Abstract
A simple yet consequential modification was made to the popular carbonization processing of citric acid - polyethylenimine precursor mixtures to produce carbon dots (CDots). The modification was primarily on pushing the carbonization processing a little harder at a higher temperature, such as the hydrothermal processing condition of around 330 °C for 6 hours. The CDots thus produced are comparable in spectroscopic and other properties to those obtained in other more controlled syntheses including the deliberate chemical functionalization of preprocessed and selected small carbon nanoparticles, demonstrating the consistency in CDots and reaffirming their general definition as carbon nanoparticles with surface passivation by organic or other species. Equally significant is the finding that the modified processing of citric acid - polyethylenimine precursor mixtures could yield CDots of record-setting fluorescence performance, approaching the upper limit of being quantitatively fluorescent. Thus, the reported work serves as a demonstration on not only the need in selecting the right processing conditions and its associated opportunities in one-pot syntheses of CDots, but also the feasibility in pursuing the preparation of quantitatively fluorescent CDots, which represents an important milestone in the development and understanding of these fluorescent carbon nanomaterials.
ToC Graphic
1. Introduction
Carbon "quantum" dots or carbon dots (CDots)1,2 have attracted so much recent attention that the research and development of CDots have emerged to become a rapidly advancing and expanding technical field.3–10 A wide variety of potential technological applications of CDots have been pursued, including bioimaging and sensing,11–14 drug and gene delivery,15,16 photodynamic therapy,17 bactericidal functions,18 photocatalytic energy conversion,8,19 and others.20–23 Most of these targeted applications mimic, compete, and/or go beyond those already derived from conventional semiconductor quantum dots (QDs). There is now extensive experimental evidence supporting the assertion that CDots represent a new class of high-performance yet benign and nontoxic fluorescent nanomaterials of unique and/or advantageous properties.
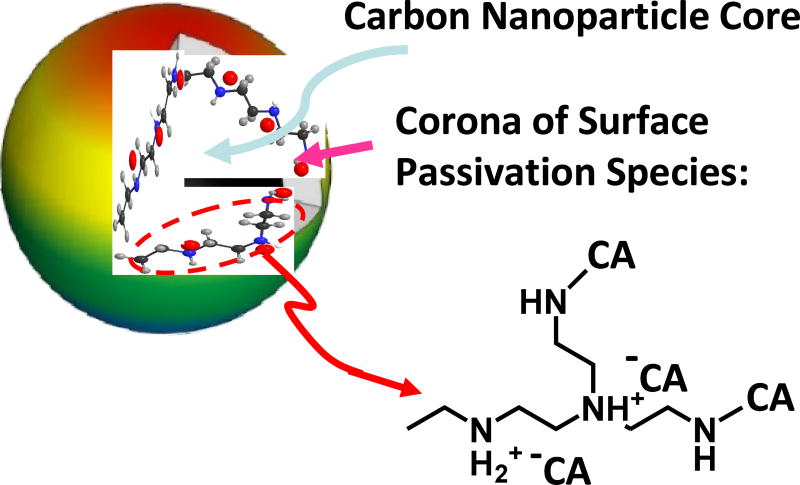
CDots are generally defined as small carbon nanoparticles with various surface passivation schemes (Figure 1).8,10,24 In the original report on CDots,1 specifically prepared carbon nanoparticles were chemically functionalized by oligomeric and polymeric molecules for the surface passivation purpose. Since then, such a synthesis, often referred to as the deliberate chemical functionalization method,8,10 has yielded some of the best-performing CDots (with observed fluorescence quantum yields beyond 50% in the visible spectral region covered by green fluorescent proteins).25,26 However, most of the reported syntheses of CDots in the literature have been based on the carbonization of organic molecules or other carbon-containing precursors, often in terms of "one-pot" processing.3–10,27–30 While a variety of molecules and materials as precursors have been studied in the literature, the use of citric acid - polyethyleneimine (PEI) binary mixtures have been particularly popular in many publications.27,31–36 The popularity is probably due to the facile nature of the synthetic protocol, such as simply the heating of a precursor mixture of citric acid and PEI up to 200 °C for a few hours.27,33 The synthesis has almost become a "classic" in the preparation of CDots, despite major issues such that the dots thus synthesized have been limited to mostly UV excitation,27,31–36 with their absorption and fluorescence emissions significantly different from those of the CDots from the deliberate chemical functionalization synthesis. Consequently, there are questions on the structural differences between the dots from the simple heating of citric acid - PEI mixtures and those from other syntheses in the effort on a more precise yet inclusive general definition on CDots and also an understanding of the structure-property relationships.
Figure 1.
A cartoon illustration on carbon dot, defined generally as a small carbon nanoparticle core with surface passivation species (a configuration similar to a soft corona), which in this work are likely zwitterionic pairs of citric acid and amino groups on polyethylenimine and the corresponding amides upon dehydration.
In the study reported here we found that the synthetic protocol widely adopted in the literature for citric acid - PEI binary mixtures as precursors should be modified, and the modification was rather simply as well, for the preparation of CDots that are not only property-wise matching those from the deliberate chemical functionalization of small carbon nanoparticles, including their comparable absorption and fluorescence spectra in the visible spectral region, but also exhibiting record-setting fluorescence performance, with observed fluorescence quantum yields in the same green spectral region overlapping with that covered by green fluorescent proteins up to at least 85%.
