Abstract
Background
Aberrant promoter DNA methylation of the cysteine dioxygenase 1 (CDO1) gene is found in various human cancers and is associated with clinical outcome. In this study, we assessed for the first time the clinicopathological significance of CDO1 methylation in primary gallbladder cancer (GBC) in comparison with non-malignant gallbladder disease.
Methods
CDO1 DNA methylation was quantified using quantitative TaqMan methylation specific PCR (Q-MSP) in 99 primary GBC patients together with the 78 corresponding non-tumor tissues and 26 benign gallbladder disease (including 7 patients with xanthogranulomatous cholecystitis) who underwent surgical resection between 1986 and 2014.
Results
The average CDO1 TaqMeth value of primary GBCs was 23.5±26. These values were significantly higher than those of corresponding non-tumor tissues (average 8±13, p < .0001) and diseased gallbladder tissues from patients with benign gallbladder diseases (average 0.98±1.6, p < .0001). CDO1 hypermethylation is also found in xanthogranulomatous cholecystitis. Using a cut-off value of 17.7, GBC cases with CDO1 hypermethylation (n = 47) showed significantly poorer prognosis than those with CDO1 hypomethylation (n = 52) (p = 0.0023). Multivariate Cox proportional hazards analysis identified that CDO1 hypermethylation was an independent prognostic factor. Notably, CDO1 hypermethylation showed prognostic relevance, especially in stage II GBC, in which it is highly anticipated to work as a predictive marker for candidates of adjuvant therapy.
Conclusions
Promoter NA methylation of CDO1 was demonstrated for the first time to be a cancer-associated methylation in primary GBC, and it has the potential to be a prognostic biomarker of GBC for high-risk patients with stage II GBC.
Introduction
Cancer statistics predicted an estimated 11,420 new gallbladder cancer (GBC) cases and 3,710 GBC deaths in the United States in 2016 [1]. GBC has been associated with a poor prognosis, and the depth of tumor invasion and the presence of lymph node metastasis have been reported to be important prognostic factors [2]. High rates of both local and distant recurrence have prompted interest in the use of adjuvant chemotherapy and radiation therapy (RT). At present, however, there is a paucity of high-quality evidence to support adjuvant treatment in GBC, and patients should be encouraged to participate in clinical trials evaluating new strategies. Therefore, molecular biomarkers are highly demanded in the clinic for tumor diagnosis and prognosis prediction, with establishment of an appropriate operative extent and optimal guidelines of postoperative adjuvant therapy.
Epigenetic gene silencing of tumor suppresser genes through promoter DNA hypermethylation is a common feature in human cancers, whereas cancer specific methylation is a relatively rare event [3, 4]. We have developed pharmacologic reversal of epigenetic silencing and thereby uncovered a myriad of transcriptionally repressed genes in human cancers [5]. Using this technique, we have identified novel tumor suppressor gene candidates including the cysteine dioxygenase type 1 (CDO1) gene in human cancers. Aberrant CDO1 promoter DNA methylation in breast cancer has firstly been reported by Dietrich et al. as a prognostic biomarker in patients who received adjuvant chemotherapy [6]. The association of CDO1 methylation with overall survival in breast cancer patients was further confirmed by Jeschke et al [7]. Furthermore, promoter DNA of the CDO1 gene was frequently methylated in esophagus, lung, bladder, gastric, and colorectal cancers [8]. Additionally, we and others investigated strong association of CDO1 gene promoter DNA methylation with poor prognosis in primary breast cancer [9], renal clear-cell cancer [10], esophageal squamous cell carcinoma [11] and Barrett esophagus adenocarcinoma [12]. Intriguingly, some previous literature has shown the availability of this methylation for diagnosis using a liquid biopsy for analysis of CDO1 promoter DNA methylation [13, 14]
In this study, we investigated for the first time the clinicopathological and prognostic relevance of promoter DNA methylation of the CDO1 gene in primary GBC in comparison with gallbladder disease.
Material and methods
GBC cell lines and tissue samples
We used 4 different human cancer cell lines in this study. The GBC cell lines (G-415 and TGBC2TKB), colorectal cancer (CRC) cell line DLD1 and the hepatoblastoma cell line HepG2 were purchased from RIKEN Bio Resource Centre (Ibaraki, Japan). DLD1 and HepG2 were used as positive and negative controls of methylation, respectively. DLD1 and G-415 were maintained in RPMI 1640 Medium (GIBCO, Carlsbad, CA) and HepG2 and TGBC2TKB were maintained in DMEM (Sigma Aldrich, St. Louis, Mo), containing 10% fetal bovine serum and Penicillin-Streptomycin (GIBCO).
We retrospectively recruited 99 primary gallbladder cancer (GBC) patients and 26 benign gallbladder disease patients, including 10 chronic cholecystitis patients (CGC), 9 adenomyomatosis patients (ADM), 7 xanthogranulomatous cholecystitis patients (XGC), who had undergone surgical resection of the primary tumors at the Kitasato University Hospital between June 1986 and September 2014. We included cases with distant metastasis or residual diseases. All 99 GBC patients had complete information available for all of the clinicopathological factors.
We extracted DNA from the formalin-fixed paraffin embedded (FFPE) 99 tumor tissues (T) and 78 corresponding non-cancerous mucosa tissues (CN) of the 99 primary GBC patients. In addition, 26 diseased gallbladder tissues (NN) from patients with benign gallbladder disease were included to compare methylation status. All tissue samples were collected at the Kitasato University Hospital, all patients had agreed to the use of their pathological specimens and written consent was obtained from all patients and healthy donors before sample collection. The present study was approved by the Ethics Committee of Kitasato University.
Clinicopathological factors
TNM classification was performed according to the 6th edition of the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS).
Operative procedures were as follows: Surgical resection was divided into 3 groups depending on the degree of resection; simple cholecystectomy (SCx), the so-called standard resection (SR), and extended resection (ER). SR refers to cholecystectomy plus liver bed resection approximately 2 cm from the gallbladder or partial resection of the liver, the lower part of segment 4 and 5. ER included the following operative procedures: hepatectomy more than segmentectomy; pancreaticoduodenectomy; and hepatopancreaticoduodenectomy.
