Abstract
Purpose
To investigate the feasibility of dose escalation and hypofractionation of pelvic lymph node intensity modulated radiation therapy (PLN-IMRT) in prostate cancer (PCa).
Methods and Materials
In a phase 1/2 study, patients with advanced localized PCa were sequentially treated with 70 to 74 Gy to the prostate and dose-escalating PLN-IMRT at doses of 50 Gy (cohort 1), 55 Gy (cohort 2), and 60 Gy (cohort 3) in 35 to 37 fractions. Two hypofractionated cohorts received 60 Gy to the prostate and 47 Gy to PLN in 20 fractions over 4 weeks (cohort 4) and 5 weeks (cohort 5). All patients received long-course androgen deprivation therapy. Primary outcome was late Radiation Therapy Oncology Group toxicity at 2 years after radiation therapy for all cohorts. Secondary outcomes were acute and late toxicity using other clinician/patient-reported instruments and treatment efficacy.
Results
Between August 9, 2000, and June 9, 2010, 447 patients were enrolled. Median follow-up was 90 months. The 2-year rates of grade 2+ bowel/bladder toxicity were as follows: cohort 1, 8.3%/4.2% (95% confidence interval 2.2%-29.4%/0.6%-26.1%); cohort 2, 8.9%/5.9% (4.1%-18.7%/2.3%-15.0%); cohort 3, 13.2%/2.9% (8.6%-20.2%/1.1%-7.7%); cohort 4, 16.4%/4.8% (9.2%-28.4%/1.6%-14.3%); cohort 5, 12.2%/7.3% (7.6%-19.5%/3.9%-13.6%). Prevalence of bowel and bladder toxicity seemed to be stable over time. Other scales mirrored these results. The biochemical/clinical failure–free rate was 71% (66%-75%) at 5 years for the whole group, with pelvic lymph node control in 94% of patients.
Conclusions
This study shows the safety and tolerability of PLN-IMRT. Ongoing and planned phase 3 studies will need to demonstrate an increase in efficacy using PLN-IMRT to offset the small increase in bowel side effects compared with prostate-only IMRT.
Summary.
Elective pelvic lymph node (PLN) radiation therapy and hypofractionation for advanced localized prostate cancer remains controversial. We report a single-center sequential cohort study using intensity modulated radiation therapy (IMRT) to deliver conventionally fractionated 50 Gy, 55 Gy, and 60 Gy to the PLN and 70 to 74 Gy (2 Gy per fraction) to the prostate. Additionally, we studied modest hypofractionation delivering 60 Gy (3 Gy per fraction) to the prostate with 47 Gy to the PLN over 4 to 5 weeks. Our findings highlight the safety of dose escalation and hypofractionation in PLN-IMRT.
Introduction
Prostate cancer is the most common cancer in men, accounting for 27% of new cancer cases in 2014, and more than 307,000 men died from prostate cancer in 2012 worldwide 1, 2. In the United Kingdom, 46,690 new cases were diagnosed in 2014 (2). Most men are now diagnosed with localized disease, but high-risk prostate cancer remains life-threatening. Treatment with external beam radiation therapy, androgen deprivation therapy (ADT), and in selected cases, high-dose-rate brachytherapy has been used in this patient group (3). Approximately 15,800 men receive radical prostate radiation therapy every year in the United Kingdom (4). However, the merit of elective pelvic lymph node radiation therapy (PLNRT) compared with treatment of prostate and seminal vesicles alone remains controversial, and present guidelines suggest that PLNRT should be considered but not mandated for high-risk disease 1, 5. This uncertainty may relate to the modest doses of radiation therapy that are usually given with PLNRT so as to avoid bowel toxicity.
Intensity modulated radiation therapy (IMRT) makes it possible to increase bowel sparing, which is the dose-limiting normal tissue when treating the pelvis 6, 7. Intensity modulated radiation therapy brings the opportunity to dose escalate, which has been linked with increased disease control in prostate cancer 8, 9, 10, 11. The low α/β ratio of prostate cancer makes hypofractionation an attractive option for treatment, with recent data demonstrating equivalent outcomes to standard dose schedules treating the prostate alone (4). Dose escalation and hypofractionation have not been adequately evaluated for pelvic LNRT, with limited data available from small case series 8, 9, 10, 12.
The aim of this study was to test the feasibility of using IMRT to deliver LNRT to patients with high-risk prostate cancer, using dose-escalated conventional and hypofractionated schedules.
Methods and Materials
Study design and participants
We performed a single-center, phase 1/2 study of IMRT to irradiate the prostate and PLN in patients with advanced localized prostate cancer. Eligible patients had prostate cancer with very high risk (T3b/T4) or node-positive disease, high-risk disease with Gleason score ≥8 or ≥2 risk factors, or an estimated risk of nodal metastases of >30% based on the Roach formula 1, 13. Postprostatectomy patients (T2-T3a, N0) with extensive Gleason score ≥8 disease and seminal vesicle or lymph node involvement were also eligible. Patients unsuitable for radical radiation therapy or with a history of pelvic surgery or inflammatory bowel disease were excluded.
Patients were sequentially assigned to receive 3 different dose schedules to the PLN of 50, 55, or 60 Gy (cohorts 1, 2, and 3, respectively) giving 70 to 74 Gy in 2-Gy fractions over 7 weeks to the prostate. An integrated boost of 5 Gy was given to radiologically suspicious PLN. Two hypofractionated cohorts (cohorts 4 and 5) were then studied, based on equivalent doses to the conventional schedule calculated assuming an α/β ratio of 2.5 Gy. They received 60 Gy to the prostate in 3-Gy fractions over 4 to 5 weeks and 47 Gy to the PLN. An integrated boost of 4 Gy was given to radiologically suspicious PLN. Patients were initially treated on a 4-week schedule (cohort 4), which was later modified to a 5-week schedule (cohort 5) because of acute gastrointestinal (GI) toxicity. Patients irradiated after prostatectomy received 64 Gy in 32 fractions in cohorts 1 and 2, 65 Gy in 35 fractions in cohort 3, or 55 Gy in 20 fractions in cohorts 4 and 5 (Table E1; available online at www.redjournal.org).