2. Results and Discussion
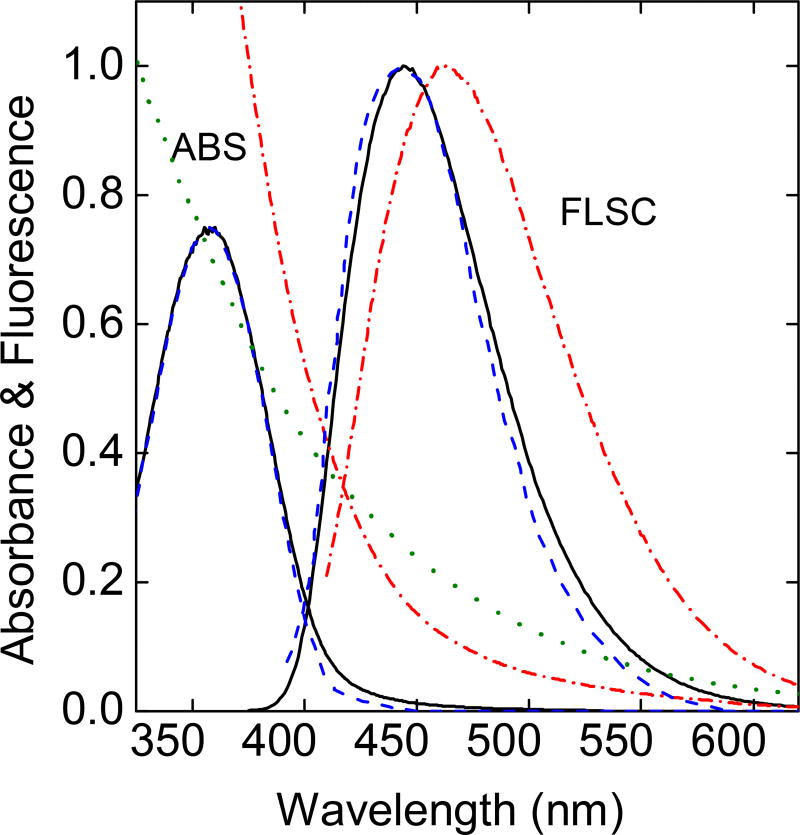
The one-pot carbonization processing with a mixture of citric acid (CA) and polyethylenimine (PEI) as precursor has been a popular preparation for fluorescent CDots,27,31–36 though the structural configurations of these dots have not been settled due in a large part to the consistent observation that the absorption and fluorescence spectral features of the dot samples thus prepared are significantly different from those of CDots from the deliberate chemical functionalization of small carbon nanoparticles. The latter with the carbon nanoparticles already in place before the dot synthesis is less ambiguous in terms of the formed dots being consistent with the general definition of CDots as surface-passivated carbon nanoparticles.8,10 For referencing purpose in this study, the experimental protocols established in the literature on the carbonization processing were adopted and evaluated, including specifically the popular reaction conditions of heating a mixture of citric acid - oligomeric PEI (branched, molecular weight ~ 1,200) either in a gel-like morphology at 200 °C for 3 h under ambient pressure or hydrothermally in an autoclave at 150 – 200 °C. The somewhat different processing conditions yielded samples of rather similar absorption and fluorescence spectral features, which are also similar to those reported in the literature (Figure 2).27,34 The absorption of these dot samples is characterized by a broad band centered at around 360 nm, having little contributions in the visible spectral region. With the known experimental results in the literature that small carbon nanoparticles are broadly absorptive in the visible,37 so are their derived CDots (Figure 2),25,37–39 it was hypothesized in this work that the synthesis protocols popular in the literature must have under-carbonized the precursor citric acid - PEI mixtures. The hypothesis was confirmed in a modified one-pot synthesis described below, where the modification was for more vigorous carbonization of the precursor mixtures at a higher temperature. As made evident by the results, the modified synthesis (or considered as a new method because in the carbonization synthesis the temperature and processing time are the synthetic method) is necessary in order to produce the desired high-quality CDots. In fact, the new processing protocol resulted in some of the most fluorescent CDots on record, approaching the limit of being quantitatively fluorescent.
Figure 2.
A comparison of absorption (ABS) and fluorescence (FLSC) spectra of the dot sample obtained by following the protocol in the literature (200 °C for 3 h, ——) with those reported in the literature (- - - -).27,33 Also for comparison are the spectra of the PEICDots (-.-.-.-)38 and the absorption of aqueous dispersed small carbon nanoparticles (·······).37
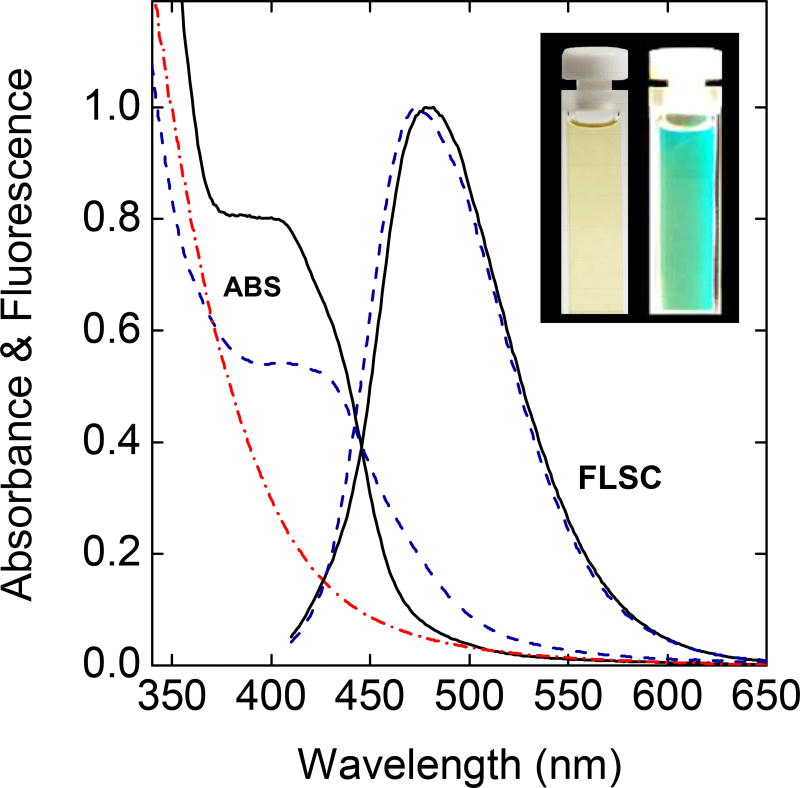
In the carbonization processing developed and evaluated in this work, a similar mixture of citric acid and the oligomeric PEI in water was processed in a stainless steel tube reactor at a temperature as high as 400 °C for up to 12 h. It was found that the hydrothermal processing conditions around 330 °C for 6 h were most favorable, and the dot samples thus produced exhibited significant absorption and bright fluorescence in the visible spectral region (Figure 3), with observed fluorescence quantum yields corresponding to the green emissions (Figure 3) readily approaching 70%. These results have validated not only the basic hypothesis of this work that the processing conditions commonly used in the literature were insufficient for the desired carbonization, but also a new synthesis approach capable of producing extremely fluorescent CDots. Again, the processing temperature and time are the synthetic method in the carbonization synthesis of CDots, determining the properties and performance levels of the resulting CDots or in some cases whether the materials produced from the carbonization processing should even be considered as the generally defined CDots.