Postoperative chemotherapy was as follows: Adjuvant therapy after surgical resection was not strictly protocol-driven and was administered in the treatment of patients at the discretion of surgeons and oncologists. The chemotherapy regimens consisted of 5-fluorouracil (5-FU)-based chemotherapy (5-FU only, or 5-FU/mitomycin-C by venous infusion, and tegafur/uracil (UFT) or 5’-deoxy-5-fluorouridine (5’DFUR), or TS-1 as oral therapy). Others were mitomycin-C or gemcitabine or gemcitabine + TS-1.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from cell lines were extracted using Rneasy Mini Kit (QIAGEN, Hilden, Germany). Reverse-transcribed with Super Script III reverse transcriptase kit (Invitrogen, Carlsbad, CA). Primers sequences are also included in S1 Table. RT-PCR was performed by 30 cycles of 95°C for 1 min, 58°C for 1 min, and 72°C for 1 min., and the PCR products were separated on 1.5–2.0% agarose gel, then visualized by ethidium bromide staining. β-actin was used as an internal control.
5-Aza-dC and TSA treatment
Cells (1×106 cells/T-75 flask) were treated with 1 or 5 μM of the demethylating agent 5-aza-2′-deoxycytidine (5-Aza-dC) (Sigma-Aldrich) dissolved in 50% acetic acid or mock-treatment with PBS including the same amount of acetic acid every 24 hr for 4 days. When combined with the histone deacetylase inhibitor trichostatin A (TSA) (Sigma-Aldrich), 300 nM TSA was added to the medium for the final 24 hr.
Bisulfite treatment of DNA
Genomic DNA of FFPE and cell lines were extracted using QIAamp DNA FFPE Tissue Kit and QIAamp DNA Mini Kit (QIAGEN). Bisulfite treatment was done by using a Methylation-Gold Kit (QIAGEN).
Quantitative-methylation-specific PCR (Q-MSP)
Quantitative-TaqMan methylation specific PCR (Q-MSP) was carried out using iQ Supermix (Bio-Rad Laboratories, Hercules, CA) in triplicate on the C1000 Touch™ Thermal Cycler CFX96 Real Time System (Bio-Rad). PCR conditions and the primer sequences are provided in S1 Table. Serial dilutions of bisulfite modified DNA from the CRC cell line DLD1 was used to construct the calibration curve on each plate as a methylation positive control, and the Hepatoblastoma cell line HepG2 was used as a negative control. The methylation value (designated as the TaqMeth value as previously described) was defined by the ratio of the amplified signal value of methylated CDO1 to the value for β-actin, which was then multiplied by 100. This ratio was used as a measure for the relative level of methylated DNA in samples. Many literatures previously assessed CpG islands structure and bisulfite-sequencing in the CDO1 gene promotor and analysis of CDO1 promotor activity by luciferase reporter assay to investigate whether CDO1 expression is regulated by the promotor methylation [7–9].
Immunohistochemistry
Formalin fixed, paraffin-embedded histological sections (3 μm thick) were immunostained using the anti-CDO1 rabbit polyclonal antibody (dilution of 1:100) (ATLAS ANTIBODIES, Bromma, Sweden) at 4°C overnight. The primary antibody was visualized using the Histofine Simple Stain PO (M) kit (Nichirei, Tokyo, Japan). And immune complexes were detected using the 3,3′-diamino-benzidine tetrahydrochloride (DAB) substrate, as a chromogen for 2 minutes. Sections were counterstained in Mayer’s Hematoxyline.
Statistical analysis
Student’s t-test was used for analysis of continuous variables, and the χ2 test was used for analysis of categorical variables. Clinicopathological characteristics and follow up data were analyzed in terms of overall survival (OS). The follow up time was calculated from the date of surgery to death. OS was calculated by the Kaplan-Meier method, and survival differences were assessed using the log-rank test. Variables suggested to be prognostic factors in univariate analysis (P<0.05) were subjected to multivariate analysis using a Cox proportional-hazards model. A P-value <0.05 was considered to indicate statistical significance. All statistical analyses were conducted using the SAS software package (JMP Pro11, SAS Institute, Cary, NC).
Results
Background of the primary GBC patients
The average age was 67 years (range, 40–86). Seventy-five (76%) of the 99 GBC patients underwent curative surgical resection (R0). Recurrence rate of the 99 primary GBC patients was 48%, and mortality was 46%. The median of postoperative follow-up period was 32 months (range, 0–243 months).
CDO1 promoter methylation level and its correlation with clinicopathological factors in GBC
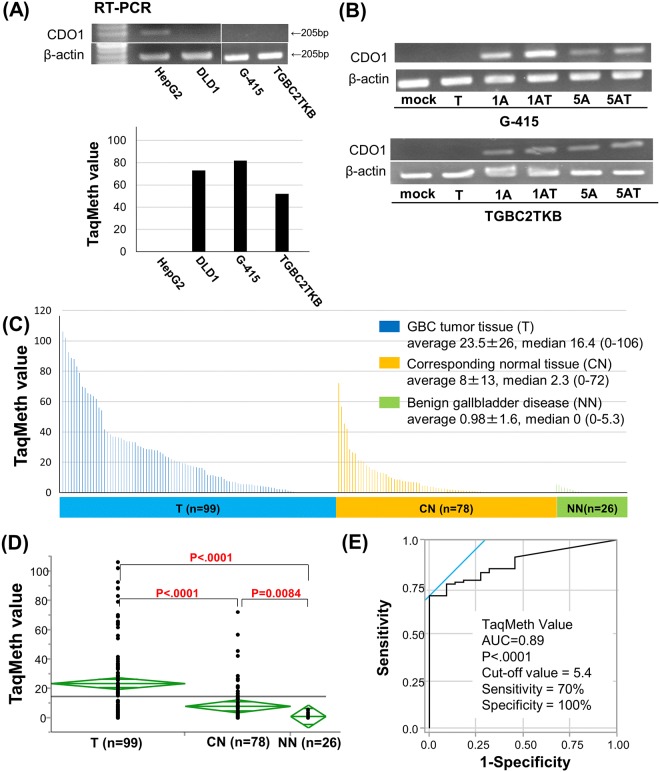
In the 2 GBC cell lines, no basal expression of CDO1 was seen (Fig 1A), and silenced expression of CDO1 gene was robustly reactivated after treatment with the demethylating agent, 5-Aza-dC, 5-Aza-dC and TSA (Fig 1B). Furthermore, CDO1 TaqMeth value in the GBC cell lines was detected highly as well as those of positive control DLD1 (Fig 1A). These results indicated that CDO1 is epigenetically inactivated in GBC cell lines.
