Abstract
Enteric pathogenic bacteria such as Vibrio cholerae and enteropathogenic Escherichia coli (E. coli) cause life-threatening diarrheal diseases that have afflicted humans for centuries. Understanding the effectors required for intestinal colonization is very important to research on bacteria pathogenesis, and is also important to testing new therapeutics and development of the novel vaccines. Here, we describe the Infant Rabbit Colonization Competition Assay, a variant method of the powerful, nonsurgical animal model reported by Ritchie et al. (2010). In our modified assay, wild type and mutant strains are mixed together and inoculated into 2-day-old New Zealand white rabbits. The competitive index for each mutant measures the colonization capacity of the mutant relative to its wild type parental strain in the gastrointestinal tract. Compared to the traditional Sucking Mice model, the clinical and histologic signs of Vibrio cholerae (V. cholera)-induced disease of infant rabbits more closely resemble human cholera. The larger input bacteria amount of this model also facilitates high-throughput screens, such as Tn-Seq technology (Fu et al., 2013).
Materials and Reagents
New Zealand white infant rabbit (2–5 days old) (Millbrook farm)
Lactating doe (Millbrook farm)
-
Vibrio cholerae
Note: Alternatively, any enteric pathogenic bacteria that can successfully colonize the infant rabbit gut system can be used in this protocol.
Zantac (GlaxoSmithKline, NDC: 0173-0363-01)
40 mEq/20 ml potassium chloride (KCl) (Hospira, NDC: 0409-6653-05)
Isoflurane (USP) (Piramal Enterprises, NDC: 66794-013-25)
Phosphate buffered saline (PBS) (Lonza, catalog number: 51225)
X-gal and/or antibiotics (depend on the selective marker of the experimental strain)
70% ethanol
2.5% sodium bicarbonate buffer (pH 8.0) (see Recipes)
Luria-Bertani broth (LB) medium and LB agar solid medium (see Recipes)
Equipment
BL2 animal facility
Fume hood
37 °C incubator or warm room
1 ml sterile syringe (BD, catalog number: 305553)
3 ml sterile syringe (BD, catalog number: DG508504)
26 G 5/8 needle (BD, catalog number: 305115)
Surgical scissors and tweezers (Fine Science Tools)
Centrifuge (Eppendorf, model: 5424)
1.5 ml sterile microcentrifuge tube
96 wells tissue culture plate (BD, catalog number: 353072)
Size 5 French catheter
Silk ligature
Shaker
Spectrophotometer
Mini-bead beater-16 (Bio Spec Products, catalog number: 607)
Procedure
Check the litters on the day when they arrive: Litters of 2–5 day old New Zealand white rabbits and the lactating doe are obtained from a commercial vendor and housed together for the duration of the experiments. A minimum of six rabbits need to be assayed for each strain to be evaluated.
Inocula preparation: A single colony from a freshly streaked plate of each mutant (lacZ+) and wild type (lacZ-) is grown in LB containing the selective antibiotic of the experimental strains for approximate 5 to 6 h at 37 °C with shaking. The bacterial cells are pelleted (9,000 rpm for 5 min), the supernatant is discarded and the pellet is resuspended in sterile sodium bicarbonate buffer. The concentration of bacteria is adjusted to about 2 x 109 cfu/ml. Wild type and mutant solutions are then mixed together (1:1).
Zantac treatment (3 h prior to inoculation): Infant rabbits are weighed and administered Zantac by intraperitoneal (i.p) injection (2 mg per kg body weight) using a 26 G needle and 1 ml syringe (this dose of anti-acid treatment has been found to transiently alter the pH of the stomach without causing ill-effects in the infant rabbits).
3 h post-Zantac treatment: Infant rabbits are oro-gastrically inoculated with 0.5 ml of bacteria using a size 5 French catheter with flexible tip. The suggested dose is 1 x 109 cfu/ml per 90 g of rabbit body weight.
Input ratio determination: Inocula are serial diluted in 1x PBS and plated on LB agar containing X-gal. CFU of white and blue colonies are counted after overnight incubation at 37 °C.
Clean the catheter: Wash the catheter with 70% ethanol, H2O and 1x PBS sequentially.
Monitor rabbits: infant rabbits are monitored for diarrhea and signs of illness (e.g. dehydration, low body temperature, scruffy fur, lethargy and decreased muscle tone) and routinely euthanized within few days.
Euthanasia: Infant rabbits are anesthetized by inhalation of isoflurane and euthanized by intracardiac injection of KCl (2 mEq/ml, 3 ml).
-
Necropsy: The entire intestinal tract from the duodenum to the rectum is removed.