Figure 3.
The absorption (ABS) and fluorescence (FLSC) spectra of the PEI/CA-CDots prepared by the modified hydrothermal processing (——) are compared with those of the EPACDots obtained from the deliberate functionalization synthesis (- - - -).39 Also for comparison is the absorption of the PEI-CDots (-.-.-.-)38 (the corresponding fluorescence spectrum already shown in Figure 2). Inset: Photos of a dilute aqueous solution of the PEI/CA-CDots under ambient room light (left) and under sunlight (right).
For the one-pot carbonization processing of a citric acid - PEI mixture as precursor, it is commonly rationalized such that the citric acid is sacrificed in the carbonization for the formation of the carbon nanoparticle core, with PEI serving more of the function of surface passivation in the generally defined structure of CDots (Figure 1). However, the PEI/CA-CDots obtained from the hydrothermal processing in this work exhibit different absorption features from those of the recently reported PEI-CDots prepared by the oligomeric PEI functionalization of pre-processed and selected small carbon nanoparticles (Figure 3).38 Interestingly, the extra absorption contributions of the PEI/CA-CDots in the blue spectral region are similar to those found in absorption spectra of CDots prepared by the deliberate chemical functionalization of small carbon nanoparticles with oligomeric poly(ethylene glycol) diamine (PEG1500N-CDots)25 and 3-ethoxypropylamine (EPA-CDots, Figure 3).39 In fact, as also reported recently,39 the extra absorption band in the blue for EPA-CDots (and similarly for PEG1500N-CDots) is correlated with the high fluorescence quantum yields in these CDots. Since EPA-CDots were well-characterized, including their spectroscopic properties associated with the extra absorption feature in the blue and relationships of these properties to structural parameters,39 they were considered as more suitable reference CDots (obtained from the deliberate chemical functionalization synthesis) for the PEI/CA-CDots (obtained from the very different carbonization synthesis in this work). Shown in Figure 3 is a comparison of the fluorescence spectra for these CDots with different surface functionalization moieties and from different syntheses, which are apparently similar. Thus, the modified hydrothermal processing of a citric acid - PEI mixture as precursor in this work could yield CDots that are not only of similar spectroscopic properties to those of the dots from various syntheses based on the deliberate chemical functionalization of small carbon nanoparticles,25,39 but also with the extra absorption spectral feature responsible for the ultrahigh fluorescence brightness. Even for the as-prepared samples of PEI/CA-CDots, their observed fluorescence quantum yields for green emissions (excitation at 400 nm – 440 nm), which share the same spectral region with those of green fluorescent proteins, are easily higher than those of the EPA-CDots and PEG1500N-CDots,25,26,39 which represent previous records.
As previously proposed1,24 and generally supported (or at least not contradicted) by the results already available in the literature, fluorescence emissions in CDots are attributed mechanistically to radiative recombinations of photogenerated electrons and holes trapped at diverse surface defect sites of the core carbon nanoparticles, which are surface-passivated by organic species, and the more effective passivation generally enhances the fluorescence brightness with higher quantum yields.8,10,39 In some special cases, represented by the EPA-CDots discussed above as a reference for the PEI/CA-CDots in this work (Figure 3), the surface passivation effect could extend to influencing the optical absorptions, with the observation of an extra absorption band into which the excitation would result in much higher fluorescence quantum yields.39 In the PEI/CA-CDots, the special passivation effect is apparently more significant, as reflected by the extra absorption band in the blue spectral region being obviously more prominent (Figure 3), with correspondingly very high fluorescence quantum yields. The same as the discussion on the EPA-CDots,39 phenomenologically, the observed high fluorescence quantum yields in the PEI/CA-CDots are correlated with the extra optical absorption features, which are likely associated with some special passivation effect (interactions of the organic species with the core carbon nanoparticles) in the dot structure, but mechanistically, an understanding of the relationship between the structural details in these CDots and the apparent special passivation effect remains a major challenge beyond this study and also for the CDots research community in general.