Fig 1. Quantitative assessment of CDO1 methylation in primary GBC.
(A) Expression level of CDO1 by RT–PCR (top panel) and CDO1 TaqMeth value in the GBC cell lines (G-415 and TGBC2TKB) (bottom panel). HepG2 cells, positive control for CDO1 expression; DLD1 cells, negative control for CDO1 expression. (B) Expression level after treatment with 5-Aza-dC alone, TSA alone or the combination by RT–PCR in the GBC cell lines. M, mock including the same volume of acetic acid; A, 5-Aza-dC (1 or 5 mM; 1A or 5A); T, TSA. (C) TaqMeth value in the 99 primary GBC tumor tissues (T), 78 corresponding normal tissues (CN) and 26 benign gallbladder disease (NN). (D) There was a significant difference in CDO1 TaqMeth values between T and CN (p = 0.0017), and between T and NN (p < .0001). (E) Receiver-operating characteristic curve of CDO1 methylation for the detection of primary gallbladder cancer. The area under the curve (AUC) represents the accuracy in discriminating T from NN in terms of its sensitivity and specificity (P < .0001).
The median TaqMeth value in the 99 primary GBC tumor tissues (T) was 16.5, with values ranging from 0 to 106 and the average methylation of them was 23.5 ± 26, while the median methylation value in corresponding normal tissues (CN) of 78 was 2.3, with values ranging from 0 to 72 and the average methylation of them was 8 ± 13. The median value in diseased gallbladder tissues (NN) of benign gallbladder disease patients was 0, with values ranging from 0 to 5.3 and the average methylation of them was 0.98 ± 1.6 (Fig 1C). There was a significant difference in CDO1 TaqMeth value between T and CN (p < .0001), and between T and NN (p < .0001) (Fig 1D). Promoter DNA methylation of the CDO1 gene showed highly discriminative receiver–operator characteristic (ROC) curve profiles, distinguishing T from NN (Fig 1E); the area under the ROC curve (AUROC) that discriminates T from NN was 0.89. In order to maximize sensitivity and specificity of detection, the optimal cut-off for methylation of the CDO1 gene was calculated from the ROC analysis (value 5.4). Using this cut-off, the sensitivity of the assay for GBC was 70%, while the specificity was 100%. In NN, CDO1 TaqMeth value of XGc (median 2.7, range 0–5.3) was significantly higher than those of others (p = 0.0010) (S2A Fig).
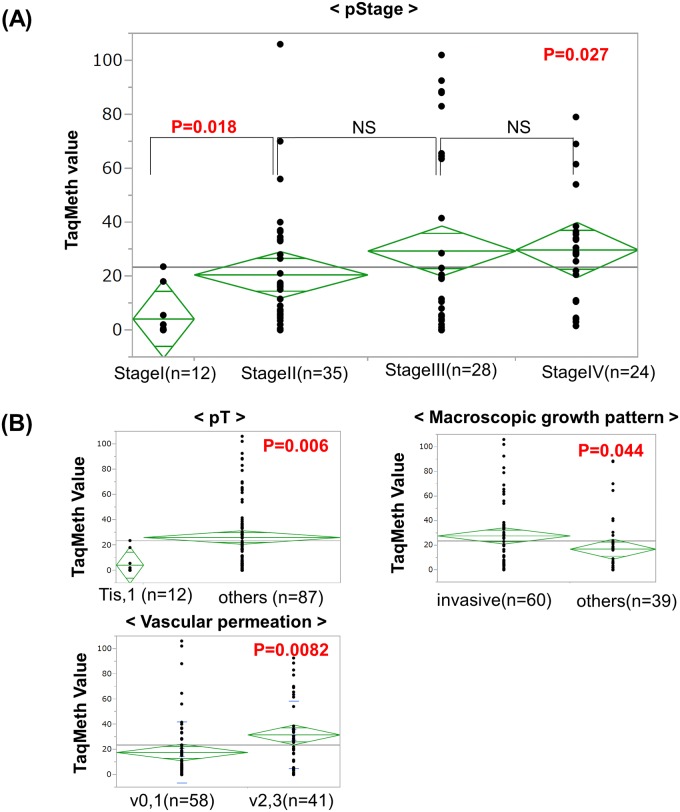
Correlation of each clinicopathological factor with CDO1 TaqMeth values of the primary GBC was determined by using Student’s t-test, and is shown in Fig 2. There were significant correlations of CDO1 TaqMeth values with pStage (p = 0.027), primary tumor factor (pT) (p = 0.0060), macroscopic growth pattern (p = 0.044), and vascular permeation (p = 0.0082). The other clinicopathological factors, such as preoperative serum values of CA19-9, lymph node metastasis, distant metastasis, and lymphatic permeation, were not significantly related to CDO1 TaqMeth values based on analysis of variance (ANOVA).
Fig 2. Correlation of the TaqMeth value of CDO1 to various clinicopathological factors in primary GBC.
(A) Correlation of the CDO1 TaqMeth value to pStage. The TaqMeth values of the CDO1 gene showed significant differences between Stage I and other stages of GBC (p = 0.018). (B) Correlation of the CDO1 TaqMeth value to clinicopathological factors other than pStage. The CDO1 TaqMeth value was significantly associated with pT, macroscopic growth pattern and vascular permeation.