Procedures
Patients received long-course (2-3 years) ADT with at least 6 months' treatment before radiation therapy commenced.
Patients underwent planning computed tomography (CT) scans with a comfortably full bladder and empty rectum. From 2011, sodium citrate enemas were used for patients with rectal dilatation. Inverse radiation therapy planning was performed for all patients, using mandatory normal tissue dose constraints (Table E2; available online at www.redjournal.org) as previously described 14, 15. Clinical target volume-1 included the prostate and any radiologically involved seminal vesicle, with a margin of 8 mm posteriorly and 10 mm in all other directions to create planning target volume-1. Clinical target volume-2 included PLN and uninvolved seminal vesicles (Text E2; available online at www.redjournal.org). A uniform margin of 5 mm was applied to create planning target volume-2. Clinical target volume-3 included any radiologically involved lymph nodes, and a uniform margin of 5 mm was applied to create planning target volume-3. All organs at risk were contoured as solid organs, by defining the outer wall of rectum, bowel, and bladder. The rectum was contoured from the anus (usually at the level of the ischial tuberosities or 1 cm below the lower margin of the planning target volume, whichever was more inferior) to the recto-sigmoid junction. Bowel was outlined separately, excluding rectum and extending 2 cm above the superior extent of planning target volume-2. The bladder was outlined from base to dome. Treatment verification was performed offline using bony anatomy for registration (Text E1; available online at www.redjournal.org).
Staging investigations included prostate-specific antigen (PSA) measurement, histologic diagnosis, radiologic or surgical lymph node assessment, and staging magnetic resonance imaging (MRI), CT, or bone scan.
Acute side effects were recorded weekly using the Radiation Therapy Oncology Group (RTOG) scoring system up to 18 weeks after initiating radiation therapy. Late toxicity was scored according to the European Organization for Research and Treatment of Cancer/RTOG and Late Effects Normal Tissue–Subjective, Objective, Management, Analytic (LENT-SOMA) late toxicity scales, and University of California, Los Angeles Prostate Cancer Index (UCLA-PCI) patient-reported outcomes 16, 17, 18. Data were collected at baseline, every 6 months up to 5 years after radiation therapy, and yearly thereafter.
Prostate-specific antigen was measured every 6 months for 8 years after the start of ADT and annually thereafter. The nadir PSA level was the lowest level recorded after radiation therapy. Biochemical failure was defined according to the Phoenix consensus guidelines as a PSA value greater than the nadir plus 2 ng/mL. Local recurrence was confirmed on MRI of the pelvis or biopsy, and postprostatectomy patients (n=34) were excluded from this endpoint. Distant relapse was confirmed on MRI, CT scan, bone scan, or choline positron emission tomography–CT scan.
Statistical considerations
The primary endpoint was late RTOG toxicity assessed 2 years after radiation therapy. Secondary endpoints included assessment of all toxicity scales during follow-up and disease recurrence. Patients were stratified by total bowel volume outlined (<450 cm3 vs ≥450 cm3). For each dose level stratified by bowel volume, at least 15 men were treated and followed up for at least 1 year. If 0 of 15 men had a grade 3 or higher (grade 3+) RTOG complication, then a 20% grade 3+ toxicity rate was excluded with 1-sided significance level of .05. Because the dose to the initial cohort was modest, patients in the low bowel volume group were recruited to the second dose level after 7 men had ≥12 months of follow-up, provided none of these had recorded a grade ≥3 complication. For other cohorts and bowel volume groups, recruitment continued at that level until such time as 15 men had been treated and followed up for at least 1 year. This strategy ensured that the low bowel volume group moved to the higher dose cohorts in advance of the high bowel volume group. Because recruitment continued in each cohort and bowel volume group until such time as the required total of men had reached ≥12 months of follow-up, in all cases the eventual sample size in each group exceeded the required total to an extent that varied according to the recruitment rate over time.
In cohorts 3 and 4, a further dose expansion phase was planned, with a target sample size of 103 patients (of any bowel volume) evaluable at 2 years, to rule out a late grade 2 or higher (grade 2+) bowel toxicity rate of ≥25%, using a 1-sided alpha 0.05 and power of 80% with an assumed true rate of toxicity not more than 15%. The sample size was expanded to a total of 123 in each of cohort 3 and 4 to allow for an expected dropout rate of 16% by 2 years. However, because of high levels of acute bowel toxicity observed in cohort 4 (4-weekly schedule), the treatment schedule was amended to 5 weeks (cohort 5) with a target of 123 patients.
Late toxicity rates were calculated using Kaplan-Meier methods, with time measured from start of radiation therapy. Rates by 1 and 2 years were calculated with 95% confidence intervals (CIs). One-sided 95% CIs were constructed for rate of RTOG grade 3+ bowel toxicity at 1 year (cohorts 1 and 2) and for rate of RTOG grade 2+ bowel toxicity at 2 years (cohorts 3 and 4), to assess safety of the primary endpoint. In addition, the number of men experiencing defined toxicity grades at each time point was reported as a percentage of all men assessed. Efficacy was assessed using Kaplan-Meier methods to calculate duration of disease control (defined as a composite endpoint of biochemical progression, local or lymph node/pelvic recurrence, or distant metastasis, or recommencement of ADT), duration of local disease control, duration of distant disease control, and disease-specific and overall survival from start of radiation therapy. For disease-specific and overall survival, patients were censored at the date they were last known to be alive. Rates at 2 and 5 years were calculated with 95% CIs. Data were extracted in September 2015 and analyzed using STATA version 13.1 (StataCorp, College Station, TX).
