Abstract
Background
The evaluation of less frequent genetic variants and their effect on complex disease pose new challenges for genomic research. To investigate whether epigenetic data can be used to inform aggregate rare-variant association methods (RVAM), we assessed whether variants more significantly associated with colorectal cancer (CRC) were preferentially located in non-coding regulatory regions, and whether enrichment was specific to colorectal tissues.
Methods
Active regulatory elements (ARE) were mapped using data from 127 tissues and cell-types from NIH Roadmap Epigenomics and Encyclopedia of DNA Elements (ENCODE) projects. We investigated whether CRC association p-values were more significant for common variants inside versus outside AREs, or 2) inside colorectal (CR) AREs versus AREs of other tissues and cell-types. We employed an integrative epigenomic RVAM for variants with allele frequency <1%. Gene sets were defined as ARE variants within 200 kilobases of a transcription start site (TSS) using either CR ARE or ARE from non-digestive tissues. CRC-set association p-values were used to evaluate enrichment of less frequent variant associations in CR ARE versus non-digestive ARE.
Results
ARE from 126/127 tissues and cell-types were significantly enriched for stronger CRC-variant associations. Strongest enrichment was observed for digestive tissues and immune cell types. CR-specific ARE were also enriched for stronger CRC-variant associations compared to ARE combined across non-digestive tissues (p-value = 9.6 × 10−4). Additionally, we found enrichment of stronger CRC association p-values for rare variant sets of CR ARE compared to non-digestive ARE (p-value = 0.029).
Conclusions
Integrative epigenomic RVAM may enable discovery of less frequent variants associated with CRC, and ARE of digestive and immune tissues are most informative. Although distance-based aggregation of less frequent variants in CR ARE surrounding TSS showed modest enrichment, future association studies would likely benefit from joint analysis of transcriptomes and epigenomes to better link regulatory variation with target genes.
Introduction
Colorectal Cancer (CRC) is a leading cause of cancer-related morbidity and mortality worldwide [1]. An understanding of the genetic etiology of CRC may inform therapeutic development and improve the effectiveness of targeted preventive strategies. To date, we know of several very rare genetic mutations that increase risk for hereditary syndromes predisposing to CRC [2]; overall these very rare high penetrance mutation account for less than 3–5% of the heritability. Recently, genome-wide association studies (GWAS) have discovered 48 independent common (minor allele frequency, MAF > 5%) autosomal genetic variants associated with CRC as well. While an estimated 12–35% of CRC risk is attributed to genetic factors [3,4] the additive variance from known common CRC variants account for 1–4% [4–7] of the narrow-sense heritability of CRC. Cumulatively, common genetic variation is estimated to explain approximately 7–8% of CRC heritability [5], suggesting that while larger CRC GWAS will discover additional common variant associations, a substantial fraction of CRC heritability may be explained by rare variants.
Although GWAS enable the discovery of common variant associations, array-based GWAS are not well-suited to evaluate the role of less frequent (0.1% < MAF < 1%) variants on CRC risk. Whole-genome sequencing (WGS) studies offer an alternative approach to array-based GWAS for investigating rare variants. Specifically, WGS can be performed on a reference set that is used to impute sequenced variants into samples with existing genome-wide genotype array data [6,7]. Recent studies have successfully applied this approach to identify novel low-frequency variants associated with complex traits [6,8,9]. Despite these successes, genotype imputation in large numbers of samples is still underpowered for detecting trait associations with less frequent genetic variants.
The power of rare-variant association methods (RVAM) can be improved in a variety of ways. By utilizing functional genomic data, tests of single variant trait associations can be restricted to regions of putative functional significance (or weighted accordingly). Power can also be improved by using aggregate testing for rare-variants. In aggregate RVAM, variants are aggregated within a set (typically a gene) and tested for association with a phenotype. To date, most RVAM have focused on the 1–2% of the genome that encodes for proteins [10,11] where reference gene annotations, such as RefSeq Genes [12], GENCODE [13], or the consensus coding sequence (CCDS) [14], provide functionally relevant units for analysis. To conduct a sufficiently powered genome-wide search for rare and less frequent variant associations, an important challenge is to identify units of analysis outside of coding regions that are biologically meaningful and enriched for associations with CRC.
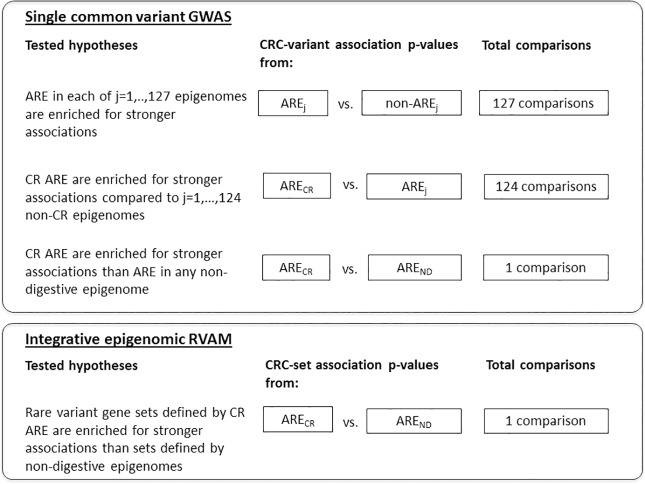
To define non-coding regulatory regions that modulate gene expression, we used functional genomic data from the NIH Roadmap Epigenome project and ENCODE in 127 different tissues and cell-types, including 3 colorectal epigenomes [15]. We then identified chromatin accessible genomic regions that overlapped nucleosome signals for enhancer and promoter states [15]. We refer to these regions as active regulatory elements (ARE) because they often demark regions of transcription factor binding that in turn modulate transcript abundance of target genes. Finally, we utilized enrichment-based methods to evaluate the extent to which ARE may be used to inform both single variant- and aggregate set-based analyses of rare and less frequent-variant associations. To do so, common single variant GWAS and integrative epigenomic RVAM analyses were performed in 12,661 CRC cases and 14,361 controls. A panel of 610 CRC cases and 309 controls with low-coverage WGS data was used to impute allelic dosages for variants that were not directly genotyped in the GWAS data. Tests of enrichment were conducted using the association results (p-values) from single-variant and aggregate RVAM (Fig 1).
