Abstract
Background
There is increasing recognition of the contribution of community-acquired cases to the global burden of Clostridium difficile infection (CDI). The epidemiology of CDI among international travellers is poorly understood, and factors associated with international travel, such as antibiotic use and changes in gut microbiota, could potentially put travellers at higher risk.
Methods
We summarized demographic, travel-associated and geographic characteristics of travellers with CDI in the GeoSentinel database from 1997 to 2015. We also surveyed GeoSentinel sites to compare various testing indications, approaches, and diagnostic modalities.
Results
We identified 260 GeoSentinel records, including 187 that satisfied criteria for analysis (confirmed cases in non-immigrant travellers aged >2 years, seen <12 weeks post-travel). CDI was reported in all age groups and in travellers to all world regions; the largest proportions of cases having destinations in Asia (31%), Central/South America or the Caribbean (30%) and Africa (24%). Our site survey revealed substantial heterogeneity of testing approaches between sites; the most commonly used test was the C. difficile toxin gene PCR.
Conclusions
CDI is encountered in returning international travellers, although there is considerable variability in testing practices. These data underscore the importance of awareness of C. difficile as a potential cause of travel-associated diarrhoea.
Keywords: Clostridium difficile, diarrhoea, travellers
Introduction
Clostridium difficile infection (CDI) is a major cause of diarrhoea-related morbidity and mortality worldwide, most commonly reported in higher-income countries. While most cases are thought to be healthcare-associated,1 community-acquired cases are recognized as a major contributor to the overall burden of disease.2,3
Risk factors for CDI may be found in returning travellers, one of the strongest being use of antibiotics. Antibiotic use is known to cause reduced resistance to C. difficile colonization through disruption of the intestinal microbiota,4 in a duration-dependent manner.5 Providing travellers with a short course of antibiotics for self-treatment of traveller’s diarrhoea is a standard of practice in travel medicine.6 A recent study of USA-based pre-travel healthcare providers by the Global TravEpiNet clinic consortium showed that 87% of international travellers received an antibiotic prescription for empiric self-treatment for presumptive travellers’ diarrhoea, although the proportion of travellers using such prescriptions during travel is unknown.7 In addition to antibiotic use, international travel itself has been associated with broad changes in intestinal microbial community structure,8 though this has not been directly associated with CDI.
CDI has been shown to be associated with international travel. In a Swedish study of 851 adult patients who presented to an infectious disease clinic with diarrhoea, CDI was found in 6% of those who travelled outside the country in the previous 2 weeks.9 In a review of 48 published cases of travel-related CDI,10 the majority acquired their disease in low- or medium-income countries, were younger than 60 years of age and were community-acquired rather than healthcare-associated infections. Among those in whom antibiotic use information was available, a large proportion (75%) had used antibiotics before developing diarrhoea, with fluoroquinolones the most commonly used. Another study11 reported C. difficile in six patients following antibiotic treatment of travellers’ diarrhoea. Each of these studies are limited by their small sample sizes and specific biases. As such, the factors associated with CDI in returning travellers remain to be determined.
Additionally, the approach to the diagnosis of C. difficile is challenging, with a number of molecular-, culture- and toxin-based assays available, and with varying algorithms proposed in guidelines from professional societies in different countries. The selection criteria for patients to test, as well as the diagnostic testing schema used influence diagnostic sensitivity and specificity.12
In this report, we used the GeoSentinel Global Surveillance Network database to describe epidemiologic characteristics of CDI and to examine the factors associated with clinical diagnosis of travel-associated CDI.