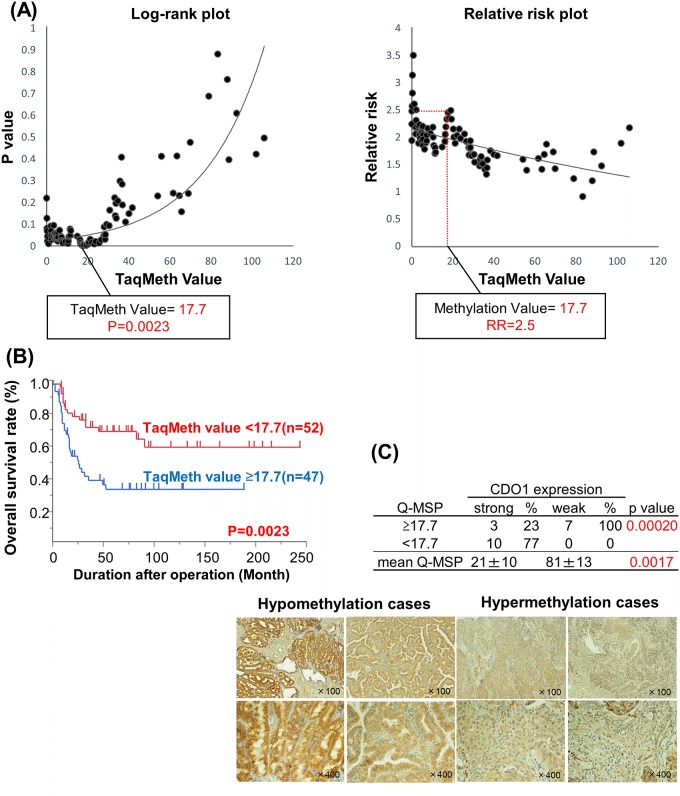
We further investigated whether the CDO1 TaqMeth value could predict prognostic outcomes of GBC. A Kaplan-Meier curve of overall survival was constructed for the 99 patients according to the CDO1 TaqMeth values, and p value and relative risk were each plotted to analyze survival differences between CDO1 TaqMeth values above and below the best optimized cut-off determined by using the log rank test (Fig 3A). We have herein based on the methods to determine the best optimal cut-off value for the prognostic stratification using the log-rank prognostic analysis [15–17]. We thereby defined the optimal cut-off value of the CDO1 TaqMeth value as 17.7 which showed the highest relative risk with statistical significance (p<0.05). Using this cut-off value to divide the patients into hyper- and hypo-methylation groups, the hypermethylation group showed a survival rate of 34% (n = 47), while the hypomethylation group showed a survival rate of 69% (n = 52). The prognostic difference between these two groups showed high statistical significance (p = 0.0023) (Fig 3B).
Fig 3. Prognostic analysis and CDO1 protein expression according to the TaqMeth value of CDO1 in primary GBC.
(A) Identification of an optimal cut-off value for the prognosis using the log-rank prognostic analysis. (B) Kaplan–Meier curves for CDO1 methylation status with value above or below 17.7 in the primary GBC (p = 0.0023). (C) Correlation between CDO1 protein expression and TaqMeth value in 20 primary GBC patients (top). Representative pictures taken from immunohistochemistry of CDO1 in methylation-positive or negative GBC samples (bottom left and right).
We then examined the differences of CDO1 protein expression in GBC tissues by immunohistochemistry with anti CDO1 polyclonal antibody, where patients with CDO1 hypomethylation (n = 10) and CDO1 hypermethylation (n = 10) were compared. Intense expression of CDO1 protein was observed in all CDO1 hypomethylation cased (n = 10), whereas weak expression of CDO1 protein was dominant in seven cases (70%) of CDO1 hypermethylation cases (n = 10). The difference of CDO1 protein expression between these two groups showed statistical significance (p = 0.0002) (Fig 3C).
Univariate and multivariate prognostic analyses including CDO1 TaqMeth values in primary GBC
The 5-years OS rate of all GBC patients was 52%. The 5-years OS in each stage was 92%, 78%, 36%, and 13%, in Stage 0/I, II, III, and IV, respectively (S1 Fig). Univariate prognostic analyses showed that preoperative serum value of CA19-9, preoperative inflammation, primary tumor factor (pT factor), lymph node metastasis (pN factor), distant metastasis (pM factor), stage, histology, mode of histological infiltration, macroscopic growth pattern, lymphatic permeation, vascular permeation, and resection status were factors indicative of poor prognosis (Table 1).
Table 1. Univariate and multivariate analysis for overall survival (OS).