Fig 1. Analysis approaches for assessing ARE enrichment of stronger CRC associations with common and less frequent variants.
ARE, Active Regulatory Elements; CR, Colorectal; ND, Non-digestive; Fig 1 describes the series of analyses performed examining the enrichment of ARE for more significant common single variant CRC associations from GWAS and less frequent variant set associations from an integrative epigenomic RVAM.
Results
ARE enrichment of stronger CRC associations with common variants
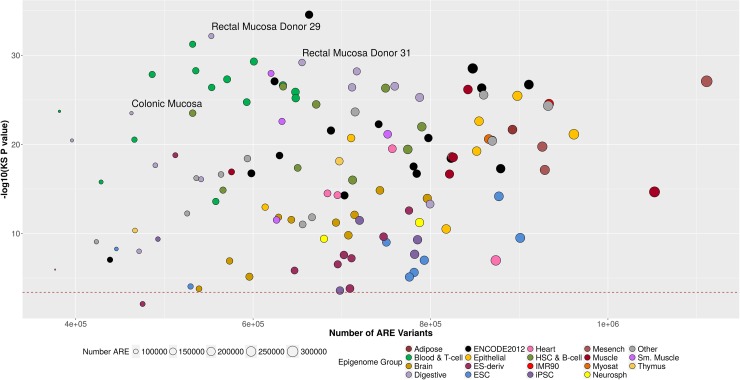
First, we tested whether ARE were enriched for common variant (MAF ≥ 1%) CRC associations (low p-values) when compared to non-ARE regions of the genome. On average there were 692,408 (ranging from 376,794 to 1,111,337) variants positioned in ARE of the 127 tissues and cell-types examined in this analysis. For comparison, there was an average of 7,585,498 (ranging from 7,901,112 to 7,166,569) common variants outside of the ARE regions. The number of ARE variants in a tissue was highly correlated with the number of ARE in a tissue (Pearson correlation r2 = 0.93). The KS test enrichment p-value, however, was not correlated with the number of ARE variants in a tissue or cell-type (Pearson correlation r2 = 0.02). For the 127 tissue and cell-type specific ARE, all but neuron cultured cells showed statistically significant (α = 0.05/127 comparisons = 3.9×10−4) enrichment of CRC associations from the one-sided Kolmogorov-Smirnov (KS) test (S1 Table). We found that ARE variants with low CRC association p-values were more significantly enriched (smaller KS p-values) in digestive tissues, including the 3 colorectal tissues, and immune cell types (Fig 2). Embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), and brain cell-types had weaker ARE enrichment.
Fig 2. ARE enrichment of stronger GWAS CRC p-values.
Each point in this scatter plot corresponds to one of the 127 tissues and cell-types examined for ARE enrichment of more significant single common variant association p-values with CRC. The x-axis shows the number of variants in ARE examined for the corresponding tissue or cell-type. The y-axis scale is the–log 10 × the p-value from the KS test comparing the distribution of CRC-variant association p-values for variants inside and outside of ARE. The size of the point corresponds to the number of ARE in the tissue or cell-type. The color of the point represents membership to an epigenomic group, based on a hierarchal clustering of the AREs. ARE of digestive tissues (in light purple) and immune cell types (in two shades of green) were more significantly enriched (lower KS test p-values). The range of ARE and ARE variants spreads across the high and low KS P-values, suggesting that the difference in number of ARE across tissues is not biasing the enrichment results. The red dash line corresponds to the Bonferroni p-value threshold correcting for 127 comparisons (0.05/127 = 4 × 10−4).
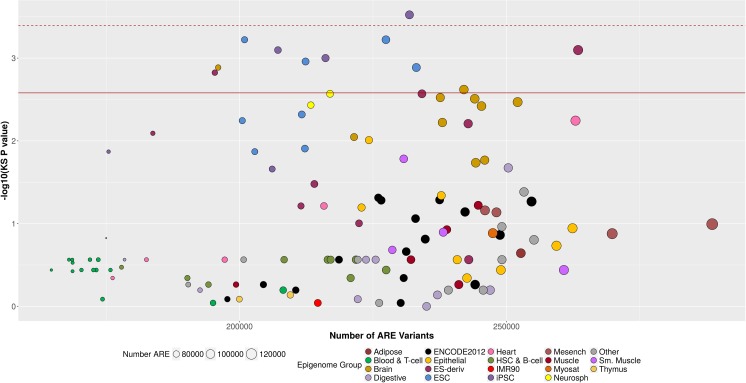
Next, we investigated whether colorectal (CR) tissue-specific ARE were enriched for low p-values of CRC-variant association in comparison to the ARE of non-CR tissues and cell-types. To do so, we pooled ARE in the three reference CR tissues into a single set and made comparisons to ARE in each of the remaining 124 tissues and cell-types. We then generated an empirical p-value using adaptive resampling with up to 10,000 iterations. After adjustment for 124 multiple comparisons, CR ARE were only enriched for low p-values of CRC-variant association in comparison to ARE in iPSC cells (p = 3 × 10−4; S2 Table, Fig 3). However, previous cluster analysis [15] revealed 19 groups of similar epigenomic landscapes (defined as the Epigenomic Group in S2 Table) that correspond to biologically relevant groups (such as digestive tissues and embryonic stem cells, S1 Fig). After adjustment for 19 comparisons (corresponding to anatomical locations with similar epigenomes), CR ARE were enriched for low p-values of CRC-variant associations in comparison to ARE from induced pluripotent stem cells (iPSCs), embryonic stem cells (ESC), and ESC-derived cells (p-values ranging from 3 × 10−4 to 0.001). These epigenomes are derived from tissues and cell-types that are anatomically distant from CR tissues and with more divergent epigenomes (S1 Fig) [15]. Finally, when pooling the ARE of 115 non-digestive tissues and cell-types and 3 CR tissues we found significant enrichment of low p-values of CRC-variant associations in CR ARE versus non-digestive ARE (KS test p-value = 0.0035, empirical p-value = 1.0×10−4 based on 10,000 resampling iterations).