Univariable Cox regression on duration of disease control was performed using factors of dose cohort, N stage (N0 vs N1-3), baseline PSA level (log transformed), clinical T stage (grouped as T1/T2; T3a; T3b+), and Gleason score (grouped as ≤6; 7; ≥8). Forward and backward stepwise selection methods were used to combine significant factors (P<.05 on univariable analysis) into a multivariable model and produce adjusted hazard ratios with 95% CIs.
Trial setup
Institutional clinical research and ethics committees approved the study, which was included in the National Cancer Research Network portfolio in December 2003. The trial was performed in accordance with the principles of good clinical practice and overseen by a trial management group. All patients provided written informed consent.
Results
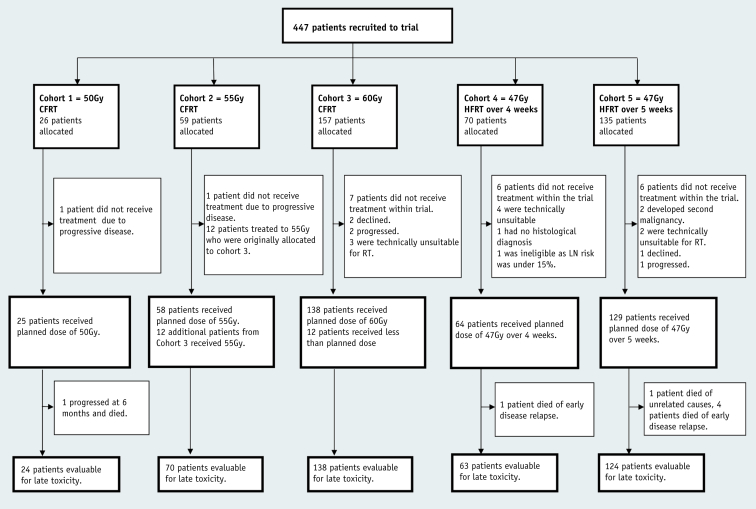
Between August 9, 2000, and June 9, 2010, 447 male patients were recruited to cohorts 1 to 5; 426 were treated according to protocol, and 421 were available for late toxicity assessment (Fig. 1). Median age was 65 years (interquartile range, 60-70 years), with median presenting PSA level of 21.4 ng/mL (10.2-42.8 ng/mL). Forty-six percent of patients had clinical T3/T4 disease, 54% had Gleason ≥8 scores, and 17% PLN involvement. Cohort 1 had a higher proportion of patients with adverse features than cohorts 2 to 5. Median duration of adjuvant hormone therapy was 35 months (33-37 months), and median follow-up was 90 months (Table 1), with 398 patients out of 426 followed up for toxicity for at least 2 years and 327 followed up for at least 5 years. Thirty-four patients (8%) were treated adjuvantly after undergoing a radical prostatectomy before entering the trial (Table E6; available online at www.redjournal.org).
Fig. 1.
Trial profile. Abbreviations: CFRT = conventionally fractionated radiation therapy; HFRT = hypofractionated radiation therapy; LN = lymph node; RT = radiation therapy.
Table 1.
Patient demographics
| Variable | Cohort 1, 50 Gy (n=25) | Cohort 2, 55 Gy (n=70) | Cohort 3, 60 Gy (n=138) | Cohort 4, 47 Gy/4 wk (n=64) | Cohort 5 47 Gy/5 wk (n=129) | Cohorts 1-5 (n=426) |
|---|---|---|---|---|---|---|
| Age at diagnosis (y) | 63 (56-67) | 62 (57-67) | 65 (59-69) | 66 (62-72) | 67 (62-71) | 65 (60-70) |
| PSA at diagnosis (ng/mL) | 39.1 (24.7-78.0) | 25.4 (12.4-44.7) | 24.5 (10.2-47.1) | 15.4 (8.5-31.4) | 18 (8.1-37.9) | 21.4 (10.2-42.8) |
| Gleason score | ||||||
| ≤7 | 13 (52) | 34 (48) | 60 (43) | 22 (35) | 56 (44) | 185 (44) |
| 8 | 4 (16) | 17 (24) | 29 (21) | 13 (20) | 11 (9) | 74 (17) |
| ≥9 | 6 (24) | 16 (22) | 48 (35) | 28 (44) | 60 (47) | 158 (37) |
| Unknown | 2 (8) | 3 (4) | 1 (1) | 1 (2) | 2 (2) | 9 (2) |
| CT/MR N stage | ||||||
| N0 | 16 (64) | 49 (70) | 115 (83) | 51 (80) | 110 (85) | 341 (80) |
| N1 | 9 (36) | 14 (20) | 22 (16) | 11 (17) | 18 (14) | 74 (17) |
| Unknown | 0 (0) | 7 (10) | 1 (1) | 2 (3) | 1 (1) | 11 (3) |
| Clinical T stage | ||||||
| cT1/T2 | 18 (32) | 23 (33) | 60 (43) | 6 (9) | 42 (32) | 156 (37) |
| cT3 | 17 (68) | 34 (49) | 57 (41) | 17 (27) | 56 (43) | 192 (45) |
| cT4 | 0 (0) | 2 (3) | 3 (2) | 28 (44) | 1 (1) | 6 (1) |
| Unknown | 0 (0) | 11 (16) | 18 (13) | 13 (20) | 30 (23) | 72 (17) |
| Duration of ADT (mo) | 36 (32-36) | 35 (33-37) | 36 (33-40) | 34 (33-36) | 35 (34-37) | 35 (33-37) |
| Median follow-up (y) | 13.9 | 11.2 | 9.0 | 7.1 | 5.7 | 7.6 |
Abbreviations: ADT = androgen deprivation therapy; CT = computed tomography; MR = magnetic resonance; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
Data are n (%) or median (interquartile range) unless otherwise stated.