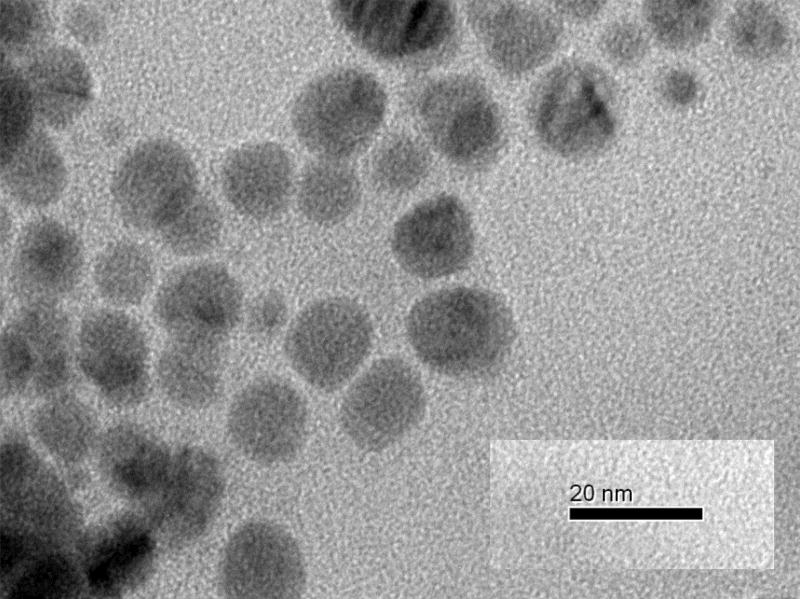
The PEI/CA-CDots were analyzed by transmission electron microscopy (TEM) from which a representative image is shown in Figure 4. Based on results obtained from multiple TEM images, a statistical analysis yielded an average dot size of about 10 nm in diameter and a size distribution standard deviation of about 3 nm. It should be emphasized that in the preparation of the TEM specimen, much effort was made to eliminate or minimize any potential aggregation of the CDots being analyzed, including dilutions of the sample solution coupled with careful sonication. However, this does not rule out the possibility or even likelihood of some CDots containing covalently clustered smaller carbon nanoparticles (perhaps via some organic species available in the dot structure). More sophisticated or special microscopy techniques are needed for the confirmation on the existence of such CDots and a probing of their structural configurations and compositions.
Figure 4.
A TEM image of the PEI/CA-CDots.
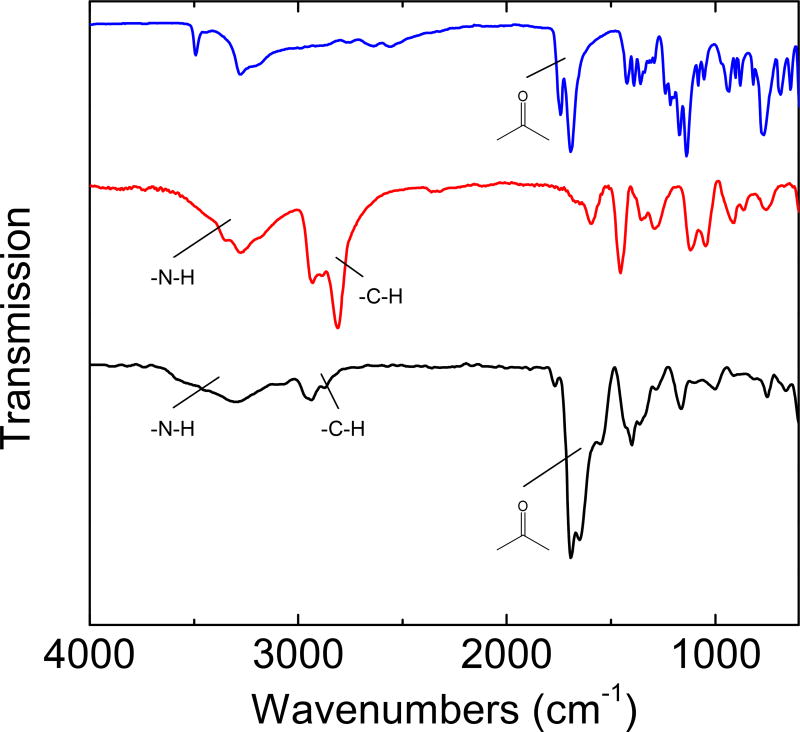
The FT-IR spectrum of the PEI/CA-CDots is compared in Figure 5 with those of neat citric acid and the oligomeric PEI. In the spectrum of citric acid, the three carboxylic acid groups in the molecule are prominently featured by the strong absorption at 1,770–1,650 cm−1. These features remain prominent in the spectrum of the PEI/CA-CDots, suggesting significant presence of carbonyl moieties. For the oligomeric PEI, on the other hand, the characteristic N-H and C-H absorptions at 3,500–3,100 cm−1 and 2,900–2,700 cm−1, respectively, are substantially weaker in the spectrum of the dot sample (Figure 5), indicative of the precursor PEI being significantly sacrificed in the hydrothermal carbonization processing. These results are generally against the simple mechanistic notion on the one-pot processing that citric acid is more for being carbonized into the carbon nanoparticle core and PEI more for the function of surface passivation in the resulting CDots, suggesting instead more complicated processes in the hydrothermal processing and also a more complex structural configuration for the CDots thus synthesized. It seems possible that the acid (citric acid) and base (amino moieties in PEI) interactions to form zwitterionic pairs in the precursor mixture could have played a major role in the hydrothermal carbonization for the CDots, effecting also the dot surface structures. For example, the carbonyl signals in the FT-IR spectrum of the PEI/CA-CDots (Figure 5) could be due to those zwitterionic pairs survived the carbonization conditions (and also the corresponding amide groups formed as a result of dehydration in the thermal processing, Figure 1). Despite the expected more complex structures in the PEI/CA-CDots, however, their rather striking similarities in spectroscopic properties to those of the dots from the deliberate chemical functionalization of small carbon nanoparticles likely reflect shared structure-property relationships among all these CDots from different syntheses. Also similar is the characteristic excitation wavelength dependence of fluorescence spectra and quantum yields in the PEI/CA-CDots (Figure 6). Such dependence was highlighted in the original report on CDots,1 and has since been found in CDots prepared by a variety of syntheses. Moreover, the hydrothermal processing in this work has apparently presented a breakthrough in pursuit of quantitatively fluorescent CDots (unity in quantum yield), as discussed below.
Figure 5.
FT-IR spectra of neat citric acid (upper), the oligomeric PEI (middle), and the PEI/CACDots (lower).