Fig 3. CR-specific ARE enrichment of stronger GWAS CRC p-values.
Each point in this scatter plot corresponds to an enrichment result comparing CR ARE (n = 108,297) to ARE in one of the 124 non-colorectal tissues and cell-types tested. The x-axis shows the number of variants in ARE examined for the corresponding non-colorectal tissue or cell-type (n = 270,030 CR ARE variants). The y-axis is the–log 10 × the p-value from the KS test comparing the distribution of CRC association p-values for variants inside CR ARE versus those inside ARE of a non-colorectal tissue and cell-type. The size of the point corresponds to the number of ARE in the tissue or cell-type. The color of the point represents membership to an epigenomic group, based on a hierarchal clustering of the AREs. In comparison to ARE of digestive tissues (in light purple) and immune cell types (in two shades of green), CR-specific ARE did not exhibit additional enrichment. The strongest enrichment was observed for induced pluripotent stem cells (iPSCs), embryonic stem cells (ESC), and ESC-derived cells, and Brain cell-types. The red dash line corresponds to the Bonferroni p-value threshold correcting for 124 comparisons (0.05/124 = 4 × 10−4). The red solid line corresponds to the Bonferroni p-value threshold correcting for 19 epigenomic group comparisons (0.05/19 = 3 × 10−3).
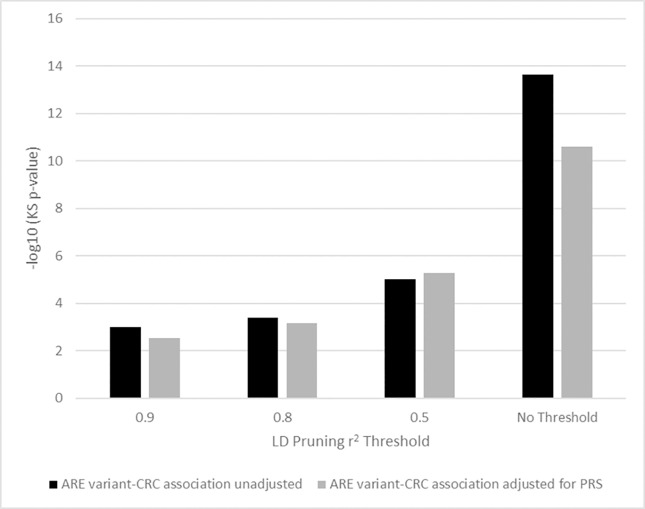
To investigate whether our results were sensitive to patterns of linkage disequilibrium (LD) near ARE, we employed a priority pruning scheme, where the variant with the lowest CRC association p-value in an LD block (defined at Pearson correlation r2 thresholds) was maintained for enrichment analysis. After LD pruning we still found significant enrichment of stronger CRC-variant associations in CR ARE in comparison to ARE combined across non-digestive tissues and cell-types at LD thresholds of r2 = 0.9, 0.8, and 0.5 (KS p-value = 9.6 × 10−4, 4.1 × 10−4, and 9.5 × 10−6, respectively; S4 Table and Fig 4). To further explore how much enrichment is driven by known CRC loci we repeated the single common variant GWAS adjusting for a polygenic risk score (PRS) based on the 48 known index variants and repeated the enrichment analysis using all variants and at the three LD pruning thresholds. At each of the pruning thresholds we observed similar enrichment for stronger CRC-variants association p-values in CR ARE compared to ARE of non-digestive tissues. In the absence of LD pruning the KS p-value was diminished after adjusting for PRS, but still highly significant (KS p-value = 2.5 × 10−11 versus 2.2 × 10−14, Fig 4).
Fig 4. CR-specific ARE enrichment of stronger GWAS CRC p-values accounting for known loci and variant correlation.
Variant-CRC association p-values for CR ARE variants were compared to ARE variants of all non-digestive tissues. The y-axis shows the–log10(KS test p-value) reflecting the significance of the enrichment. Enrichment analyses were performed for single variant-CRC association p-values from GWAS unadjusted for known loci (shown in black) and from GWAS that included a polygenic risk score (PRS) in the model (shown in gray). Results across different LD pruning schemes are shown using three different correlation r2 thresholds. For each threshold, LD blocks were defined as sets of correlated CR ARE variants or ARE variants from non-digestive tissues with r2 greater than or equal to 0.9, 0.8, or 0.5. For each LD block, a priority pruning scheme was employed selecting the ARE variant with the strongest CRC association p-value. Enrichment tests were repeated for each pruning threshold and compared to enrichment results without LD pruning (‘No Threshold’).
CR ARE enrichment of rare variant CRC associations from aggregate tests
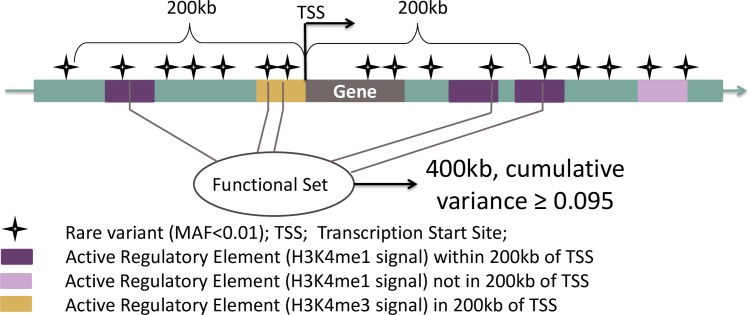
To explore whether CR ARE were enriched for low p-values for aggregate RVAM CRC associations, we leveraged evidence suggesting that regulatory elements are often located within 200kb of their target gene(s) [16]. Accordingly, we defined sets of rare variants (MAF < 1%) by pooling ARE within 200kb upstream or downstream of transcription start sites (TSS) from RefSeq [12], (Fig 5 and S3 Fig). We pooled CR-specific ARE as well as ARE in non-digestive tissues/cell-types and ran set-based association tests for CRC (see Methods) in both. We refer to this as an integrative epigenomic RVAM.CR-specific sets were enriched for rare-variant CRC-associations compared to sets specific to non-digestive tissues and cell-types (p-value = 0.029).
