, as expected. This finding is consistent with previously published data12,13,16 and suggests that the clinical data obtained in this study are representative. In addition, we observed interesting nonsignificant trends in our physician-reported data regarding prior treatment. For instance, in ATT-naïve patients, prior treatment with docetaxel increased the incidence of AR-V7 to 40% (v 21% in the chemotherapy-naïve group). This finding is corroborated by the recent biomarker data from the ARMOR3-SV (A Study of Galeterone Compared with Enzalutamide in Men Expressing Androgen Receptor Splice Variant-7 mRNA [AR-V7] Metastatic CRPC) trial, in which first-line patients with mCRPC who had previously received docetaxel for metastatic hormone-sensitive disease had a higher prevalence of AR-V7 detection compared with chemotherapy-naïve patients.22 Moreover, 79% of men with newly developed mCRPC had prior exposure to bicalutamide. Interestingly, the incidence of AR-V7 was 27% in patients who had received bicalutamide compared with 0% in patients with no prior bicalutamide treatment. The higher trend of AR-V7+ tests after treatment with docetaxel or bicalutamide, in the absence of a novel ATT, may suggest that total number of therapies (ie, more advanced disease) contributes to AR-V7 expression in addition to the known relationship with prior ATT exposure. Another provocative hypothesis might be that docetaxel works as an AR-modulating therapy in prostate cancer, inhibiting microtubule-dependent nuclear transport of wild-type AR but not AR-V7, thereby selecting for the emergence of AR-V7–expressing clones during or after chemotherapy treatment.
This study had some additional limitations. First, the prevalence of AR-V7 was probably overestimated compared with earlier reports because providers were more likely to order a test if the clinical scenario suggested that the test might be positive. Other significant weaknesses of this analysis were its retrospective nature and reliance on self-reporting by providers. To this end, we did not review the medical records of all patients to confirm their prior treatment history or their PSA50 response. An audit of 14 randomly selected questionnaires determined that providers gave accurate responses to the questionnaire in most instances (see Appendix), suggesting that our data represent the true clinical course for each patient. Finally, we concede that many patients will receive all ATTs approved by the US Food and Drug Administration, regardless of AR-V7 status, because clinical responses to ATTs are sometimes observed in AR-V7+ patients.13 However, the clinical utility of AR-V7 testing may potentially be clearest in AR-V7+ patients who have additional ATTs still available for treatment. By identifying high-risk patients (ie, AR-V7+) who have adequate performance status earlier in their mCRPC treatment, this would allow them to be treated with taxane therapy before the chemotherapy window closes. AR-V7– tests are probably less clinically useful because patients could receive either chemotherapy or ATTs at their physician’s discretion.
We propose a decision algorithm using serial AR-V7 testing across the mCRPC landscape. This algorithm is based largely on published data suggesting that the presence of AR-V7 confers resistance to ATTs but does not influence response to taxane chemotherapy.11,13,14,16 We note that at this time, there is no clinical trial evidence to support the use of ATTs in AR-V7+ patients who convert to AR-V7– status after chemotherapy. In the setting of patients who have received abiraterone and enzalutamide, AR-V7 testing may be considered if AR-V7-selected clinical trials are available. In our study, 17 of 22 AR-V7+ patients were enrolled on a clinical trial that mandated AR-V7 detection as an entry criterion, suggesting that many of the tests in this study were ordered for the purpose of screening for a clinical trial. In these instances, the clinical utility of AR-V7 testing is primarily to allow enrollment on an AR-V7–directed trial. Finally, there is ongoing debate in the prostate cancer community about whether AR-V7 detection is merely a proxy for AR amplification or overexpression and not an independent predictor of response to therapy or clinical outcomes.21 Although several studies have shown that AR-V7 detection is indeed correlated with AR-FL expression, AR-V7 still remains independently prognostic in multivariable analysis after controlling for AR-FL levels.11,13,23
In conclusion, to our knowledge, this is the first study to examine the preliminary real-world clinical utility of CLIA-grade AR-V7 testing in patients with mCRPC. We show that AR-V7 testing influenced clinical decision making overall (regardless of test results) but that its utility was greatest in the setting of AR-V7+ results. Additional prospective studies are needed and are ongoing (eg, NCT02269982; Prospective Circulating Prostate Cancer Predictors in Higher Risk mCRPC Study [PROPHECY]).