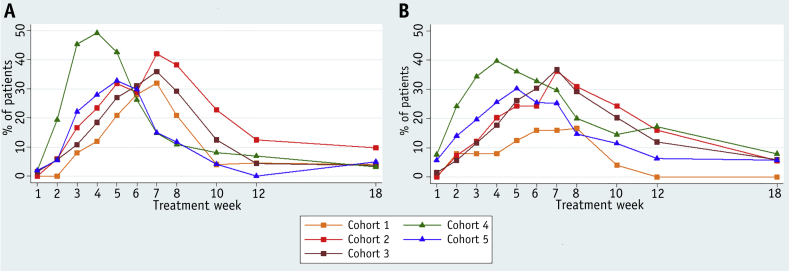
Acute bowel toxicity peaked at 6 to 8 weeks in the conventionally fractionated (CFRT) cohorts 1 to 3, compared with 4 to 5 and 5 to 6 weeks in the hypofractionated (HFRT) cohorts 4 and 5, respectively. Peak grade 2+ toxicity was recorded in 40%, 56%, and 54% of cohorts 1 to 3 respectively. Patients in cohort 4 developed the highest acute bowel toxicity rates, with 66% reporting grade 2+ bowel toxicity, compared with 48% in cohort 5. However, by 18 weeks after treatment the incidence of grade 2+ RTOG bowel toxicity was similar in all cohorts (Fig. 2 and Table E3 [available online at www.redjournal.org]). Acute grade 3+ peak toxicity occurred in 0 (0%), 1 (1%), 5 (4%), 3 (5%), and 9 (7%) patients in cohorts 1 to 5, respectively. One patient in each of cohorts 4 and 5 developed grade 4 acute toxicity, and there was 1 death (recorded as grade 5 toxicity), determined at autopsy to have resulted from perforation of an undiagnosed caecal carcinoma.
Fig. 2.
Acute Radiation Therapy Oncology Group (RTOG) grade 2 or worse toxicity by time point and treatment group. Prevalence of (A) acute RTOG grade 2+ bowel toxicity and (B) acute RTOG grade 2+ bladder toxicity.
Acute bladder toxicity was related to dose in the CFRT cohorts, with peak grade 2+ toxicity recorded in 28%, 44%, and 53% of patients in cohorts 1 to 3, respectively. Patients in cohort 4 experienced higher rates of bladder toxicity of grade 2+ (61%) than patients in cohort 5 (53%). However, rates of grade 2+ bladder toxicity at 18 weeks were low and similar in all cohorts (Fig. 2).
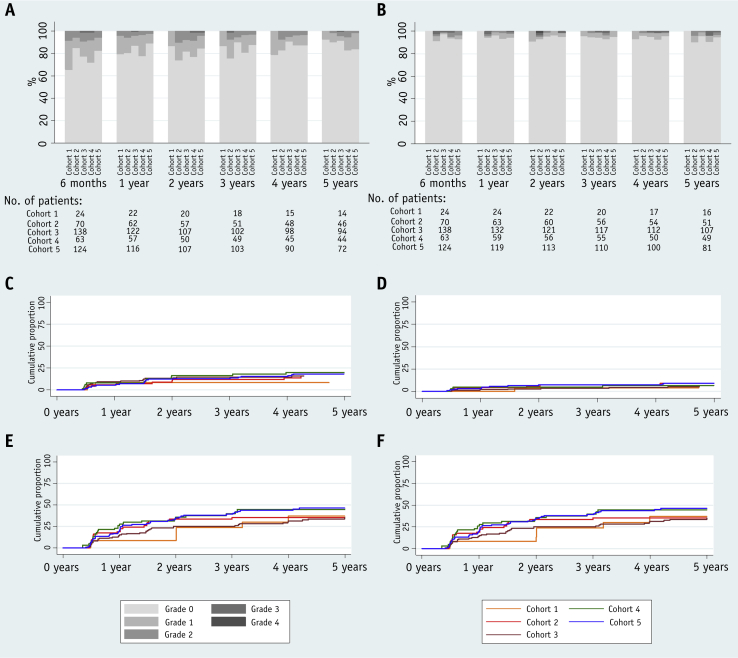
The 2-year cumulative rate of RTOG grade 2+/grade 3+ bowel toxicity was 8.3% (95% CI 2.7%-24.3%)/0%, 8.9% (4.1%-18.7%)/1.5% (0.2%-10.4%), and 13.2% (8.6%-20.2%)/2.2% (0.7%-6.7%) in cohorts 1 to 3 (CFRT), respectively. In the HFRT cohorts 4 and 5, the 2-year rate of grade 2+/grade 3+ bowel toxicity was 16.4% (9.2%-28.4%)/6.6% (2.5%-16.7%) and 12.2% (7.6%-19.5%)/0.8% (0.1%-5.7%), respectively (Fig. 3 and Tables E4 and E5 [available online at www.redjournal.org]). A comparable 12.2% (4.7%-29.3%) of postprostatectomy patients experienced grade 2+ bowel toxicity, with no clear difference between the cohorts in view of the small numbers included (Table E6; available online at www.redjournal.org).
Fig. 3.
Late bowel and bladder toxicity by time point, assessment, and treatment group. Grade distribution of (A) bowel adverse events and (B) bladder adverse events measured with Radiation Therapy Oncology Group (RTOG) scale. Cumulative incidence of (C) grade 2 or worse bowel adverse events measured with RTOG scale and (E) small or worse bowel symptom scores measured with University of California, Los Angeles Prostate Cancer Index (UCLA-PCI). Cumulative incidence of (D) grade 2+ bladder adverse events measured with RTOG scale and (F) small or worse bladder symptom scores measured with UCLA-PCI.