Methods
Data were collected by reporting clinics of the GeoSentinel Global Surveillance Network, a global surveillance network of 59 travel and tropical medicine clinics on 6 continents operating at the time the analysis was conducted.13 To be eligible for inclusion in the GeoSentinel surveillance database, patients must have crossed an international border within the 10 years preceding their clinical visit and have sought medical care from a GeoSentinel clinician for a presumed travel-associated illness. Clinics submit surveillance-related data into an encrypted, structured query database devoid of unique identifiers. Final diagnoses are assigned by clinicians and chosen from a standard list of >500 surveillance diagnosis codes, which are grouped into 21 broad syndrome categories. GeoSentinel’s data collection protocol has been reviewed by the institutional review board officer at CDC’s National Center for Emerging and Zoonotic Infectious Diseases and is classified as public health surveillance and not human subjects research. Where needed, because of national regulations at individual GeoSentinel sites, ethical clearance has been obtained.
The GeoSentinel database was examined to identify patients with a laboratory-confirmed diagnosis of CDI and a clinic visit date from 1 January 1997 to 31 August 2015. The GeoSentinel definition for C. difficile-associated disease is ‘Antibiotic associated diarrhoea with or without colitis with or without pseudo-membrane formation’, although clinician adherence to this definition may vary in practice. We extracted demographic, travel-associated, geographic and clinical variables. We included cases classified as confirmed by the diagnosing medical provider. Excluded from analysis were records of patients with immigration as the reason for travel, age ≤2 year, GeoSentinel visits occurring during travel, clinic visits >12 weeks after returning home from travel, and missing data on travel exposures.
The annual frequency of cases of CDI was compared with the annual frequency of three other etiologic intestinal disease diagnoses (giardiasis, cryptosporidiosis, and campylobacteriosis) among CDI-reporting GeoSentinel sites during the study period. We only included in this analysis records contributed by sites that had also reported at least 1 case of confirmed CDI, limited to the years in which full-year data were available (1997–2014). We applied the same inclusion and exclusion criteria to these three diagnoses as were applied to CDI.
We examined differences in diagnostic approach to CDI through an online site survey. SurveyMonkey™ (SurveyMonkey, Inc., Palo Alto, USA) was used to conduct an online survey of the 59 GeoSentinel sites active during October 2015. We queried the various testing indications/approaches, the types of C. difficile lab diagnostic tests used, the types of laboratory facilities available, and whether or not testing was performed on unformed stool only STATA 14 software (StataCorp, College Station, TX) was used to carry out descriptive and analytic statistics. Figures were created in Microsoft Excel for Mac version 14.6.0.
Results
Clostridium difficile Infections
Of the 260 patients with a CDI diagnosis in the GeoSentinel database, 187 satisfied criteria for inclusion and were analyzed (Figure 1). An increase in the number of reported CDI diagnoses over time was observed (Figure 2), that paralleled the increases in cases of giardiasis, cryptosporidiosis, and campylobacteriosis reported over the same time period. Of note, the number of sites participating in GeoSentinel increased from 9 in 1997 to 31 in 2006 to 56 in 2015, reflective of a steady growth in the GeoSentinel network. CDI cases were reported with a similar frequency as cases of Cryptosporidium.
Figure 1.
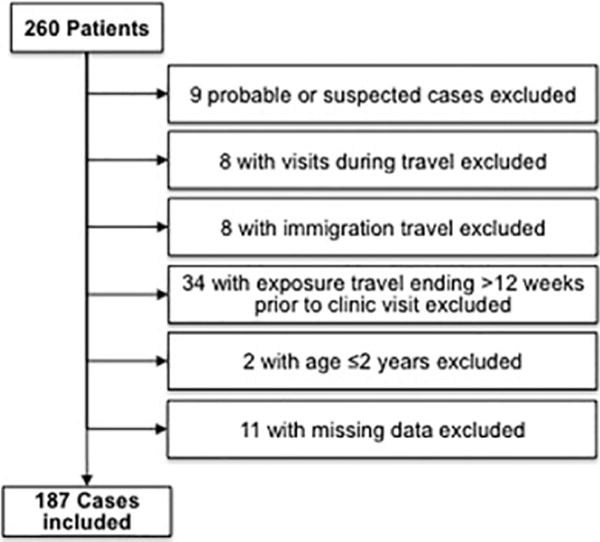
Exclusion criteria for cases of travel-associated C. difficile-associated diarrhoea in GeoSentinel travellers, 1997–2015
Figure 2.