| Clinicopathological parameters | Number | Univariate analysis | Multiivariate analysis | |||
|---|---|---|---|---|---|---|
| OS (%) | p valuea | HR | 95%CI | p valueb | ||
| Age | ||||||
| <65 / ≥65 | 38 / 61 | 48 / 54 | 0.448 | |||
| Gender | ||||||
| male / female | 38 / 61 | 44 / 57 | 0.084 | |||
| Preopertive serum CA19-9 | ||||||
| <37 / ≥37 | 56 / 43 | 68 / 32 | 0.0001 | 1.7 | 0.84–3.7 | 0.05 |
| Preoperative inflammation | ||||||
| absence / presence | 83 / 16 | 58 / 25 | 0.013 | 1.6 | 0.53–5.8 | 0.40 |
| Preoperative jaundice | ||||||
| absence / presence | 91 / 8. | 54 / 38 | 0.46 | |||
| Preoperative biliary drainage | ||||||
| absence / presence | 86 / 13 | 58 / 15 | 0.0007 | 2.1 | 0.56–8.0 | 0.27 |
| Tumor location | ||||||
| Gfb / GnC | 64 / 35 | 53 / 49 | 0.68 | |||
| Histology | ||||||
| well, mod, pap / others | 74 / 25 | 62 / 24 | 0.0004 | 1.2 | 0.60–2.4 | 0.58 |
| Mode of histological Infiltration | ||||||
| α / βγ | 18 / 81 | 81 / 47 | 0.017 | 1.6 | 0.42–8.1 | 0.52 |
| Macroscopic growth pattern | ||||||
| others / invasive | 39 / 60 | 68 / 42 | 0.0082 | 0.63 | 0.27–1.5 | 0.29 |
| Lymphatic permeation (ly) | ||||||
| ly0, 1 / ly2, 3 | 73 / 26 | 69. / 4 | <.0001 | 2.4 | 1.1–5.5 | 0.028 |
| Vascular permeation (v) | ||||||
| v0, 1 / v2, 3 | 58 / 41 | 71 / 25 | <.0001 | 1.33 | 0.54–3.2 | 0.53 |
| Operative procedures | ||||||
| SCx / SR / ER | 36 / 47 / 16 | 43 / 63 / 41 | 0.092 | |||
| Lymph node dissection (D) | ||||||
| D1, 2 / D0 | 74 / 25 | 57 / 38 | 0.060 | |||
| Resection status (R) | ||||||
| R0 / R1, 2 | 75 / 24 | 67. / 6 | <.0001 | 2.6 | 1.0–6.5 | 0.042 |
| Postoperative chemotherapy | ||||||
| absence / presence | 51 / 48 | 59 / 45 | 0.23 | |||
| TaqMeth Value | ||||||
| <17.7 / ≥17.7 | 52 / 47 | 69 / 34 | 0.0023 | 2.1 | 1.0–4.4 | 0.047 |
| pStage* | ||||||
| I | 12 | 92 | <.0001 | Reference | 0.54 | |
| II | 35 | 78 | 2.2 | 0.30–45 | ||
| III | 28 | 36 | 3.7 | 0.53–77 | ||
| IV | 24 | 13 | 3.8 | 0.44–86 | ||
Abbreviations: HR, Hazard Ratio; CI, confidence interval; Gfb, fundus and body of gallbladder; GnC, gallbladder neck and cystic duct; SCx, simple cholecystectomy; SR, standard resection; ER, extended resection.
* Classification of biliary tract cancers established by Japanease Society of Hepato-Biliary Pancreatic Surgery (JSHBPS): The 6th edition.
a Log-rank test.
b Cox-proportional hazard model.
The clinicopathological factors related to prognosis were then applied to a multivariate Cox proportional hazards model. Individual TNM factors were excluded, because they are considered to be confounding factors. The multivariate analyses indicated that CDO1 DNA hypermethylation (p = 0.047), lymphatic permeation (ly2/3) (p = 0.028), and resection status (R1/2) (p = 0.042) were independent prognostic factors that were significantly related to OS in primary GBC (Table 1), and stage was eliminated as a prognostic factor. Kaplan-Meier curves of the overall survival of the patients according to the independent prognostic factors of lymphatic permeation and resection status are shown (S1B Fig).
Correlation of clinicopathological factors to the CDO1 TaqMeth value status divided by the prognostically optimized cut-off value in primary GBC
Correlation of clinicopathological factors in primary GBC according to CDO1 promoter DNA methylation status, using a CDO1 TaqMeth cut-off value 17.7, was determined using a χ2 test. pT factor (≥T2 advanced), advanced stage, mode of histological infiltration (β/γ), lymphatic permeation (ly2/3), and vascular permeation (v2/3) were significantly related to CDO1 promotor DNA hypermethylation status (Table 2).
Table 2. Correlation of clinicopathological factors and CDO1 methylation.
| Clinicopathological parameters | CDO1 TaqMeth value | p valuea | ||||
|---|---|---|---|---|---|---|
| low (<17.7) | high (≥17.7) | |||||
| n = 52 | n = 47 | |||||
| No. | % | No. | % | |||
| Preoperative factor | ||||||
| Age | <65 | 22 | 42 | 16 | 34 | 0.40 |
| ≥65 | 30 | 58 | 31 | 66 | ||
| Gender | male | 22 | 42 | 16 | 34 | 0.40 |
| female | 30 | 58 | 31 | 66 | ||
| Preopertive serum CA19-9 | <37 | 30 | 58 | 26 | 55 | 0.81 |
| ≥37 | 22 | 42 | 21 | 45 | ||
| Preoperative inflammation | absence | 44 | 85 | 39 | 83 | 0.83 |
| presence | 8 | 15 | 8 | 17 | ||
| Preoperative jaundice | absence | 47 | 90 | 44 | 94 | 0.56 |
| presence | 5 | 10 | 3 | 6 | ||
| Preoperative biliary drainage | absence | 44 | 85 | 42 | 89 | 0.49 |
| presence | 8 | 15 | 5 | 11 | ||
| Pathological factor | ||||||
| pT | Tis, T1 | 10 | 19 | 2 | 4 | 0.023 |
| other | 42 | 81 | 45 | 96 | ||
| pN | N0 | 37 | 71 | 28 | 60 | 0.23 |
| N1 | 15 | 29 | 19 | 40 | ||
| pM | M0 | 46 | 88 | 37 | 79 | 0.19 |
| M1 | 6 | 12 | 10 | 21 | ||
| pStage* | I | 10 | 19 | 2 | 4 | 0.023 |
| others | 42 | 81 | 45 | 96 | ||
| Tumor location | Gfb | 35 | 67 | 29 | 62 | 0.56 |
| GnC | 17 | 33 | 18 | 38 | ||
| Histology | well, mod, pap | 42 | 81 | 32 | 68 | 0.15 |
| other | 10 | 19 | 15 | 32 | ||
| Mode of histological Infiltration | α | 14 | 27 | 4 | 9 | 0.018 |
| β, γ | 38 | 73 | 43 | 91 | ||
| Macroscopic growth pattern | invasive | 27 | 52 | 33 | 70 | 0.063 |
| others | 25 | 48 | 14 | 30 | ||
| Lymphatic permeation (ly) | ly0, 1 | 44 | 85 | 29 | 62 | 0.0097 |
| ly2, 3 | 8 | 15 | 18 | 38 | ||
| Vascular permeation (v) | v0, 1 | 38 | 73 | 20 | 43 | 0.0021 |
| v2, 3 | 14 | 27 | 27 | 57 | ||
| Treatment factor | ||||||
| Operative procedures | Standard resection | 26 | 50 | 20 | 43 | 0.46 |
| others | 26 | 50 | 27 | 57 | ||
| Lymph node dissection (D) | D0 | 15 | 29 | 16 | 34 | 0.99 |
| D1, 2 | 27 | 52 | 29 | 62 | ||
| Resection status (R) | R0 | 42 | 81 | 33 | 70 | 0.22 |
| R1, 2 | 10 | 19 | 14 | 30 | ||
| Postoperative chemotherapy | absence | 26 | 50 | 25 | 53 | 0.75 |
| presence | 26 | 50 | 22 | 47 | ||
Abbreviations: Gfb, fundus and body of gallbladder; GnC, gallbladder neck and cystic duct
* Classification of biliary tract cancers established by Japanease Society of Hepato-Biliary Pancreatic Surgery (JSHBPS): The 6th edition.