The 2-year cumulative rates of grade 2+/grade 3+ bladder toxicity were 4.2% (0.6%-26.1%)/4.2% (0.6%-26.1%), 5.9% (2.3%-15.0%)/2.9% (0.7%-11.3%), and 2.9% (1.1%-7.7%)/2.2% (0.7%-6.8%) in cohorts 1 to 3 (CFRT), respectively. In cohorts 4 and 5 (HFRT), rates were 4.8% (1.6%-14.3%)/1.6% (0.2%-10.9%), and 7.3% (3.9%-13.6%)/1.2% (0.4%-6.4%), respectively (Fig. 3 and Tables E4-E6 [available online at www.redjournal.org]). Postprostatectomy patients had a higher rate of urinary symptoms at 9.0%, albeit with a large CI (3.0%-25.4%), with no clear differences between cohorts (Table E6; available online at www.redjournal.org).
The prevalence of bowel and bladder toxicity seemed to be stable over time (Fig. 3 and Tables E4 and E5 [available online at www.redjournal.org]). At 5 years' follow-up, 0/0 (0%/0%), 1/0 (2%/0%), 5/1 (5%/1%), 3/0 (6%/0%), and 2/0 (2%/0%) men had grade 2+/3+ RTOG bowel toxicity in cohorts 1 to 5, respectively. The 5-year prevalence of grade 2+ bladder toxicity was 0/0 (0%/0%), 2/0 (4%/0%), 1/0 (1%/0%), 2/2 (4%/4%), and 3/1 (3%/1%) in cohorts 1 to 5, respectively.
All estimates of late toxicity met predefined safety criteria. Results using the Royal Marsden Hospital (RMH) and LENT-SOMA assessments are given in Tables E4 and E5 (available online at www.redjournal.org). Table E6 (available online at www.redjournal.org) details rates of late symptoms in patients treated after prostatectomy.
Patient-reported outcomes (PRO) were obtained with the UCLA-PCI instrument (Tables E4 and E5; available online at www.redjournal.org). The cumulative 5-year rate of small or worse bowel/bladder bother was 26% (95% CI 13%-50%)/37% (19%-63%), 49% (37%-63%)/35% (24%-49%), 38% (30%-48%)/35% (27%-45%), 56% (43%-69%)/45% (32%-59%), and 54% (44%-64%)/46% (37%-57%) in cohorts 1 to 5, respectively. Prevalence of moderate/severe bowel problems at 2 years was 1 of 22 patients (5%), 4 of 47 (9%), 6 of 84 (7%), 4 of 45 (9%), and 10 of 85 (12%) in cohorts 1 to 5, respectively. Moderate/severe urinary problems at 2 years were reported by 1 of 22 patients (5%), 5 of 47 (10%), 6 of 85 (7%), 6 of 47 (13%), and 10 of 85 (12%) in cohorts 1 to 5, respectively. At 5 years, prevalence rates for moderate/severe bowel problems were 0 of 12 patients (0%), 1 of 42 (2%), 1 of 76 (1%), 2 of 35 (6%), and 2 of 54 (4%) in cohorts 1 to 5, respectively. No severe bowel problems were reported at 5 years. Prevalence of moderate/severe urinary problems at 5 years was 0 of 12 patients (0%), 2 of 42 (4%), 6 of 78 (7%), 2 of 35 (6%), and 3 of 57 (5%) in cohorts 1 to 5, respectively. No men in the HFRT cohorts had severe urinary problems at 5 years.
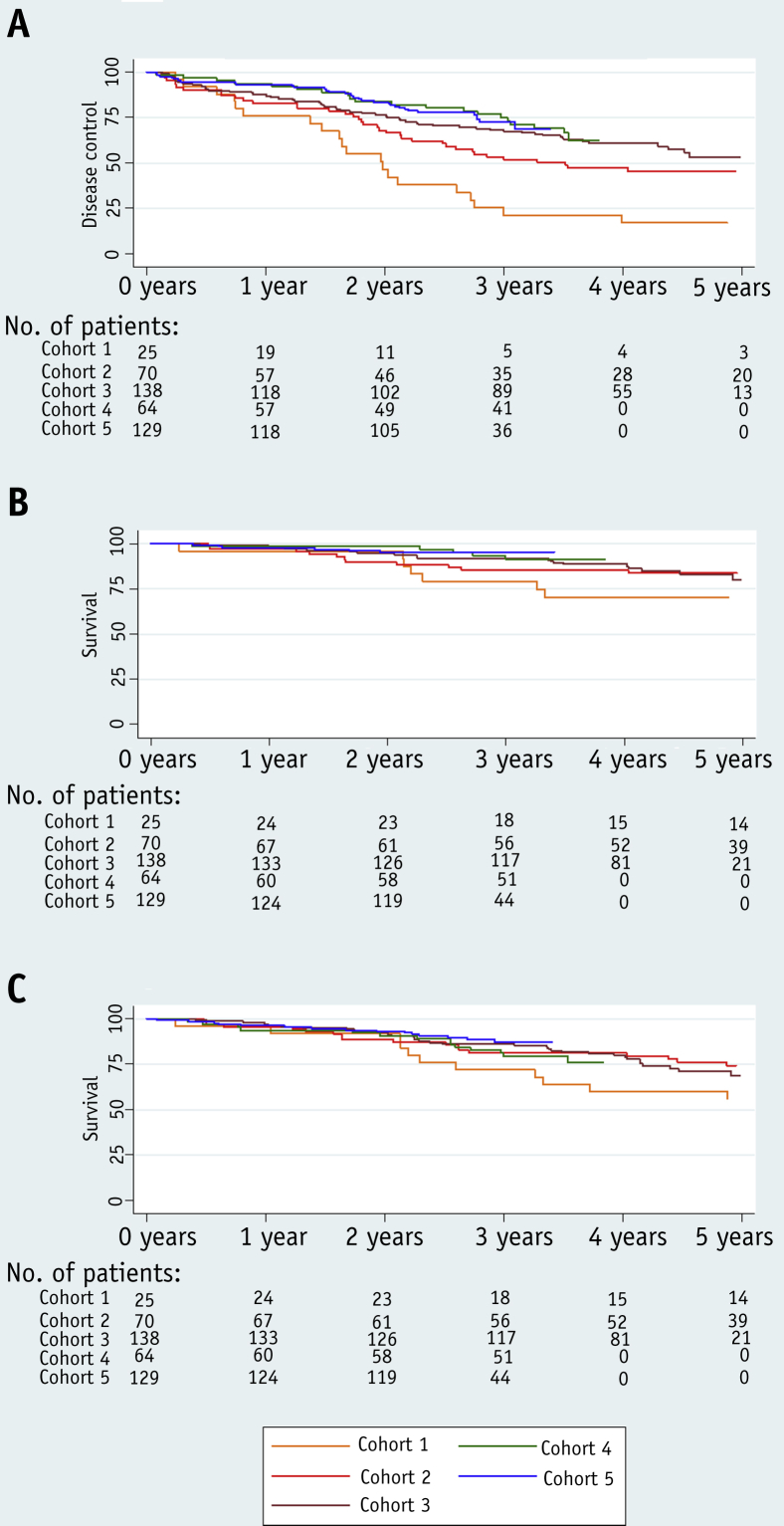
Biochemical or clinical progression occurred in 169 of 426 patients (39.7%). At first relapse, biochemical failure alone occurred in 141 of 169 patients (59%), local recurrence in 11 of 169 patients (7%), distant metastases in 7 of 169 patients (4%), and 3 of 169 patients (2%) commenced salvage hormone therapy in the absence of radiologic confirmation of sites of disease. On subsequent follow-up there were 41 of 426 patients (10%) with confirmed relapses within the prostate, 26 of 426 (6%) with PLN recurrences, 39 of 426 (9%) with relapses in distant nodal groups, and 99 of 426 (23%) with relapses at other metastatic sites. The biochemical/clinical failure–free rate was 71% (95% CI 66%-75%) at 5 years for the whole group, with 38%, 61%, 70%, 80%, and 78% remaining recurrence free in cohorts 1 to 5, respectively.