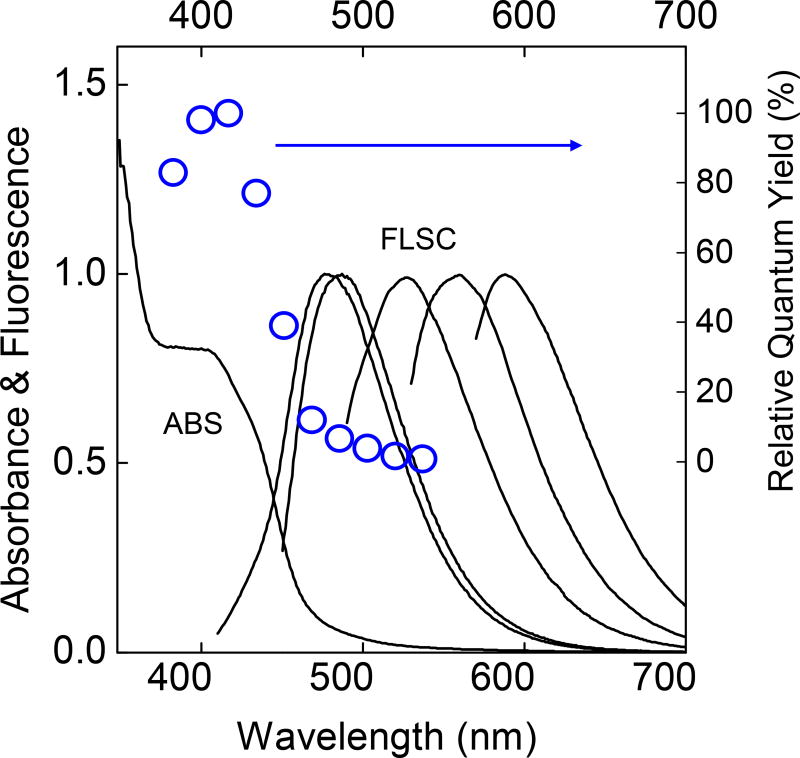
Figure 6.
Absorption (ABS) and fluorescence (FLSC, with corresponding excitation wavelengths from left to right: 400 nm, 440 nm, 480 nm, 520 nm, and 560 nm) spectra of a representative sample of the PEI/CA-CDots in aqueous solution, and the fluorescence quantum yields at a series of excitation wavelengths (○, relative to that at 420 nm excitation).
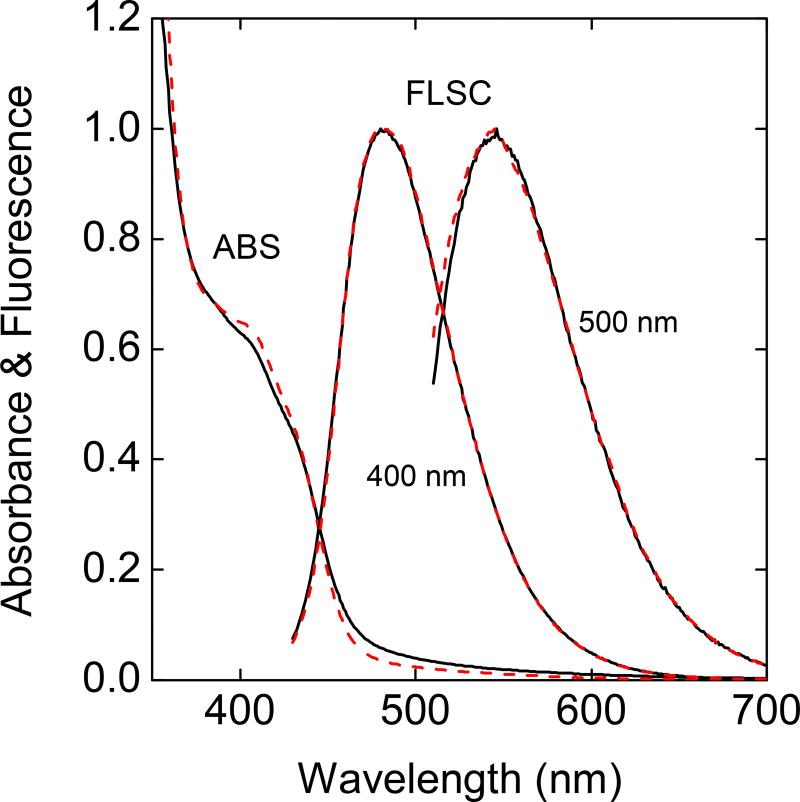
The as-prepared samples of PEI/CA-CDots were expected to be mixtures of dots with different fluorescence quantum yields, so the observed yields of around 70% should simply represent the averages. Similar mixtures were found for as-prepared samples of CDots from other syntheses.25,26,38–40 For the EPA-CDots and PEG1500N-CDots as examples, the separation of the as-synthesized samples on an aqueous gel column (Sephadex G-100) yielded fractions of significantly higher fluorescence quantum yields.25 The same gel column and separation protocol were applied to the fractionation of the as-prepared samples of PEI/CA-CDots, with some of the collected fractions reaching fluorescence quantum yields of 85–90%. The absorption and fluorescence spectra of these more fluorescent fractions are not meaningfully different from those of the as-prepared samples (Figure 7), similar to what were observed in the fractionation of EPA-CDots and PEG1500N-CDots.25,39 These results are consistent with the mechanistic notion that the effectiveness of surface passivation in CDots dictates their fluorescence brightness.10,39 The aqueous gel column is likely selective toward different dot surface moieties and configurations, picking out some fractions in which the dots are surface-passivated more effectively and thus more fluorescent. In this regard, it may be argued that the as-prepared samples of PEI/CA-CDots contain fractions at or close to being quantitatively fluorescent, whose separation likely requires methods beyond the Sephadex G-100 gel column fractionation. Such separation methods will be pursued in further investigations.
Figure 7.