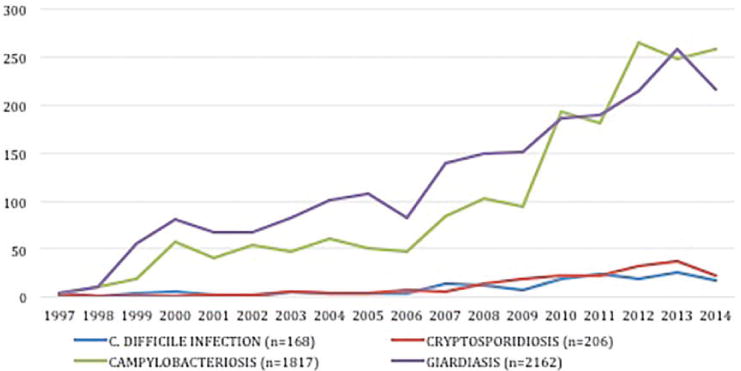
Relative occurrence of C. difficile infection compared with giardiasis, cryptosporidiosis and campylobacteriosis, GeoSentinel 1997–2014
Of the 187 travellers with CDI, 113 (62%) were female (Table 1). The median age was 34 years old (range 6–89 years old). The majority of CDI diagnoses were made by clinics in North America [58 (31%) in the USA and 37 (20%) in Canada], followed by 22 (12%) in Germany, 18 (10%) in France and 15 (8%) in Sweden (Table 2).
Table 1.
Characteristics of patients with travel-associated C. difficile infection, Geosentinel, 1997–2015
| Sex | n | % |
|---|---|---|
| Male | 70 | 38 |
| Female | 113 | 62 |
| Age (years) | ||
| 3 to 9 | 1 | <1 |
| 10 to 19 | 3 | 2 |
| 20 to 29 | 62 | 34 |
| 30 to 39 | 43 | 23 |
| 40 to 49 | 27 | 15 |
| 50 to 59 | 22 | 12 |
| 60 to 69 | 15 | 8 |
| 70 to 79 | 8 | 4 |
| 80 to 89 | 4 | 2 |
| Median | 34 | |
| Range | 6 – 89 | |
|
| ||
| Reason for travel | ||
| Business | 20 | 11 |
| Missionary/volunteer/Researcher/aid work | 31 | 17 |
| Student | 11 | 6 |
| Tourism | 115 | 62 |
| Visiting friends and relatives | 10 | 5 |
|
| ||
| Pre-travel visit | ||
| Yes | 78 | 45 |
| No | 58 | 34 |
| Unknown | 36 | 21 |
|
| ||
| Travel duration | Days | |
| Median | 20.5 | |
| IQR | 13, 42 | |
|
| ||
| Time from return to presentation | Days | |
| Median | 14.0 | |
| IQR | 5, 30 | |
|
| ||
| Region of travel | n | % |
| Asia | 57 | 31 |
| North East Asia | 4 | 2 |
| South Central Asia | 33 | 18 |
| South East Asia | 20 | 11 |
| Americas | 58 | 31 |
| North America | 2 | 1 |
| Caribbean | 17 | 9 |
| Central America | 18 | 10 |
| South America | 21 | 11 |
| Africa | 44 | 24 |
| North Africa | 13 | 7 |
| Sub-Saharan Africa | 31 | 17 |
| Europe | 14 | 7 |
| Western Europe | 12 | 6 |
| Eastern Europe | 2 | 1 |
| Middle East | 5 | 3 |
| Pacific | 2 | 1 |
| Australia/New Zealand | 1 | 0.5 |
| Oceania | 1 | 0.5 |
| Multiple | 7 | 4 |
Table 2.