Disease-specific survival at 5 years was 92% (95% CI 89%-94%) for the whole cohort and 79%, 88%, 92%, 97%, and 95% in cohorts 1 to 5, respectively. The 5-year overall survival was 87% (95% CI 84%-90%) and 76%, 87%, 86%, 89%, and 91% in cohorts 1 to 5, respectively (Fig. 4).
Fig. 4.
Biochemical failure–free survival (A), disease-specific survival (B), and overall survival (C).
Multivariate analysis identified pretreatment PSA level (P=.004), PLN involvement (P=.02), T stage (P=.05), and dose cohort (P=.05) as factors associated with duration of disease control. Patients treated in cohorts 4 and 5 had similar outcomes (Table 2).
Table 2.
Multivariate Cox regression analysis, for duration of disease control (n=326)
| Factor | Hazard ratio (95% CI) | P |
|---|---|---|
| Dose cohort | .05 | |
| Cohort 1, 50 Gy | 1 (NA) | |
| Cohort 2, 55 Gy | 0.71 (0.40-1.26) | |
| Cohort 3, 60 Gy | 0.45 (0.26-0.80) | |
| Cohort 4, HFRT 4 wk | 0.50 (0.25-1.01) | |
| Cohort 5, HFRT 5 wk | 0.45 (0.24-0.84) | |
| Log max pretreatment PSA | <.01 | |
| Continuous, ng/mL | 1.30 (1.08-1.57) | |
| Clinical T stage | .05 | |
| T1/T2 | 1 (NA) | |
| T3a | 1.22 (0.78-1.91) | |
| T3b+ | 1.70 (1.11-2.60) | |
| Radiologic N stage | .02 | |
| N0 | 1 (NA) | |
| N+ | 1.65 (1.09-2.48) |
Abbreviations: CI = confidence interval; HFRT = hypofractionated; NA = not available.
Discussion
We found acceptable acute and late GI/genitourinary (GU) toxicity measured using both clinician-reported outcomes (CRO) and PRO in all patient cohorts. To assess the impact of PLNRT, we compared these results with a large contemporaneous group of patients treated in the CHHiP phase 3 trial, which used IMRT to treat the prostate alone using similar CFRT/HFRT schedules and scored side effects with the same compendium of CRO and PRO (4). We also used comparable data reported in a recent systematic review by Holch et al (19), which included no studies with PLNRT. We found that acute grade 2+ GI toxicity occurred in 40% to 56% of CFRT patients in cohorts 1 to 3, compared with 25% in CHHiP and 21% to 60% in Holch et al, with a rate of 66% in cohort 4 (4-week HFRT), compared with 30% in the CHHiP HFRT group and 36% in Holch et al. Increasing the overall treatment time to 5 weeks reduced the rate to 48% in cohort 5. However, these side effects settled rapidly in all groups. There were no differences in grade 2+ toxicity by 18 weeks, although some increase in mild grade 1+ side-effect rates persisted with PLNRT (25%-36%, compared with 21% in CHHiP). There were no clear differences between grade 2+ peak/week-18 or grade 1+ week-18 GU toxicities among cohorts 1 to 5 or when comparing with the CHHiP or Holch et al GU toxicity rates (Table E3; available online at www.redjournal.org).