Absorption (ABS) and fluorescence (FLSC, excited at indicated wavelengths) spectra of as-prepared (——) and separated more fluorescent fraction with quantum yield around 85% (- - - -) of the PEI/CA-CDots in aqueous solutions.
3. Conclusions
The results obtained in this work suggest that a simple yet consequential modification (primarily somewhat more vigorous processing at a higher temperature) is necessary to the onepot carbonization protocol widely adopted in the literature in order to correct apparently the under-carbonization of precursor CA - PEI mixtures, so as to yield CDots that are comparable in spectroscopic and other properties to those synthesized via the better-controlled deliberate chemical functionalization of pre-processed and selected small carbon nanoparticles. The comparability is also very significant in terms of reaffirming the general definition of CDots as carbon nanoparticles with various surface passivation schemes. Equally important is the finding that the modified processing method could produce CDots of record-setting fluorescence performance (quantum yields higher than 85% for emissions in green spectral region), offering an opportunity to potentially isolate quantitatively fluorescent (100% in quantum yield) CDots.
4. Experimental Section
4.1. Materials
Citric acid was purchased from Alfa Aesar, and polyethylenimine (PEI, branched, average molecular weight ~1,200) from Polyscience, Inc. The dialysis membrane tubing (molecular weight cut-off ~ 500 or 1,000) was supplied by Spectrum Laboratories. Water was deionized and purified by being passed through a Labconco WaterPros water purification system.
4.2. Measurement
UV/vis absorption spectra were recorded on a Shimadzu UV-3600 spectrophotometer. Fluorescence spectra were acquired on a Jobin-Yvon emission spectrometer equipped with a 450 W xenon source, Gemini-180 excitation and Tirax-550 emission monochromators, and a photon counting detector (Hamamatsu R928P PMT at 950 V). Unless specified otherwise in the description of results, the excitation and emission slits on the spectrometer were both set at 1.0 mm. FT-IR spectra were measured on a Shimadzu IRAffinity-1S spectrometer with the single reflection ATR accessory for solid samples. Transmission electron microscopy (TEM) imaging was performed on a Hitachi H-9500 high-resolution TEM system.
The determination of fluorescence quantum yields was based on the established relative method, in which 9,10-bis(phenylethynyl)-anthracene (BPEA) in cyclohexane was used as a standard (unity in fluorescence quantum yield, calibrated against the established quinine sulfate standard). The selection of BPEA was primarily for its similar excitation and emission spectral regions to those of the CDots, thus enabling the use of same excitation wavelength for both sample and standard and minimizing the potential inaccuracy associated with the corrections between sample and standard for nonlinear instrumental response of the emission spectrometer. In the quantum yield determination for a selected sample, solutions of the sample and standard were prepared for the same absorbance (<0.1) at the excitation wavelength, and their fluorescence spectra at the same excitation were collected, corrected for nonlinear instrumental response by using pre-determined correction factors specific to the emission spectrometer, and integrated to obtain the total emission intensities (areas under the corrected fluorescence spectra of the sample and standard) for the calculation of the sample fluorescence quantum yield.
4.3. Carbon Dots
Two experimental procedures with different carbonization reaction conditions were used for the dot preparation. One was designed for referencing purpose by following the popular protocols reported in the literature.27 The other was developed in this work for the extremely fluorescent CDots.
In the experiment for the first purpose designed to mimic what have been widely used in the literature, citric acid (1 g) and PEI (0.5 g) were dissolved in hot water (~50 °C, 10 mL), and the aqueous mixture was heated with stirring under ambient pressure to 200 °C, which took about 20 min and resulted in a nearly complete evaporation of the water. The sample mixture became gel-like with a pale-yellow color, to which was added water (1 mL) with the same heating and stirring, again until a nearly complete evaporation of water. The same procedure of adding water and heating to 200 °C with stirring was repeated for about 3 h until the color of the gel-like reaction mixture turned orange. The orange-colored mixture was collected and dispersed in water (10 mL). According to what have been reported in the literature,27,33 the aqueous solution thus obtained should be considered as a solution of CDots. Alternatively, also based on the protocol reported in the literature,34,35 the same citric acid - PEI mixture in water (2 mL) was heated in a sealed stainless steel reactor up to 200 °C for 3 h. The reaction mixture back at ambient temperature and pressure was collected by washing with water (10 mL) to obtain a light brown solution, again considered as a solution of CDots.34,35
In the other procedure developed in this work for extremely fluorescent CDots, citric acid (1 g) was dissolved in water (2 mL) with mild sonication (30 seconds in a VWR 250D ultrasonic cleaner). The solution was added to PEI (0.5 g) with stirring for a good mixing. The aqueous mixture was loaded into a stainless steel tube reactor (1.9 cm in OD × 0.165 cm in wall thickness × 30.5 cm in length, by Swagelok, Inc). The reactor was sealed and heated in a tube furnace (Lindberg/Blue-M by Thermal Product Solutions, Inc.) at a temperature up to 400 °C for 6 h. After the hydrothermal processing, the reactor was cooled to ambient temperature, and the reaction mixture in the reactor was collected by washing with water (10 mL). The resulting brownish aqueous solution was cleaned via dialysis (tubing cut-off molecular weight ~ 500 or 1,000) against fresh water to obtain the as-synthesized sample of PEI/CA-CDots for characterization and/or further processing. For microscopy analyses, some of the PEI/CA-CDots were doped with a small amount of silver via simple visible-light photolysis in an aqueous solution of silver nitrate (the doping level kept very low, as monitored in terms of the very onset in the initial emergence of the silver plasmon absorption band).41
For the fractionation of the as-prepared sample, a gel column was packed with the commercially supplied Sephadex G-100 gel by following the previously reported protocol.25 Briefly, the gel (15 g) was soaked in water for 3 days, and the supernatant (including the suspended ultrafine gel) was discarded. The remaining gel was washed until no gel was suspended in the supernatant. Air bubbles were removed under vacuum. Separately, a glass column (2.5 cm inner diameter) was filled with water to remove air bubbles, and then closed. The gel suspension described above was poured into the column until reaching about 2 cm in height, and then the column was opened for the continuous addition of the gel suspension. The gel-filled column was washed by water until no change in height (35 cm), followed by the testing and calibration.26 In the fractionation, a concentrated solution of the as-prepared PEI/CA-CDots sample was added to the gel column and eluted with water. Colored fractions (around 40 drops per fraction) were collected for characterization and further investigation. In a typical gel-column separation, an as-prepared PEI/CA-CDots sample with observed fluorescence quantum yield in the 60–70% range was used as the feed sample for separation. Post-separation, the fractions of higher fluorescence quantum yields than that of the feed sample collectively accounted for more than half in weight of the feed sample, and those of the highest fluorescence quantum yield accounted for about 10%.