Fig 5. Rare variant test set.
Variant sets were anchored on Transcription Start Sites (TSS) as defined by protein coding gene transcripts with validated RefSeq records. If a gene had multiple TSS, the 5'-most and 3'-most TSS were used as anchors. Accordingly, variants overlapping ARE within 200Kb of a TSS were pooled into a test set.
Discussion
Consistent with the results from previous studies [17], we found that ARE (defined using the Roadmap and ENCODE epigenomic data) were enriched for variants associated with a complex human disease, in our case, CRC. Interestingly, when comparing CR ARE directly to ARE defined in other tissues and cell-types we found significant enrichment of low p-values in comparison to anatomically distant tissues and cell-types. We did not observe enrichment when comparing CR ARE to ARE defined in other digestive tissues. Contrary to our expectations, we also observed minimal enrichment in comparison to most of the other tissues and cell-types. This suggests that although presence of the ARE in CR tissue is important, there may be low specificity of the ARE harboring risk variants in CR tissue in comparison to ARE of other epigenomes, particularly those from similar anatomical locations and regulatory landscapes. That being said, we did observe stronger enrichment of low CRC-variant association p-values inside ARE of digestive and immune cell-types when compared to regions outside of ARE relative to the enrichment observed from other cell-types and tissues. This suggests that these epigenomes may be most relevant for honing in on putative functional variants. It is possible that in light of the limited sample sizes that are currently available for CR tissues (n = 3), the reference ARE maps are incomplete. If this is true, inclusion of all digestive tissues with similar epigenomic landscapes may help identify missed CR ARE that are shared across all digestive tissues.
In addition, for aggregate testing of rare-variant associations, we found that sets based on CR-specific ARE were enriched compared to sets based on ARE from non-digestive cell-types. We acknowledge that physical proximity of enhancers to genes has limited predictive accuracy for identifying the gene targets [18]. However, even though the rare-variant analysis was anchored on genes by considering ARE within 200kb of TSS, our method for defining units of analysis represents a significant advance over the exome-only analyses that are the current standard. Given that most of the common CRC loci identified thus far are positioned outside of the exome, our enrichment results suggest that rare variant associations will similarly implicate non-protein coding regulatory mechanisms. The lower enrichment observed for rare variant associations in comparison to common variants likely reflects both incomplete mapping of ARE and the employed pooling scheme. Future approaches could benefit from integration of epigenomic, transcriptomic, and genomic data in order to group ARE linked to regulation of the same target gene(s) through expression quantitative trait mapping.
Our findings have broad implications for future studies aimed at understanding the genetic etiology of CRC. First, we demonstrated that regulatory variation may play an important role in the inherited susceptibility to CRC. Specifically, using an LD r2 ≥ 0.5 threshold, we considered all variants that tag one of the 48 known, common-variant loci for CRC risk (S3 Table). We found that 36 of the 48 index variants (73%) tagged a variant located in a CR ARE. Of those not positioned within a CR ARE, 5 were in the AREs of stomach mucosa, duodenum mucosa or relevant immune cells. Second, by showing that CRC associations are enriched in ARE, we demonstrate that methods for discovering new associations that prioritize variants in ARE may gain statistical power over agnostic approaches. Finally, with rare-variant association studies expanding beyond the exome, there are as yet no gold-standard approaches for constructing non-coding units of analysis for rare-variant association testing. The ARE we defined represent a logical non-coding unit of analysis that permit aggregate rare-variant association tests such as Mixed Effects Score Test (MiST) [19], Sequence Kernel Association Tests (SKAT) [20], or Combined and Multivariate Collapsing Method (CMC) [21].
This study has a number of strengths. We utilized a large collection of WGS data to investigate rare, low-frequency, and common variants across the genome. We are also confident that the p-values for association with CRC risk are well-calibrated due to the large sample sizes in GECCO and CCFR (over 27,000 samples). Finally, we were able to leverage high-quality epigenetic data that have been uniformly processed by the Roadmap and ENCODE epigenome consortia. By utilizing DHS available across a subset of the 127 Roadmap epigenomes, the resolution of predicted functional elements was greatly improved and thus aided in reducing background variation less likely to be related to CRC.
Although these data aided in honing in on likely functional regions, the ARE we investigated only cover a small portion of the genome. On average, each cell-type specific ARE covered 3% of the autosome, leaving 97% of the genome untested in this analysis. In addition, the rare variants in this analysis were mainly imputed from a panel of 919 samples with 6x coverage WGS as opposed to more expensive sequencing at higher depths. While this internal sequencing panel has the advantage of being potentially enriched for risk variants given the higher proportion of CRC cases, lower coverage sequencing has decreased ability to confidently call rarer variants. Consequentially, we were unable to impute and test the CRC association for many of the true rare variants present in our study population. Based on the QC assessment we are confident in the calling of the variants from the WGS panel that were included in this study, and in the subsequent imputation. However, it should be noted that assessing the imputation quality of rarer variants remains an active area of study. Balancing sample size with sequencing coverage is an important consideration for rare variant studies. In light of the decreasing cost of WGS, we would recommend future studies to use higher coverage sequencing in order to better assess the burden of rarer regulatory variation. Another important consideration is the role of other types of functional elements both within the coding region (such as frameshift or splice variants) and in the non-coding regions (such as silencers, long non-coding RNA, and micro RNA). Furthermore, we had access to the epigenomes from only 3 non-diseased colorectal tissues. With more epigenetic data from relevant tissues and cell-types, the predicted ARE from CR and other digestive tissues would be more comprehensive and precise.