Late GI side effects seemed highest in cohort 4 using CRO scales, both 2 and 5 years after treatment. For example, 2-year estimated cumulative proportions with grade 2+ (CRO) or small or worse bowel problems (PRO) were 16%, 16%, 34%, and 53% using the RTOG, RMH, LENT-SOMA, and UCLA-PCI scales, respectively, compared with 8% to 13%, 8% to 15%, 13% to 25%, and 21% to 43% for the other cohorts. The rates for the comparator CHHiP group were 8% to 9%, 10% to 11%, 16% to 18%, and 25% to 27%, respectively. Applicable results in the Holch et al systematic review (19) were similar to those in CHHiP. The increased acute and late GI toxicity seen in cohort 4 would be consistent with a consequential late side effect 20, 21. Extending treatment duration to 5 weeks by treating 4 times per week seems to reduce any impact of hypofractionation (Tables E4 and E5; available online at www.redjournal.org).
Late GU side effects, assessed using RTOG and RMH CRO scales, were similar among all groups, with no obvious impact from dose, fractionation schedule, or use of PLNRT. However, the cumulative proportion of patients with grade 2+ toxicity (LENT-SOMA, CRO) or small or worse bladder bother (UCLA-PCI, PRO) at 2 years was somewhat higher than in the CHHiP groups, suggesting these scales are more sensitive. Any differences had disappeared by 5 years, when the prevalence of small or worse bladder bother was 8% to 20% in cohorts 1 to 5 and 17% in the CHHiP comparator group (Tables E4 and E5; available online at www.redjournal.org).
Late bowel and bladder side effects did not show consistent differences when the subgroup of patients treated after prostatectomy was analyzed, either with CRO or PRO data (Table E6; available online at www.redjournal.org). However, these results should be interpreted with caution given that only 34 patients were treated adjuvantly in this trial, and limited conclusions can be drawn.
The low level of side effects seen in the present series probably relates to the use of a strict IMRT protocol and the mandating of dose constraints for both bowel and bladder. However, the doses delivered in cohorts 3 to 5 are at least 10% higher than used in past and contemporary practice (Fig. E1; available online at www.redjournal.org). Similar dose increments have been shown to improve disease outcome in trials treating the prostate alone 22, 23.
The 5-year overall survival in this series (87%; 95% CI 84%-90%) is at least comparable to a recent retrospective series from the National Cancer Database, in which 7606 patients were treated with PLNRT, with 5-year overall survival of 81.6% (24). In the group randomized to PLNRT in the RTOG 94-13 trial, a 4-year overall survival of 84% was reported. The 5-year biochemical/clinical failure–free rate of 71% for our entire series is similar to the control group treated with radiation therapy in the contemporaneous MRC STAMPEDE trial, which showed an estimated 75%/50% 5-year control in patients with N0/N1 disease, respectively (25).
The low PLN recurrence rate of 6% is reassuring, but further efforts to improve local control in the prostate for patients with aggressive bulky disease seem warranted (hazard ratio for local disease control in T3b+: 1.70, 95% CI 1.11-2.60; Table 2). Approaches using high-dose focal radiation therapy boosts, prostatectomy, or additional ablative focal therapies using, for example, high intensity focused ultrasound or cryotherapy can be considered (26). Avoidance of toxicity, however, is important, because a considerable majority of patients have disease controlled by IMRT and ADT or, alternatively, relapse with metastatic disease outside the pelvis, making additional measures to improve local control futile. The development of biomarkers to predict the response to radiation therapy and define patient groups destined to develop metastatic disease would therefore be invaluable in guiding treatment individualization (27). Treatment intensification with additional systemic treatments, such as docetaxel or the new generation of hormonal therapies, can be considered (28). Additionally, radiogenomic and dosimetric studies are aiming to refine estimates of an individual's risk of developing side effects 29, 30.
Conclusion
This study has provided the safety data to encourage further investigation of high-dose LNRT. The treatment techniques described have been generalized in a United Kingdom national phase 2 randomised pilot study, PIVOTAL (ISRCTN48709247), which compares prostate and pelvis with prostate-alone IMRT. Hypofractionated radiation therapy will become the United Kingdom standard of care after the CHHiP trial (4). The safety data of hypofractionated schedules in the present study is encouraging, and the use of HFRT in a new trial, PIVOTALboost, is planned. It will assess the value of pelvic IMRT as well as the effects of a focal high-dose intraprostatic boost to dominant lesions. These studies will complement other ongoing phase 3 studies, RTOG 09-24 (NCT01368588) and PEACE 2 (NCT01952223), which should finally determine the role of PLNRT in prostate cancer. An increase in efficacy will need to be demonstrated to offset the small but expected adverse effects of pelvic IMRT.
Acknowledgments
The authors thank the patients and all investigators and research support staff. Recognition goes to all the trials unit staff at the Bob Champion Unit and Royal Marsden Hospital Trial Unit, who contributed to the central coordination of the study.
Footnotes
Supported by Cancer Research UK (C46/A2131, C46/A3976, C46/A10588, C33589/A19727), the Department of Health, the National Institute for Health Research (NIHR) Cancer Research Network, and by National Health Service (NHS) funding to the NIHR Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London. M.R.F. was supported by the Calouste Gulbenkian Foundation, the Fundação para a Ciência e a Tecnologia, and the Champalimaud Foundation.
M.R.F. and A.K. contributed equally to this work.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
Supplementary Data
References
- 1.NCCN clinical practice guidelines in oncology (NCCN guidelines) for prostate cancer. Version 1.2016. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 15, 2017.
- 2.Cancer Research UK. Prostate cancer statistics—key facts. Available at: www.cancerresearchuk.org/health-professional/prostate-cancer-statistics. Accessed March 23, 2017.