Cecal fluid: Cecal fluid (1–2 ml per rabbit) is collected by first isolating the cecum from the rest of the intestine with silk ligatures; then, the cecal contents are collected by snipping the end of the cecum and allowing the contents to drain under gravity into a collection tube. 1 ml cecal fluid of each rabbit is used for the CFU counting.
Distal small intestine and colon: a 1 cm sample is cut and removed, and then homogenized (e.g. bead beater or slides scissoring) in 1 ml of 1x PBS.
Output ratio determination: Serial dilutions of each sample are plated in LB solid media with antibiotic and X-gal and incubated overnight at 37 °C to enumerate the output ratio of the wild type and mutant strain.
Competitive Index (C.I) counting: The competitive index for each mutant is defined as the input ratio of mutant/WT strain divided by the output ratio of mutant/WT strain. (Figure 1)
Figure 1. Infant rabbit competition assays of intestinal colonization related mutants of Vibrio cholerae C6706 strain.
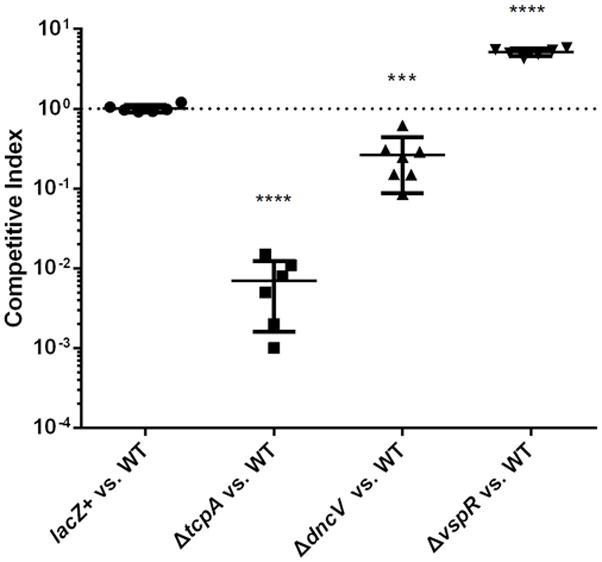
In vivo competition experiments were performed comparing the colonization related mutants to parental strain C6706 lacZ− (WT). Left, negative control mutant C6706 lacZ+; Middle left, severe colonization defective mutant ΔtcpA; Middle right, intermediate defect mutant ΔdncV and Right, hyper-colonization mutant ΔvspR. Statistical significance was determined by t test relative to the C.I. determined for a competition between C6706 lacZ+ versus WT with **** indicating a P value < 0.0001, *** P< 0.001. Mean with SEM was shown.
Representative data
Example of infant rabbit competitive index counting
Notes
-
Ethics statement
All animal experiments were performed with protocols approved by the Harvard Medical School Office for Research Protection Standing Committee on Animals in accordance to NIH guidelines. The Harvard Medical School animal management program is also accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). The institution also accepts as mandatory the PHS Policy on Humane Care and Use of Laboratory Animals by Awardee Institutions and NIH Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training. The office of Laboratory Animal Welfare (OLAW) has the approved Assurance of Compliance (A3431-01) on file.
Recipes
-
2.5% sodium bicarbonate buffer (pH 8.0) (100 ml)
Mix 2.5 g Sodium Bicarbonate with 80 ml dH2O
pH to 8.0 with NaOH
Add dH2O to 100 ml
Filter sterilize (0.2 μm)
Stored at room temperature
-
Luria-Bertani broth (LB) medium (1 L)
Mix of 10 g tryptone, 10 g NaCl and 5 g yeast extract
Add dH2O to 1 L
For LB agar add agar to a final concentration of 1.5%
Heat the mixture to boiling to dissolve agar and sterilize by autoclaving at 15 psi, from 121–124 °C for 15 min
Stored at room temperature
Acknowledgments
This protocol was first described and used in our previous Vibrio cholerae intestinal colonization study (Fu et al., 2013), and was adapted from a precious infant rabbit model, which was described by Ritchie et al. (2010). We thank Dr. Waldor and Dr. Ritchie for helpful suggestions and comments. This work was funded by grants AI-018045 and AI-26289 to J.J.M. from the National Institute of Allergy and Infectious Disease.
References
- Fu Y, Waldor MK, Mekalanos JJ. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe. 2013;14(6):652–663. doi: 10.1016/j.chom.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Rui H, Bronson RT, Waldor MK. Back to the future: studying cholera pathogenesis using infant rabbits. MBio. 2010;1(1) doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