a χ2 test or Fisher exact test
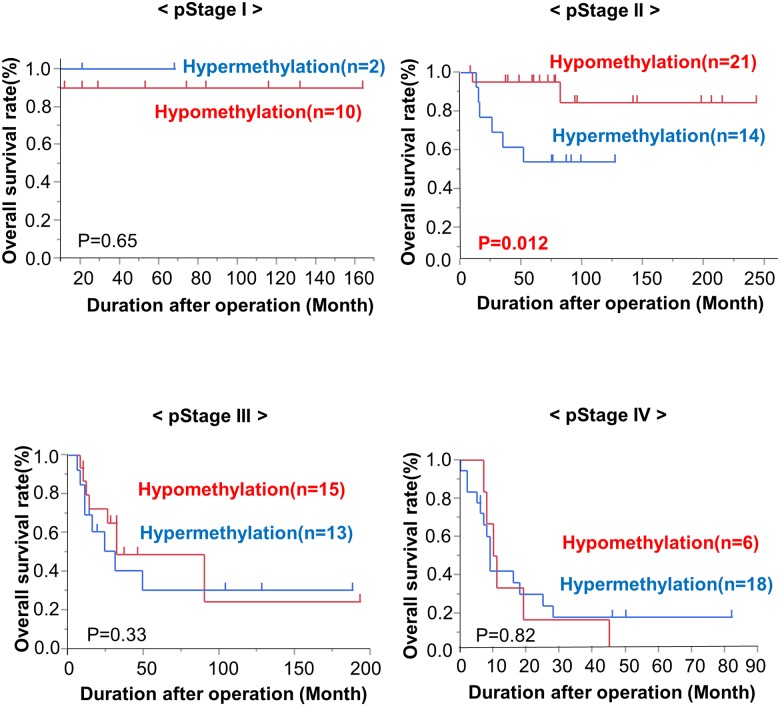
Prognostic relevance of the CDO1 TaqMeth value in each stage of GBC
The TaqMeth values of the CDO1 gene showed significant differences between Stage I and other stages of GBC (Fig 2B). We also examined the OS of each pStage (stage I, stage II, stage III and stage IV) according to CDO1 TaqMeth values. This analysis showed that the hypermethylation group showed poorer prognosis than the hypomethylation group, especially in Stage II (p = 0.023) (Fig 4). In addition, we performed a subgroup prognostic analysis for stage II GBC CDO1 hypomethylation group vs. CDO1 hypermethylation group. Lymphatic permeation (ly2/3) was significantly related to CDO1 hypermethylation status in Stage II (p = 0015).
Fig 4. Kaplan-Meier curve for overall survival of each pathological stage according to the CDO1 TaqMeth value.
The CDO1 gene hypermethylation group showed poorer prognosis than the CDO1 gene hypomethylation group, especially in Stage II (p = 0.023), but not in other Stages.
Discussion
We recently confirmed the CDO1 gene as being specifically methylated in various human cancers [8] following our development of an original algorithm designated as a pharmacological unmasking microarray (PUM), which suggested that CDO1 plays a tumor suppressive role in human carcinogenesis [5]. Promoter DNA of the CDO1 gene has been reported to be frequently methylated in various cancers, including breast [6–9], esophagus [11], lung [8, 18, 19], bladder [8], gastric [8], colorectal [8, 20], biliary tract [13], hepatocyte [21], renal clear-cell cancer [10], and testicular germ cell cancers [22]. Furthermore, we previously described detection of CDO1 methylation in the plasma of colorectal cancer (CRC) using methylation specific PCR (Q-MSP) and extensive analysis of the PCR reaction [23]. We showed that analysis of plasma CDO1 methylation in combination with CEA/CA19-9 levels increases the detection rate of curable CRC patients. In the present study, we investigated for the first time the promoter DNA methylation status of the CDO1 gene in primary GBC. We found that CDO1 promoter hypermethylation was cancer prone and strongly associated with tumor malignancy. However, CDO1 TaqMeth value of CN was significantly higher than those of NN (p = 0.0084), and the AUC of CDO1 methylation to discriminate T from CN was 0.71, which is the lowest value compared to those reported to date in the literature for cancers such as breast (AUC, 0.84), esophagus (AUC, 0.91), lung (AUC, 0.87), bladder (AUC, 0.87), and stomach (AUC, 0.95) [8]. Furthermore, TaqMeth value of CN with CDO1 hypermethylation status in T was significantly higher than them of CN with CDO1 hypomethylation status in T by the χ2 test (p = 0.011). Frequent CDO1 methylation of the CN may suggest the existence of a potentially precancerous lesion around the main lesion.