Appendix
Analysis of PSA50 Response Rate Stratified by AR-V7 Status and Change/No Change Designation
We further queried our database to investigate the difference in the 50% decline in prostate-specific antigen (PSA50) response rate (RR) between the change and no-change groups on the basis of androgen receptor splice variant 7 (AR-V7) status. For patients with AR-V7+ disease, the PSA50 RR in those who did not change therapy was 5.3% (one of 19) compared with 38.7% (12 of 31) in the change group (Table A3). In the AR-V7− patients, the PSA50 RR was 45.45% (15 of 33) in the no-change group versus 68.88% (22 of 32) in the change group. We asked whether there was a difference in the number of lines of therapy (ie, more advanced disease) between the change and no-change groups. Each group was subdivided by AR-V7 status (Table A3). Within both the AR-V7+ and AR-V7– groups, there was no discernible difference in number of lines of therapy between the change and no-change group. We also examined PSA50 RR by treatment choice in those patients who had a change in treatment (these data were not solicited for patients who did not have a treatment change in the questionnaire). In AR-V7+ patients, the PSA50 RR to chemotherapy (either changed or confirmed) was 73.33% (11 of 15). The PSA50 RR in clinical trials for AR-V7+ patients was one (6.7%) of 15 in the change group. In AR-V7– patients who had treatment changed (or confirmed), the PSA50 RR was 70.8% (17 of 24) on AR-targeted therapy (chemotherapy, 100% [three of three]; clinical trial, 0% [zero of three]; observation, 100% [one of one]; unknown, 100% [one of one].
Audit of Randomly Selected Questionnaires Completed by Johns Hopkins University Providers
We (M.C.M.) performed a medical record audit of 14 randomly selected questionnaires completed by Johns Hopkins University providers to assess for accuracy. Clinical data from other sites were not accessible for audit. We investigated only objective questionnaire responses: clinical stage, prior therapies, most recent treatment, PSA50 response on next-line therapy, and clinical trial eligibility. In the 14 questionnaires audited, we found two minor discrepancies: (1) bicalutamide was listed in error as a prior therapy (one time), and (2) the correct clinical trial was misidentified for a patient (one time). Subjective responses (ie, clinical helpfulness and change in management) were not assessed. Although this audit reflects a small sample size (approximately 10% of the entire population included in this study), it suggests that providers documented accurate responses to the questionnaire in almost all instances.
Table A1.
AR-V7–Directed Clinical Trials for Patients With mCRPC
Table A2.
Next-Line Systemic Therapy in Patients After Abiraterone and Enzalutamide on the Basis of AR-V7 Status and Prior Treatment With Docetaxel
Table A3.
PSA50 RR and Lines of Therapy Subgrouped by AR-V7 Status in Patients With mCRPC Who Had a Change or No Change in Management
Footnotes
This work was supported by National Institutes of Health, National Cancer Institute Grants No. R01 CA185297 (E.S.A. and J.L.) and P30 CA006973 (E.S.A.), Department of Defense Prostate Cancer Research Program Grants No. W81XWH-15-2-0050 and W81XWH-12-1-0605 (J.L.), Johns Hopkins Prostate Specialized Programs of Research Excellence Grant P50 CA058236 (J.L.), the Patrick C. Walsh fund (E.S.A. and J.L.), the Prostate Cancer Foundation (E.S.A, J.L., and M.C.M.), and by an ASCO/Conquer Cancer Foundation Young Investigator Award (M.C.M).