ARE defined by the Roadmap and ENCODE epigenetic data represent empirical estimates of the genomic location of enhancers and promoters. These elements regulate multiple genes and operate as nodes in gene expression networks. As is, we are treating the ARE as independent, distinct units. A logical next step is to define gene-enhancer links, where the entire regulatory-coding module can be treated as a unit of analysis (for each gene, define exons + promoter + enhancer). As new gene expression and epigenetic data become available, we will be able to define gene-enhancer links [22,23] and construct meaningful, statistically valid units of analysis for aggregate RVAM outside of the exome. In this study, we found that CR ARE were enriched for more significant CRC associations with both common and rare variants. Furthermore we found that this enrichment was not restricted to ARE of colorectal tissues. These findings may help guide novel approaches to discover biologically meaningful genetic associations for colorectal cancer and other complex diseases.
Materials and methods
Study participants and whole genome sequence imputed GWAS
Ethics statement
All participants gave written informed consent and this study has been approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board.
GWAS participants
Study-specific eligibility criteria, details about genotyping, and quality control (QC) analyses can be found in Peters et al [24]. Briefly, we included individual-level genotype data pooled from a total of 12,661 CRC cases and 14,361 controls of European ancestry from 16 studies within the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) and the Colorectal Cancer Family Registry (CCFR). Study characteristics are described in S5 Table. All cases were defined as invasive colorectal adenocarcinoma and confirmed by medical record, pathology report, or death certificate. Variants that were not directly genotyped on the GWAS platforms were imputed using an internal reference panel with a 2 to 1 CRC cases to control ratio with the intent of enriching for CRC risk variants.
Quality control of GWAS and WGS
610 CRC cases and 309 controls from the WHI study were sequenced with low-pass coverage and served as the internal WGS imputation panel for this study (S5 Table). Details about sequence data QC and imputation are described in Supporting Information text under Whole Genome Sequence Data and Genotype Imputation to GECCO-CCFR GWAS. In brief, multi-sample calling was performed at the University of Michigan. After removal of external duplicates, standard QC steps were used to examine for potential sample swap and contamination, e.g. sample heterozygosity, identity by descent (IBD) and principal component analyses. Only three samples were excluded from the analysis before imputation. For internal duplicates, the sample with the higher sequencing depth was selected for analysis. Gender checking confirmed all samples were female. Genotyping concordance between WGS and our previous GWAS were checked as well. Monomorphic, non-biallelic variants, and variants with low multi-sample calling quality (beagle R2<0.3) were removed from subsequent analysis. Distributions of sequencing depth, mapping quality, beagle allelic R2, minor allele frequency and minor allele count were examined for QC purposes.
Definition of active regulatory elements, known loci, and rare variant sets
Description of active regulatory elements
Annotations for active regulatory elements (AREs) ARE were downloaded from Wouter Meuleman Reg2Map (http://www.broadinstitute.org/~meuleman/reg2map/HoneyBadger2_release/). Chromatin accessible regions were defined using the union of DNaseI-hypersensitivity sequencing (DNase-seq) peaks across 53 tissues and cell types. Enhancer and promoter states were previously annotated for the epigenomes of 127 tissues and cell-types (Roadmap + ENCODE) using a 5-mark 15-state Hidden Markov Model (ChromHMM) [25]. Active regulatory elements correspond to regions of chromatin accessibility within enhancer and promoter regions. DNase-seq signal scores of -log10(Poisson p-value) ≥ 10 were used as a threshold for statistically significant AREs because this was previously shown to provide very good separation between signal and noise [15].
We defined CR AREs using 2 rectal mucosa tissues (E101, E102) and one colon mucosa tissue (E075). We defined non-digestive AREs by excluding AREs from 12 digestive tissues (E075, E077, E079, E084, E085, E092, E094, E101, E102, E106, E109, E110), due to similarity with CR epigenomes [15]. Pairwise comparisons were made for ARE signal scores from each of the 127 tissues and cell types. Broad categorization of the 127 tissues into and cell-types was derived using hierarchically clustering using Pearson correlation as the distance measure and complete linkage followed by optimal ordering of leaves.
Defining rare variant sets
We anchored variant sets on transcription start sites (TSS), as defined by dbXref MySQL from UCSC Genome Browser (Human Feb. 2009 GRCh37/hg19 Assembly, data available at http://genome.ucsc.edu/cgi-bin/hgTables). For each set, we pooled variants within 200kb upstream and downstream of the TSS (S3 Fig). Only variant sets with a cumulative variance of ≥0.095 were analyzed, which is equivalent to analyzing single variants with MAF = 0.05. We calculated cumulative variance instead of cumulative MAF because variance can better account for genotype dosage imputation than MAF. In total, set-based CRC association tests were performed for 13,861 CR-ARE sets with 388,140 less frequent variants and 14,047 non-digestive ARE sets with 425,610 less-frequent variants. For CR tissue sets there was a median of 137 (interquartile range-IQR 91–197) variants per set. Similarly, for non-digestive sets there was a median of 94 (IQR 75–119) variants per set. The median cumulative variance across sets was 0.26 (IQR 0.17–0.38) and 0.17 (IQR 0.13–0.22) for CR sets non-digestive sets, respectively (S6 Table).
Defining known CRC loci
We defined the known colorectal cancer loci as 48 index variants likely to have independent effects on CRC risk and variants in LD with index variants (r2 ≥ 0.5; S3 Table). We calculated LD using Haploreg V3 [26] using EUR 1000 Genomes Phase 1 data and a 500kb maximum distance upstream and downstream for each LD region. Five of the loci were in LD (r2 ≥ 0.5) with a missense coding variant. Two of the five loci contained missense mutations (rs10936599, rs1789961) predicted by PolyPhen2 [27] (S3 Table) to have damaging effects. Given that none of the known loci have been confirmed through laboratory evidence to confer deleterious structural changes to the encoded protein, all 48 variants were included in the analysis. A polygenic risk score (PRS) was calculated as the sum of the imputed risk allele doses (ie the count of CRC risk increasing alleles carried).