Interestingly, CDO1 hypermethylation was also found in xanthogranulomatous cholecystitis (S2A Fig). Xanthogranulomatous cholecystitis (XGC) is an uncommon variant of chronic cholecystitis characterized by xanthogranulomatous inflammation of the gallbladder. Intramural accumulation of lipid-laden macrophages and acute and chronic inflammatory cells is the hallmark of the disease. The xanthogranulomatous inflammation of the gallbladder can be very severe and can spill over to the neighbouring structures like liver, bowel and stomach resulting in dense adhesions, perforation, abscess formation, fistulous communication with adjacent bowel. Striking gallbladder wall thickening and dense local adhesions can be easily mistaken for carcinoma of the gallbladder, both intraoperatively as well as on preoperative imaging [24]. In addition, although XGC is not believed to be a premalignant lesion, the frequency of coexisting XGC and GB cancer is nearly 10% [25]. There were no patients with coexisting XGC and GB cancer in this study, but this result propose further study whether aberrant CDO1 DNA methylation in XGC mean possibility to be an onset factor of GBC or the result of accumulation of severe inflammation of the gallbladder wall.
There was no significant difference in CDO1 methylation between Stage0/I and the corresponding non-cancerous mucosa tissues (CN) (S2B Fig). However, the CDO1 methylation value increased in a stepwise manner towards advanced stages of GBC; in particular there was a significant difference in CDO1 methylation between Stage I and Stage II (p = 0.018) (Fig 2A). Prognosis of Stage I was very much better than that of Stage II (S1A Fig) as reported previously [26, 27]. The gallbladder has unique histological characteristics including the fact that it does not have lamina muscularis mucosae and that cancer cells tend to more easily infiltrate into the thin intrinsic muscle layer and serosa in comparison with other cancers. That is the reason why the increase in CDO1 methylation from Stage I to Stage II is important.
More importantly, we also showed that CDO1 hypermethylation was significantly associated with poor prognosis in Stage II GBC patients, where all patients underwent R0 resection, and when the optimal cut-off value of CDO1 methylation was determined according to the log-rank plot analysis for total cases. This finding indicated that the CDO1 methylation cut-off value of 17.7 has clinically meaningful significance, because the stage II patients with a CDO1 methylation value below 17.7 showed excellent prognosis that was similar to that of stage I patients. There have been numerous clinical reports describing that GBC patients with either Stage I or Stage II are potentially resectable with radical surgery and have a reasonable prognosis, but that some of the Stage II patients showed a poor prognosis. When postoperative surveillance strategy including adaptation of postoperative adjuvant therapy is considered, CDO1 hypermethylation could be a beneficial predictor for patients at risk for recurrence. Moreover, in the log-rank plot analysis of the CDO1 TaqMeth value, the higher this value was, the worse the prognosis was, and this held true for almost all cut-off values among the TaqMeth value range from 1 to 30. Therefore, CDO1 methylation status is regarded as an ideal prognostic indicator of primary GBC.
In this study, CDO1 hypermethylation was significantly associated with GBC tumor progression parameters, if the CDO1 methylation cut-off value of 17.7, which was meaningful from a prognostic point of view, was used. These findings suggested that CDO1 methylation was significantly associated with phenotypic acquisition of invasive and metastatic properties in primary GBC. Brait et al. reported that forced expression of full-length CDO1 in human cancer cells markedly decreased anchorage independent growth and/or tumorigenesis even in a mouse model, whereas knockdown of CDO1 inversely increased cell growth in both states. These functional data suggested that CDO1 hypermethylation may play a causative role in tumor progression of primary GBC, rather than in the results of progression.
Limitation
The limitations of this study included a retrospective study over 20 years with 99 GBC patients and loss of statistical power in the stage-specific prognostic sub analysis. So, this study might suffer from bias, i.e., change in treatment strategy such as range of lymph node dissection and application of chemoradiotherapy. Prospective validation is further needed to clarify the relationship between CDO1 promotor DNA methylation and prognosis in primary gallbladder cancer.
Conclusions
Promoter DNA methylation of the CDO1 gene was, for the first time, proven to be cancer-prone in primary GBC, and it could be a useful biomarker independent of stage in primary GBC.
Supporting information
(A) Comparison of overall survival according to pStage. (B) The Kaplan-Meier curve of the overall survival of the patients are shown according to lymphatic permeation and resection status.
(TIF)
(A) In the diseased gallbladder tissues (NN) of benign gallbladder disease patients, CDO1 TaqMeth value of XGc was significantly higher than those of others (p = 0.0010). (B) There was no significant difference in CDO1 methylation between tumor tissues (T) of Stage 0, I patients and the corresponding non-cancerous mucosa tissues.
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. Epub 2016/01/09. doi: 10.3322/caac.21332 . [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum DJ, Vigano L, Russolillo N, Langella S, Ferrero A, Capussotti L. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Annals of surgical oncology. 2015;22(3):811–8. Epub 2014/09/10. doi: 10.1245/s10434-014-4044-4 . [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92. Epub 2007/02/27. doi: 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–54. Epub 2003/11/25. doi: 10.1056/NEJMra023075 . [DOI] [PubMed] [Google Scholar]
- 5.Yamashita K, Upadhyay S, Osada M, Hoque MO, Xiao Y, Mori M, et al. Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell. 2002;2(6):485–95. Epub 2002/12/25. . [DOI] [PubMed] [Google Scholar]