Acknowledgments
Financial support from NIH (R15GM114752) and the South Carolina Space Grant Consortium is gratefully acknowledged. Y.H. was a visiting student from Beijing Jiaotong University in China sponsored by the China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen BL, Veca LM, Xie S-Y. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y-P. Fluorescent Carbon Nanoparticles. 7,829,772 U.S. Patent.
- 3.Luo PG, Yang F, Yang S-T, Sonkar SK, Yang L, Broglie JJ, Liu Y, Sun Y-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014;4:10791–10807. [Google Scholar]
- 4.Hola K, Zhang Y, Wang Y, Giannelis EP, Zboril R, Rogach AL. Carbon dots— emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today. 2014;9:590–603. [Google Scholar]
- 5.Wang Y, Hu A. Carbon quantum dots: synthesis, properties and applications. J. Mater. Chem. C. 2014;2:6921–6939. [Google Scholar]
- 6.Miao P, Han K, Tang Y, Wang B, Lin T, Cheng W. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7:1586–1595. doi: 10.1039/c4nr05712k. [DOI] [PubMed] [Google Scholar]
- 7.Lim SY, Shen W, Gao Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015;44:362–381. doi: 10.1039/c4cs00269e. [DOI] [PubMed] [Google Scholar]
- 8.Fernando KAS, Sahu S, Liu Y, Lewis WK, Guliants EA, Jafariyan A, Wang P, Bunker CE, Sun Y-P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces. 2015;7:8363–8376. doi: 10.1021/acsami.5b00448. [DOI] [PubMed] [Google Scholar]
- 9.Du Y, Guo S. Chemically doped fluorescent carbon and graphene quantum dots for ACCEPTED MANUSCRIPT bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale. 2016;8:2532–2543. doi: 10.1039/c5nr07579c. [DOI] [PubMed] [Google Scholar]
- 10.LeCroy GE, Yang S-T, Yang F, Liu Y, Fernando KAS, Bunker CE, Hu Y, Luo PG, Sun Y-P. Functionalized carbon nanoparticles: syntheses and applications in optical bioimaging and energy conversion. Coord. Chem. Rev. 2016;320:66–81. [Google Scholar]
- 11.Yang S-T, Cao L, Luo PG, Lu F, Wang X, Wang H, Meziani MJ, Liu Y, Qi G, Sun Y-P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009;131:11308–11309. doi: 10.1021/ja904843x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong B, Zhu A, Ding C, Zhao X, Li B, Tian Y. Carbon dot-based inorganic-organic nanosystem for two-photon imaging and biosensing of pH variation in living cells and tissues. Adv. Mater. 2012;24:5844–5848. doi: 10.1002/adma.201202599. [DOI] [PubMed] [Google Scholar]
- 13.Liu J-H, Cao L, LeCroy GE, Wang P, Meziani MJ, Dong Y, Liu Y, Luo PG, Sun Y-P. Carbon "quantum" dots for fluorescence labeling of cells. ACS Appl. Mater. Interfaces. 2015;7:19439–19445. doi: 10.1021/acsami.5b05665. [DOI] [PubMed] [Google Scholar]
- 14.Loo AH, Sofer Z, Bousa D, Ulbrich P, Bonanni A, Pumera M. Carboxylic carbon quantum dots as a fluorescent sensing platform for DNA detection. ACS Appl. Mater. Interfaces. 2016;8:1951–1957. doi: 10.1021/acsami.5b10160. [DOI] [PubMed] [Google Scholar]
- 15.Karthik S, Saha B, Ghosh SK, Pradeep Singh ND. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery. Chem. Comm. 2013;49:10471–10473. doi: 10.1039/c3cc46078a. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, Li Y, Zhai X, Xu B, Cao Z, Liu W. Polycation-b-polyzwitterion copolymer grafted luminescent carbon dots as a multifunctional platform for serum-resistant gene delivery and bioimaging. ACS Appl. Mater. Interfaces. 2014;6:20487–20497. doi: 10.1021/am506076r. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Lin J, Wang X, Wang Z, Zhang C, He M, Wang K, Chen F, Li Z, Shen G, Cui D, Chen X. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv. Mater. 2012;24:5104–5110. doi: 10.1002/adma.201200650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meziani MJ, Dong X, Zhu L, Jones LP, LeCroy GE, Yang F, Wang S, Wang P, Zhao Y, Yang L, Tripp RA, Sun Y-P. Visible-light-activated bactericidal functions of carbon "quantum" dots. ACS Appl. Mater. Interfaces. 2016;8:10761–10766. doi: 10.1021/acsami.6b01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Sahu S, Anilkumar P, Bunker CE, Xu J, Fernando KAS, Wang P, Guliants EA, Tackett KN, II, Sun Y-P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J. Am. Chem. Soc. 2011;133:4754–4757. doi: 10.1021/ja200804h. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Chen YH, Liu CY, Ma DG. White light-emitting devices based on carbon dots' electroluminescence. Chem. Comm. 2011;47:3502–3504. doi: 10.1039/c0cc05391k. [DOI] [PubMed] [Google Scholar]
- 21.Veca LM, Diac A, Mihalache I, Wang P, LeCroy GE, Pavelescu EM, Gavrila R, Vasile E, Terec A, Sun Y-P. Electroluminescence of carbon ‘quantum’ dots – from materials to devices. Chem. Phys. Lett. 2014;613:40–44. [Google Scholar]