Statistical analyses
For variants with MAF ≥ 0.01 and imputation R2 ≥ 0.3, we ran marginal association analyses of the GWAS data using log-additive logistic regression of dosage effect on CRC risk with adjustment for age, sex (when appropriate), center (when appropriate), batch effects (ASTERISK only), and the first three PCs from EIGENSTRAT to account for population substructure within each individual study. To conduct functional set-based association analysis of rare variants, MAF < 0.01 and cumulative variance in set ≥ 0.095, we tested for association with CRC risk using MiST [19], adjusting for the same covariates as the single variant analysis. To test for enrichment, we compared the distribution of p-values for CRC association using the KS test. To test the hypotheses that, 1) ARE would be enriched for stronger CRC association p-values compared to non-ARE regions; and 2) ARE specific to CR would be enriched for stronger CRC association p-values compared to ARE specific to non-digestive cell-types, we tested enrichment using a one-sided KS test implemented using R version 3.2.2 (stats package function ‘ks.test’)[28].
Due to LD among variants, the asymptotic distribution for the KS test is not valid. We therefore used the resampling technique to estimate the p-value. Specifically, suppose there are X AREs in group 1 and Y AREs in group 2. A KS test was performed and the p-value was denoted as Pobs. For each resampling, a random set of size X was drawn from the total X+Y AREs and assigned to group 1. The remaining AREs were assigned to group 2. The p-value from KS test was obtained, and denoted by Psim. The number of resamples varied from 10, 100, 1000, and up to 10,000 until 20% of the total number of p-values (Psim) were less than or equal to Pobs. The empirical p-value was calculated as the ratio of the number of Psim ≤ Pobs and the total number of resamples.
A p-value ≤ 0.05 was considered statistically significant. For 124 tissue comparisons the significance threshold was set to 0.05/124 and suggestive threshold was set to 0.05/19 given that several of the tissues examined were either biological duplicates (e.g. rectal mucosa samples from two donors, E101 and E102) or have highly similar epigenomes and cellular attributes (samples from the left versus right ventricles, E095 and E105).
For the comparison of CR versus non-digestive tissues the significance threshold was considered to be 0.05. For the 124 tissue comparisons the significance threshold was set to 0.05/124 and suggestive threshold was set to 0.05/19 given that many of the epigenomes were highly correlated.
Supporting information
AREs were defined as accessible chromatin regions (http://www.broadinstitute.org/~meuleman/reg2map/HoneyBadger2_release/DNase/p2/regions_all.bed, downloaded 2/9/2016) overlapping enhancer and promoter states marked by the H3K4me1 histone modification. Clustering was performed using the average H3K4me1 signal confidence scores, -log10(Poisson p-value), in the ARE of consolidated epigenomes for 127 tissues and cell-types (http://egg2.wustl.edu/roadmap/data/byFileType/signal/consolidated/macs2signal/pval/; downloaded 2/9/2016). The tissues and cell types were hierarchically clustered using Pearson correlation as the distance measure and complete linkage followed by optimal ordering of leaves. The leaves are colored by the broad categorization of each epigenome. Digestive tissues are colored in light purple. Immune cell-types are colored in green.
(PDF)
The negative logarithm of the Colorectal ARE (y axis) and the combined Non-digestive ARE (x axis) CRC association P-value is plotted for each variant (dot). The red line indicates the null hypothesis that the two distributions of p-values are the same.
(TIF)
UCSC genome browser image of an example variant set in the known CDH1 locus. The first track shows the RefSeq gene annotations. The following 5 tracks are Roadmap ChIPseq histone modifications for colon mucosa. The following track ‘ChromHMM’ demarks the enhancer regions derived from the 15-state hidden markov modeling of these histone modifications. Next, the DHS marks across 52 Roadmap and ENCODE cell lines used to further refine likely transcription factors. The intersect of enhancer/promoter ChromHMM segments and DHS marks were used to define the Active Regulatory Elements (ARE) of colon mucosa. Variant sets were anchored on TSS as defined by protein coding gene transcripts with validated RefSeq records. If a gene had multiple TSS, the 5'-most and 3'-most TSS were used as anchors. Accordingly, variants overlapping ARE within 200Kb of a TSS were pooled into a test set, as shown in the highlighted blue region. Only sets with a cumulative variance of greater than 0.095 were analyzed.
(PDF)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in ARE vs those not in ARE are presented for 127 cell-types and tissues.
(XLS)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in ARE of 3 CR tissues in comparison to ARE of another cell-type or tissues presented for 124 cell-types and tissues.
(XLS)
Functional annotation of 48 known loci defined as the Index variants and variants tagged by index with r2≥0.5 in all European 1000 Genomes Project populations (CEU+FIN+GBR+IBS+TSI).
(XLS)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in CR ARE vs non-digestive tissue ARE across 124 cell-types and tissues.
(XLS)
(XLS)
(XLS)
(DOCX)
Acknowledgments
The authors would like to thank the following:
ASTERISK: We are very grateful to Dr. Bruno Buecher without whom this project would not have existed. We also thank all those who agreed to participate in this study, including the patients and the healthy control persons, as well as all the physicians, technicians and students.
DACHS: We thank all participants and cooperating clinicians, and Ute Handte-Daub, Utz Benscheid, Muhabbet Celik and Ursula Eilber for excellent technical assistance.
Galeon: GALEON wishes to thank the Department of Surgery of University Hospital of Santiago (CHUS), Sara Miranda Ponte, Carmen M Redondo, and the staff of the Department of Pathology and Biobank of CHUS, Instituto de Investigación Sanitaria de Santiago (IDIS), Instituto de Investigación Sanitaria Galicia Sur (IISGS), SERGAS, Vigo, Spain, and Programa Grupos Emergentes, Cancer Genetics Unit, CHUVI Vigo Hospital, Instituto de Salud Carlos III, Spain.