- 6.Dietrich D, Krispin M, Dietrich J, Fassbender A, Lewin J, Harbeck N, et al. CDO1 promoter methylation is a biomarker for outcome prediction of anthracycline treated, estrogen receptor-positive, lymph node-positive breast cancer patients. BMC cancer. 2010;10:247 Epub 2010/06/03. doi: 10.1186/1471-2407-10-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeschke J, O'Hagan HM, Zhang W, Vatapalli R, Calmon MF, Danilova L, et al. Frequent inactivation of cysteine dioxygenase type 1 contributes to survival of breast cancer cells and resistance to anthracyclines. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(12):3201–11. doi: 10.1158/1078-0432.CCR-12-3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brait M, Ling S, Nagpal JK, Chang X, Park HL, Lee J, et al. Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. PloS one. 2012;7(9):e44951 Epub 2012/10/03. doi: 10.1371/journal.pone.0044951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minatani N, Waraya M, Yamashita K, Kikuchi M, Ushiku H, Kojo K, et al. Prognostic Significance of Promoter DNA Hypermethylation of cysteine dioxygenase 1 (CDO1) Gene in Primary Breast Cancer. PloS one. 2016;11(1):e0144862 Epub 2016/01/20. doi: 10.1371/journal.pone.0144862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckers IA, Schouten LJ, Van Neste L, van Vlodrop IJ, Soetekouw PM, Baldewijns MM, et al. Promoter Methylation of CDO1 Identifies Clear-Cell Renal Cell Cancer Patients with Poor Survival Outcome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(15):3492–500. Epub 2015/04/24. doi: 10.1158/1078-0432.ccr-14-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushiku H, Yamashita K, Katoh H, Ema A, Minatani N, Kikuchi M, et al. Promoter DNA methylation of CDO1 gene and its clinical significance in esophageal squamous cell carcinoma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2017;30(2):1–9. Epub 2016/09/16. doi: 10.1111/dote.12496 . [DOI] [PubMed] [Google Scholar]
- 12.Kojima K, Yamashita K, Ushiku H, Katoh H, Ishii S, Tanaka T, et al. The clinical significance of cysteine dioxygenase type 1 methylation in Barrett esophagus adenocarcinoma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2017;30(3):1–9. Epub 2017/02/12. doi: 10.1093/dote/dow001 . [DOI] [PubMed] [Google Scholar]
- 13.Andresen K, Boberg KM, Vedeld HM, Honne H, Jebsen P, Hektoen M, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61(5):1651–9. Epub 2015/02/04. doi: 10.1002/hep.27707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushiku H, Yamashita K, Ema A, Minatani N, Kikuchi M, Kojo K, et al. DNA diagnosis of peritoneal fluid cytology test by CDO1 promoter DNA hypermethylation in gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017. (in press). Epub 2017/03/01. doi: 10.1007/s10120-017-0697-6 . [DOI] [PubMed] [Google Scholar]
- 15.Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Kokubo K, et al. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene. 2010;29(22):3263–75. Epub 2010/03/17. doi: 10.1038/onc.2010.76 . [DOI] [PubMed] [Google Scholar]
- 16.Waraya M, Yamashita K, Katagiri H, Ishii K, Takahashi Y, Furuta K, et al. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Annals of surgical oncology. 2009;16(5):1231–40. Epub 2009/03/06. doi: 10.1245/s10434-009-0415-7 . [DOI] [PubMed] [Google Scholar]
- 17.Waraya M, Yamashita K, Katoh H, Ooki A, Kawamata H, Nishimiya H, et al. Cancer specific promoter CpG Islands hypermethylation of HOP homeobox (HOPX) gene and its potential tumor suppressive role in pancreatic carcinogenesis. BMC cancer. 2012;12:397 Epub 2012/09/11. doi: 10.1186/1471-2407-12-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrangle J, Machida EO, Danilova L, Hulbert A, Franco N, Zhang W, et al. Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(7):1856–64. Epub 2014/02/04. doi: 10.1158/1078-0432.ccr-13-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Lagares A, Mendez-Gonzalez J, Hervas D, Saigi M, Pajares MJ, Garcia D, et al. A Novel Epigenetic Signature for Early Diagnosis in Lung Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(13):3361–71. Epub 2016/02/05. doi: 10.1158/1078-0432.ccr-15-2346 . [DOI] [PubMed] [Google Scholar]
- 20.Vedeld HM, Andresen K, Eilertsen IA, Nesbakken A, Seruca R, Gladhaug IP, et al. The novel colorectal cancer biomarkers CDO1, ZSCAN18 and ZNF331 are frequently methylated across gastrointestinal cancers. Int J Cancer. 2015;136(4):844–53. Epub 2014/06/21. doi: 10.1002/ijc.29039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Fan YC, Gao S, Dou CY, Zhang JJ, Sun FK, et al. Methylated cysteine dioxygenase-1 gene promoter in the serum is a potential biomarker for hepatitis B virus-related hepatocellular carcinoma. Tohoku J Exp Med. 2014;232(3):187–94. Epub 2014/03/22. . [DOI] [PubMed] [Google Scholar]
- 22.Martinelli C, Lengert AVH, Carcano FM, Silva ECA, Brait M, Lopes LF, et al. MGMT and CALCA promoter methylation are associated with poor prognosis in testicular germ cell tumor patients. Oncotarget. 2017;8(31):50608–17. Epub 2017/09/09. doi: 10.18632/oncotarget.11167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita K, Waraya M, Kim MS, Sidransky D, Katada N, Sato T, et al. Detection of methylated CDO1 in plasma of colorectal cancer; a PCR study. PloS one. 2014;9(12):e113546 Epub 2014/12/04. doi: 10.1371/journal.pone.0113546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh VP, Rajesh S, Bihari C, Desai SN, Pargewar SS, Arora A. Xanthogranulomatous cholecystitis: What every radiologist should know. World journal of radiology. 2016;8(2):183–91. Epub 2016/03/17. doi: 10.4329/wjr.v8.i2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Wada S, Araki K, Kubo N, Watanabe A, Tsukagoshi M, et al. Xanthogranulomatous cholecystitis: Difficulty in differentiating from gallbladder cancer. World journal of gastroenterology. 2015;21(35):10166–73. Epub 2015/09/25. doi: 10.3748/wjg.v21.i35.10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayahara M, Nagakawa T, Nakagawara H, Kitagawa H, Ohta T. Prognostic factors for gallbladder cancer in Japan. Ann Surg. 2008;248(5):807–14. Epub 2008/10/25. doi: 10.1097/SLA.0b013e31818a1561 . [DOI] [PubMed] [Google Scholar]
- 27.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93(8):670–81. Epub 2006/05/26. doi: 10.1002/jso.20535 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Comparison of overall survival according to pStage. (B) The Kaplan-Meier curve of the overall survival of the patients are shown according to lymphatic permeation and resection status.
(TIF)
(A) In the diseased gallbladder tissues (NN) of benign gallbladder disease patients, CDO1 TaqMeth value of XGc was significantly higher than those of others (p = 0.0010). (B) There was no significant difference in CDO1 methylation between tumor tissues (T) of Stage 0, I patients and the corresponding non-cancerous mucosa tissues.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.