Presented at the ASCO 2017 Genitourinary Cancers Symposium, Orlando, FL, February 16-18, 2017.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Mark C. Markowski, Mario A. Eisenberger, Jun Luo, Emmanuel S. Antonarakis
Financial support: Jun Luo, Emmanuel S. Antonarakis
Provision of study materials or patients: Jun Luo, James R. Eschleman
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
Mark C. Markowski
No relationship to disclose
John L. Silberstein
No relationship to disclose
James R. Eshleman
Patents, Royalties, Other Intellectual Property: Palb2 gene testing licensed to Myriad Genetics
Mario A. Eisenberger
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Astellas Pharma, Ipsen Biopharmaceuticals, Bayer, Sanofi, Pfizer
Research Funding: Sanofi, Tokai Pharmaceuticals, Genentech
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals, Astellas Pharma, Sanofi, Pfizer
Jun Luo
Honoraria: Gilead Sciences, Sanofi
Consulting or Advisory Role: Tokai Pharmaceuticals, Sun Pharmaceutical Industries, Janssen Oncology
Research Funding: Sanofi (Inst), Orion Pharma (Inst), Mirati Therapeutics (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor of an AR-V7 biomarker technology assigned to Johns Hopkins University who licensed to Tokai Pharmaceuticals; co-inventor of a technology licensed to Qiagen
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium Pharmaceuticals (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
REFERENCES
- 1.Montgomery RB, Mostaghel EA, Vessella R, et al. : Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res 68:4447-4454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-1482013. 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maughan BL, Luber B, Nadal R, et al. : Comparing sequencing of abiraterone and enzalutamide in men with metastatic castration-resistant prostate cancer: A retrospective study. Prostate 77:33-40, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Schrader AJ, Boegemann M, Ohlmann CH, et al. : Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 65:30-36, 2014 [DOI] [PubMed] [Google Scholar]
- 6. Noonan KL, North S, Bitting RL, et al: Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 24:1802-1807, 2013 . [DOI] [PubMed]
- 7.Silberstein JL, Taylor MN, Antonarakis ES: Novel insights into molecular indicators of response and resistance to modern androgen-axis therapies in prostate cancer. Curr Urol Rep 17:29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehm SM, Schmidt LJ, Heemers HV, et al. : Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 68:5469-5477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu R, Dunn TA, Wei S, et al. : Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 69:16-22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Armstrong AJ, Dehm SM, et al. : Androgen receptor variant-driven prostate cancer: Clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis 19:231-241, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonarakis ES, Lu C, Wang H, et al. : AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371:1028-1038, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.18632/oncotarget.3925. Steinestel J, Luedeke M, Arndt A, et al: Detecting predictive androgen receptor modifications in circulating prostate cancer cells. J Clin Oncol 33, 2015 (suppl; abstr 5067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonarakis ES, Lu C, Luber B, et al. : Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol 35:2149-2156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Lu C, Luber B, et al. : Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol 1:582-591, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onstenk W, Sieuwerts AM, Kraan J, et al. : Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol 68:939-945, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Scher HI, Lu D, Schreiber NA, et al. : Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol 2:1441-1449, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakazawa M, Lu C, Chen Y, et al: Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol 26:1859-1865, 2015. [DOI] [PMC free article] [PubMed]
- 18. Sprenger C, Uo T, Plymate S: Androgen receptor splice variant V7 (AR-V7) in circulating tumor cells: A coming of age for AR splice variants? Ann Oncol 26:1805-1807, 2015. [DOI] [PMC free article] [PubMed]
- 19.Markowski MC, Frick KD, Eshleman JR, et al. : Cost-savings analysis of AR-V7 testing in patients with metastatic castration-resistant prostate cancer eligible for treatment with abiraterone or enzalutamide. Prostate 76:1484-1490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokhandwala PM, Riel SL, Haley L, et al. : Analytical validation of androgen receptor splice variant 7 detection in a Clinical Laboratory Improvement Amendments (CLIA) laboratory setting. J Mol Diagn 19:115-125, 2017 [DOI] [PubMed] [Google Scholar]
- 21. doi: 10.1016/j.eururo.2017.06.002. Gillessen S, Attard G, Beer TM, et al: Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol . [Epub ahead of print on June 24, 2017] [DOI] [PubMed] [Google Scholar]
- 22. Taplin ME, Antonarakis ES, Ferrante KJ, et al: Clinical factors associated with AR-V7 detection in ARMOR3-SV, a randomized trial of galeterone (Gal) vs enzalutamide (Enz) in men with AR-V7+ metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 35, 2017 (suppl; abstr 5005) [Google Scholar]
- 23. Silberstein J, Luber B, Wang H, et al: Clinical significance of AR mRNA quantification from circulating tumor cells (CTCs) in men with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone (Abi) or enzalutamide (Enza). J Clin Oncol 35, 2017 (suppl; abstr 132) [DOI] [PMC free article] [PubMed]