GECCO: The authors would like to thank all those at the GECCO Coordinating Center for helping bring together the data and people that made this project possible. The authors also acknowledge Deanna Stelling, Mark Thornquist, Greg Warnick, Carolyn Hutter, and team members at COMPASS (Comprehensive Center for the Advancement of Scientific Strategies) at the Fred Hutchinson Cancer Research Center for their work harmonizing the GECCO epidemiological data set. The authors acknowledge Dave Duggan and team members at TGEN (Translational Genomics Research Institute), the Broad Institute, and the Génome Québec Innovation Center for genotyping DNA samples of cases and controls, and for scientific input for GECCO.
HPFS, NHS and PHS: We would like to acknowledge Patrice Soule and Hardeep Ranu of the Dana Farber Harvard Cancer Center High-Throughput Polymorphism Core who assisted in the genotyping for NHS, HPFS, and PHS under the supervision of Dr. Immaculata Devivo and Dr. David Hunter, Qin (Carolyn) Guo and Lixue Zhu who assisted in programming for NHS and HPFS, and Haiyan Zhang who assisted in programming for the PHS. We would like to thank the participants and staff of the Nurses' Health Study and the Health Professionals Follow-Up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
PLCO: The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff or the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O’Brien and staff, Westat, Inc., and Drs. Bill Kopp and staff, SAIC-Frederick. Most importantly, we acknowledge the study participants for their contributions to making this study possible. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
PMH: Study participants and staff of the Hormones and Colon Cancer study.
WHI: WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
Data Availability
The PLCO genetic data can be accessed with appropriate approval through the dbgap online resource (Prostate Cancer https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000207.v1.p1 and PanScan (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000206.v5.p3), accession numbers phs000207v.1p1 and phs000206.v3.p2, respectively. Lung cancer datasets are available at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000093.v2.p2, accession number phs000093.v2.p2. Data from the gecco studies are available from dbgap at the following accession number: phs001078.v1.p1.
Funding Statement
ASTERISK: a Hospital Clinical Research Program (PHRC) and supported by the Regional Council of Pays de la Loire, the Groupement des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC), the Association Anne de Bretagne Génétique and the Ligue Régionale Contre le Cancer (LRCC). COLO2&3: National Institutes of Health (R01 CA60987). CCFR: This work was supported by grant UM1 CA167551 from the National Cancer Institute and through cooperative agreements with the following CCFR centers: Australasian Colorectal Cancer Family Registry (U01 CA074778 and U01/U24 CA097735), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01/U24 CA074800), Ontario Familial Colorectal Cancer Registry (U01/U24 CA074783), Seattle Colorectal Cancer Family Registry (U01/U24 CA074794), University of Hawaii Colorectal Cancer Family Registry (U01/U24 CA074806), and USC Consortium Colorectal Cancer Family Registry U01/U24 CA074799).The Colon CFR GWAS was supported by funding from the National Cancer Institute, National Institutes of Health (U01 CA122839 and R01 CA143237 to Graham Casey). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Colon Cancer Family Registry (CCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CCFR. DACHS: German Research Council (Deutsche Forschungsgemeinschaft, BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 117/1-1), and the German Federal Ministry of Education and Research (01KH0404 and 01ER0814). DALS: National Institutes of Health (R01 CA48998 to M. L. Slattery). GECCO: National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (U01 CA137088; R01 CA059045; R01 CA120582; U01 CA164930). HPFS is supported by the National Institutes of Health (P01 CA 055075, UM1 CA167552, R01 137178, R01 CA151993 and P50 CA127003), NHS by the National Institutes of Health (UM1 CA186107, R01 CA137178, P01 CA87969, R01 CA151993 and P50 CA127003,) and PHS by the National Institutes of Health (R01 CA042182). MEC: National Institutes of Health (R37 CA54281, P01 CA033619, and R01 CA63464). OFCCR: National Institutes of Health, through funding allocated to the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783); see CCFR section above. Additional funding toward genetic analyses of OFCCR includes the Ontario Research Fund, the Canadian Institutes of Health Research, and the Ontario Institute for Cancer Research, through generous support from the Ontario Ministry of Research and Innovation. PLCO: Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. Additionally, a subset of control samples were genotyped as part of the Cancer Genetic Markers of Susceptibility (CGEMS) Prostate Cancer GWAS (Yeager, M et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 2007 May;39(5):645-9), Colon CGEMS pancreatic cancer scan (PanScan) (Amundadottir, L et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009 Sep;41(9):986-90, and Petersen, GM et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010 Mar;42(3):224-8), and the Lung Cancer and Smoking study (Landi MT, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009 Nov;85(5):679-91). The prostate and PanScan study datasets were accessed with appropriate approval through the dbGaP online resource (http://cgems.cancer.gov/data/) accession numbers phs000207.v1.p1 and phs000206.v3.p2, respectively, and the lung datasets were accessed from the dbGaP website (http://www.ncbi.nlm.nih.gov/gap) through accession number phs000093.v2.p2. Funding for the Lung Cancer and Smoking study was provided by National Institutes of Health (NIH), Genes, Environment and Health Initiative (GEI) Z01 CP 010200, NIH U01 HG004446, and NIH GEI U01 HG 004438. For the lung study, the GENEVA Coordinating Center provided assistance with genotype cleaning and general study coordination, and the Johns Hopkins University Center for Inherited Disease Research conducted genotyping. PMH: National Institutes of Health (R01 CA076366). VITAL: National Institutes of Health (K05 CA154337). WHI: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Peters U, Bien S, Zubair N. Genetic architecture of colorectal cancer. Gut. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99(2):260–266. doi: 10.1002/ijc.10332 [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201 [DOI] [PubMed] [Google Scholar]