- 22.Han X, Zhong S, Pan W, Shen W. A simple strategy for synthesizing highly luminescent carbon nanodots and application as effective down-shifting layers. Nanotechnology. 2015;26:065402. doi: 10.1088/0957-4484/26/6/065402. [DOI] [PubMed] [Google Scholar]
- 23.Xu ZQ, Lan JY, Jin JC, Dong P, Jiang FL, Liu Y. Highly photoluminescent nitrogen-doped carbon nanodots and their protective effects against oxidative stress on cells. ACS Appl. Mater. Interfaces. 2015;7:28346–28352. doi: 10.1021/acsami.5b08945. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Meziani MJ, Sahu S, Sun Y-P. Photoluminescence properties of graphene versus other carbon nanomaterials. Acc. Chem. Res. 2013;46:171–180. doi: 10.1021/ar300128j. [DOI] [PubMed] [Google Scholar]
- 25.Anilkumar P, Wang X, Cao L, Sahu S, Liu J-H, Wang P, Korch K, Tackett KN, II, Parenzan A, Sun Y-P. Toward quantitatively fluorescent carbon-based "quantum" dots. Nanoscale. 2011;3:2023–2027. doi: 10.1039/c0nr00962h. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Cao L, Yang S-T, Lu F, Meziani MJ, Tian L, Sun KW, Bloodgood MA, Sun Y-P. Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angew. Chem., Int. Ed. 2010;122:5438–5442. doi: 10.1002/anie.201000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y, Wang R, Li G, Chen C, Chi Y, Chen G. Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal. Chem. 2012;84:6220–6224. doi: 10.1021/ac3012126. [DOI] [PubMed] [Google Scholar]
- 28.Zhai X, Zhang P, Liu C, Bai T, Li W, Dai L, Liu W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Comm. 2012;48:7955–7957. doi: 10.1039/c2cc33869f. [DOI] [PubMed] [Google Scholar]
- 29.Hsu PC, Chang HT. Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem. Comm. 2012;48:3984–3986. doi: 10.1039/c2cc30188a. [DOI] [PubMed] [Google Scholar]
- 30.Stan CS, Albu C, Coroaba A, Popa M, Sutiman D. One step synthesis of fluorescent carbon dots through pyrolysis of N-hydroxysuccinimide. J. Mater. Chem. C. 2015;3:789–795. [Google Scholar]
- 31.Dong Y, Wang R, Li H, Shao J, Chi Y, Lin X, Chen G. Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon. 2012;50:2810–2815. [Google Scholar]
- 32.Wang R, Li G, Dong Y, Chi Y, Chen G. Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal. Chem. 2013;85:8065–8069. doi: 10.1021/ac401880h. [DOI] [PubMed] [Google Scholar]
- 33.Dong Y, Wang R, Tian W, Chi Y, Chen G. “Turn-on” fluorescent detection of cyanide based on polyamine-functionalized carbon quantum dots. RSC Adv. 2014;4:3685–3689. [Google Scholar]
- 34.Liu J, Liu X, Luo H, Gao Y. One-step preparation of nitrogen-doped and surface-passivated carbon quantum dots with high quantum yield and excellent optical properties. RSC Adv. 2014;4:7648–7654. [Google Scholar]
- 35.Wang C, Xu Z, Zhang C. Polyethyleneimine-functionalized fluorescent carbon dots: water stability, pH sensing, and cellular imaging. ChemNanoMat. 2015;1:122–127. [Google Scholar]
- 36.Pierrat P, Wang R, Kereselidze D, Lux M, Didier P, Kichler A, Pons F, Lebeau L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials. 2015;51:290–302. doi: 10.1016/j.biomaterials.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wang P, Fernando KAS, LeCroy GE, Maimaiti H, Harruff-Miller BA, Lewis WK, Bunker CE, Hou Z-L, Sun Y-P. Enhanced fluorescence properties of carbon dots in polymer films. J. Mater. Chem. C. 2016;4:6967–6974. doi: 10.1039/C6TC01932C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Al Awak MM, Yang F, Yan S, Xiong Q, Wang P, Tang Y, Yang L, LeCroy GE, Hou X, Bunker CE, Xu L, Tomlinson N, Sun Y-P. Photoexcited state properties of carbon dots from thermally induced functionalization of carbon nanoparticles. J. Mater. Chem. C. 2016;4:10554–10561. doi: 10.1039/C6TC03666J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang F, LeCroy GE, Wang P, Liang W, Chen J, Fernando KAS, Bunker CE, Qian H, Sun Y-P. Functionalization of carbon nanoparticles and defunctionalization—toward structural and mechanistic elucidation of carbon “quantum” dots. J. Phys. Chem. C. 2016;120:25604–25611. [Google Scholar]
- 40.LeCroy GE, Sonkar SK, Yang F, Veca LM, Wang P, Tackett KN, II, Yu J-J, Vasile E, Qian H, Liu Y, Luo PG, Sun Y-P. Toward structurally defined carbon dots as ultracompact fluorescent probes. ACS Nano. 2014;8:4522–4529. doi: 10.1021/nn406628s. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Sahu S, Cao L, Bunker CE, Peng G, Liu Y, Fernando KAS, Wang P, Guliants EA, Meziani MJ, Qian H, Sun Y-P. Efficient fluorescence quenching in carbon dots by surface-doped metals - disruption of excited state redox processes and mechanistic implications. Langmuir. 2012;28:16141–16147. doi: 10.1021/la302506e. [DOI] [PubMed] [Google Scholar]