- 3.Mohiuddin J.J., Baker B.R., Chen R.C. Radiotherapy for high-risk prostate cancer. Nat Rev Urol. 2015;12:145–154. doi: 10.1038/nrurol.2015.25. [DOI] [PubMed] [Google Scholar]
- 4.Dearnaley D., Syndikus I., Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook J. Prostate cancer: Elective pelvic nodal radiotherapy: Is the jury still out? Nat Rev Urol. 2016;13:10–11. doi: 10.1038/nrurol.2015.283. [DOI] [PubMed] [Google Scholar]
- 6.Nutting C.M., Convery D.J., Cosgrove V.P. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:649–656. doi: 10.1016/s0360-3016(00)00653-2. [DOI] [PubMed] [Google Scholar]
- 7.Dawson L.A., Sharpe M.B. Image-guided radiotherapy: Rationale, benefits, and limitations. Lancet Oncol. 2006;7:848–858. doi: 10.1016/S1470-2045(06)70904-4. [DOI] [PubMed] [Google Scholar]
- 8.Dolezel M., Odrazka K., Zouhar M. Comparing morbidity and cancer control after 3D-conformal (70/74 Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate cancer. Strahlenther Onkol. 2015;191:338–346. doi: 10.1007/s00066-014-0806-y. [DOI] [PubMed] [Google Scholar]
- 9.Safdieh J.J., Schwartz D., Weiner J. Long-term tolerance and outcomes for dose escalation in early salvage post-prostatectomy radiation therapy. Radiat Oncol J. 2014;32:179–186. doi: 10.3857/roj.2014.32.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox S.W., Aherne N.J., Benjamin L.C. Long-term outcomes from dose-escalated image-guided intensity-modulated radiotherapy with androgen deprivation: Encouraging results for intermediate- and high-risk prostate cancer. Onco Targets Ther. 2014;7:1519–1523. doi: 10.2147/OTT.S65238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creak A., Hall E., Horwich A. Randomised pilot study of dose escalation using conformal radiotherapy in prostate cancer: Long-term follow-up. Br J Cancer. 2013;109:651–657. doi: 10.1038/bjc.2013.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCammon R., Rusthoven K.E., Kavanagh B. Toxicity assessment of pelvic intensity-modulated radiotherapy with hypofractionated simultaneous integrated boost to prostate for intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:413–420. doi: 10.1016/j.ijrobp.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Roach M., 3rd, Marquez C., Yuo H.S. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28:33–37. doi: 10.1016/0360-3016(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 14.Adams E.J., Convery D.J., Cosgrove V.P. Clinical implementation of dynamic and step-and-shoot IMRT to treat prostate cancer with high risk of pelvic lymph node involvement. Radiother Oncol. 2004;70:1–10. doi: 10.1016/j.radonc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Clark C.H., Mubata C.D., Meehan C.A. IMRT clinical implementation: Prostate and pelvic node irradiation using Helios and a 120-leaf multileaf collimator. J Appl Clin Med Phys. 2002;3:273–284. doi: 10.1120/jacmp.v3i4.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.LENT SOMA scales for all anatomic sites. Int J Radiat Oncol Biol Phys. 1995;31:1049–1091. doi: 10.1016/0360-3016(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 18.Litwin M.S., Hays R.D., Fink A. The UCLA Prostate Cancer Index: Development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Holch P., Henry A.M., Davidson S. Acute and late adverse events associated with radical radiation therapy prostate cancer treatment: A systematic review of clinician and patient toxicity reporting in randomized controlled trials. Int J Radiat Oncol Biol Phys. 2017;97:495–510. doi: 10.1016/j.ijrobp.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Dörr W., Hendry J.H. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–231. doi: 10.1016/s0167-8140(01)00429-7. [DOI] [PubMed] [Google Scholar]
- 21.Michalski J.M., Yan Y., Watkins-Bruner D. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou Z., Li G., Bai S. High dose versus conventional dose in external beam radiotherapy of prostate cancer: A meta-analysis of long-term follow-up. J Cancer Res Clin Oncol. 2015;141:1063–1071. doi: 10.1007/s00432-014-1813-1. [DOI] [PubMed] [Google Scholar]
- 23.Dearnaley D.P., Jovic G., Syndikus I. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 24.Amini A., Jones B.L., Yeh N. Survival outcomes of whole-pelvic versus prostate-only radiation therapy for high-risk prostate cancer patients with use of the National Cancer Data Base. Int J Radiat Oncol Biol Phys. 2015;93:1052–1063. doi: 10.1016/j.ijrobp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 25.James N.D., Spears M.R., Clarke N.W. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348–357. doi: 10.1001/jamaoncol.2015.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lips I.M., van der Heide U.A., Haustermans K. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): Study protocol for a randomized controlled trial. Trials. 2011;12:255. doi: 10.1186/1745-6215-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudreau P.O., Stagg J., Soulières D. The present and future of biomarkers in prostate cancer: Proteomics, genomics, and immunology advancements. Biomark Cancer. 2016;8(Suppl. 2):15–33. doi: 10.4137/BIC.S31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James N.D., Sydes M.R., Clarke N.W. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fachal L., Gómez-Caamaño A., Barnett G.C. A three-stage genome-wide association study identifies a susceptibility locus for late radiotherapy toxicity at 2q24.1. Nat Genet. 2014;46:891–894. doi: 10.1038/ng.3020. [DOI] [PubMed] [Google Scholar]
- 30.Gulliford S.L., Partridge M., Sydes M.R. Parameters for the Lyman Kutcher Burman (LKB) model of Normal Tissue Complication Probability (NTCP) for specific rectal complications observed in clinical practise. Radiother Oncol. 2012;102:347–351. doi: 10.1016/j.radonc.2011.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.