- 5.Jiao S, Peters U, Berndt S, Brenner H, Butterbach K, Caan BJ et al. Estimating the heritability of colorectal cancer. Hum Mol Genet. 2014;23(14):3898–3905. doi: 10.1093/hmg/ddu087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auer PL, Johnsen JM, Johnson AD, Logsdon BA, Lange LA, Nalls MA et al. Imputation of exome sequence variants into population- based samples and blood-cell-trait-associated loci in African Americans: NHLBI GO Exome Sequencing Project. Am J Hum Genet. 2012;91(5):794–808. doi: 10.1016/j.ajhg.2012.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du M, Auer PL, Jiao S, Haessler J, Altshuler D, Boerwinkle E et al. Whole-exome imputation of sequence variants identified two novel alleles associated with adult body height in African Americans. Hum Mol Genet. 2014;23(24):6607–6615. doi: 10.1093/hmg/ddu361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnsen JM, Auer PL, Morrison AC, Jiao S, Wei P, Haessler J et al. Common and rare von Willebrand factor (VWF) coding variants, VWF levels, and factor VIII levels in African Americans: the NHLBI Exome Sequencing Project. Blood. 2013;122(4):590–597. doi: 10.1182/blood-2013-02-485094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auer PL, Teumer A, Schick U, O'Shaughnessy A, Lo KS, Chami N et al. Rare and low-frequency coding variants in CXCR2 and other genes are associated with hematological traits. Nat Genet. 2014;46(6):629–634. doi: 10.1038/ng.2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruitt KD, Tatusova T, Klimke W, Maglott DR. NCBI Reference Sequences: current status, policy and new initiatives. Nucleic Acids Res. 2009;37(Database issue):D32–D36. doi: 10.1093/nar/gkn721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruitt KD, Harrow J, Harte RA, Wallin C, Diekhans M, Maglott DR et al. The consensus coding sequence (CCDS) project: Identifying a common protein-coding gene set for the human and mouse genomes. Genome Res. 2009;19(7):1316–1323. doi: 10.1101/gr.080531.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–330. doi: 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mifsud B, Tavares-Cadete F, Young AN, Sugar R, Schoenfelder S, Ferreira L et al. Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet. 2015;47(6):598–606. doi: 10.1038/ng.3286 [DOI] [PubMed] [Google Scholar]
- 17.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. doi: 10.1126/science.1222794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–113. doi: 10.1038/nature11279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Zheng Y, Hsu L. A unified mixed-effects model for rare-variant association in sequencing studies. Genet Epidemiol. 2013;37(4):334–344. doi: 10.1002/gepi.21717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X. Sequence kernel association tests for the combined effect of rare and common variants. Am J Hum Genet. 2013;92(6):841–853. doi: 10.1016/j.ajhg.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83(3):311–321. doi: 10.1016/j.ajhg.2008.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vucicevic D, Corradin O, Ntini E, Scacheri PC, Orom UA. Long ncRNA expression associates with tissue-specific enhancers. Cell Cycle. 2015;14(2):253–260. doi: 10.4161/15384101.2014.977641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47(9):1091–1098. doi: 10.1038/ng.3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters U, Jiao S, Schumacher FR, Hutter CM, Aragaki AK, Baron JA et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology. 2013;144(4):799–807. doi: 10.1053/j.gastro.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012;9(3):215–216. doi: 10.1038/nmeth.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–D934. doi: 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AREs were defined as accessible chromatin regions (http://www.broadinstitute.org/~meuleman/reg2map/HoneyBadger2_release/DNase/p2/regions_all.bed, downloaded 2/9/2016) overlapping enhancer and promoter states marked by the H3K4me1 histone modification. Clustering was performed using the average H3K4me1 signal confidence scores, -log10(Poisson p-value), in the ARE of consolidated epigenomes for 127 tissues and cell-types (http://egg2.wustl.edu/roadmap/data/byFileType/signal/consolidated/macs2signal/pval/; downloaded 2/9/2016). The tissues and cell types were hierarchically clustered using Pearson correlation as the distance measure and complete linkage followed by optimal ordering of leaves. The leaves are colored by the broad categorization of each epigenome. Digestive tissues are colored in light purple. Immune cell-types are colored in green.
(PDF)
The negative logarithm of the Colorectal ARE (y axis) and the combined Non-digestive ARE (x axis) CRC association P-value is plotted for each variant (dot). The red line indicates the null hypothesis that the two distributions of p-values are the same.
(TIF)
UCSC genome browser image of an example variant set in the known CDH1 locus. The first track shows the RefSeq gene annotations. The following 5 tracks are Roadmap ChIPseq histone modifications for colon mucosa. The following track ‘ChromHMM’ demarks the enhancer regions derived from the 15-state hidden markov modeling of these histone modifications. Next, the DHS marks across 52 Roadmap and ENCODE cell lines used to further refine likely transcription factors. The intersect of enhancer/promoter ChromHMM segments and DHS marks were used to define the Active Regulatory Elements (ARE) of colon mucosa. Variant sets were anchored on TSS as defined by protein coding gene transcripts with validated RefSeq records. If a gene had multiple TSS, the 5'-most and 3'-most TSS were used as anchors. Accordingly, variants overlapping ARE within 200Kb of a TSS were pooled into a test set, as shown in the highlighted blue region. Only sets with a cumulative variance of greater than 0.095 were analyzed.
(PDF)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in ARE vs those not in ARE are presented for 127 cell-types and tissues.
(XLS)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in ARE of 3 CR tissues in comparison to ARE of another cell-type or tissues presented for 124 cell-types and tissues.
(XLS)
Functional annotation of 48 known loci defined as the Index variants and variants tagged by index with r2≥0.5 in all European 1000 Genomes Project populations (CEU+FIN+GBR+IBS+TSI).
(XLS)
Results for the kolmogorov-smirnov (KS) test for p-values of common variants in CR ARE vs non-digestive tissue ARE across 124 cell-types and tissues.
(XLS)
(XLS)
(XLS)
(DOCX)
Data Availability Statement
The PLCO genetic data can be accessed with appropriate approval through the dbgap online resource (Prostate Cancer https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000207.v1.p1 and PanScan (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000206.v5.p3), accession numbers phs000207v.1p1 and phs000206.v3.p2, respectively. Lung cancer datasets are available at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000093.v2.p2, accession number phs000093.v2.p2. Data from the gecco studies are available from dbgap at the following accession number: phs001078.v1.p1.