Countries where C. difficile infection diagnoses were made, GeoSentinel, 1997–2015
| Cases of CDI
|
||
|---|---|---|
| n | % | |
| USA | 58 | 31 |
| Canada | 37 | 20 |
| Germany | 22 | 12 |
| France | 18 | 10 |
| Sweden | 15 | 8 |
| Switzerland | 7 | 4 |
| Australia | 8 | 4 |
| Spain | 6 | 3 |
| Japan | 4 | 2 |
| The Netherlands | 4 | 2 |
| Norway | 4 | 2 |
| Israel | 2 | 1 |
| UK | 1 | <1 |
| New Zealand | 1 | <1 |
CDI was reported with travel to Asia in 57 (31%) travellers, particularly South Central Asia (n = 33, 18%), and Southeast Asia (n = 20, 11%). Thirty-one (17%) were associated with travel to sub-Saharan Africa and 56 (30%) with travel to Central/South America or the Carribean. Tourism was the most commonly reported reason for travel (n = 115, 62%), followed by missionary/volunteer/researcher/aid work (n = 31, 17%), business travel (20, 11%), and student travel (n = 11, 6%). Seventy-eight (45%) travellers had a pre-travel health consultation. The median travel duration was 20.5 days (range 3–1235 days). The median time from return from travel to GeoSentinel clinic presentation was 15 days (range 0–83 days).
GeoSentinel Site Survey
Fifty-six of the 59 (95%) GeoSentinel sites responded to the on-line survey, accounting for >98% of the reported CDI cases. These sites represent 26 countries on 6 continents. Results were stratified into one of the three geographic reporting groups: North America, Europe and Rest of the World to reflect the global distribution of participating clinics.
In evaluating testing approaches stratified by group (Table 3), 15 sites (7 in Europe, 5 in North America) reported testing of patients with acute diarrhoea who failed empiric treatment for presumed bacterial diarrhoea. Forty-two sites (18 in Europe, 13 in North America) reported testing patients with acute diarrhoea and known risk factors for CDI (e.g. recent antibiotic use, healthcare exposure). Thirty-five sites (17 in Europe, 11 in North America) reported testing patients with chronic diarrhoea and known risk factors for CDI. Most notably, 23 of the 56 sites (42%) reported that the testing approach varied by individual clinician practices at the reporting site.
Table 3.
Testing indications and diagnostic testing methods by regional groups for travel-associated C. difficile infection at GeoSentinel sites (n = 56)
| Testing approaches1 | Europe (n = 21) | North America (n = 19) | Other (n = 16) | Total (n = 56) | |
|---|---|---|---|---|---|
| All or most patients with acute diarrhoea are tested | 2 (10%) | 5 (26%) | 2 (13%) | 9 | |
| Any patient with acute diarrhoea who failed empiric treatment for presumed bacterial diarrhoea | 7 (33%) | 5 (26%) | 3 (19%) | 15 | |
| Patients with acute diarrhoea and risk factors for (recent antibiotic exposure, healthcare exposure) | 18 (86%) | 13 (68%) | 11 (69%) | 42 | |
| All or most patients with chronic diarrhoea | 7 (33%) | 8 (42%) | 4 (25%) | 19 | |
| Patients with chronic diarrhoea and risk factors for (recent antibiotic exposure, healthcare exposure) | 17 (81%) | 11 (58%) | 7 (44%) | 35 | |
| Patients with chronic diarrhoea who failed empiric treatment for presumed parasitic infection | 5 (24%) | 3 (16%) | 5 (31%) | 13 | |
| Elderly patients (age 65 or older) | 4 (19%) | 2 (11%) | 1 (6%) | 7 | |
| Patients with active malignancy or other immunocompromising condition | 6 (29%) | 2 (11%) | 2 (13%) | 10 | |
| Patients with evidence of organ dysfunction due to the severity of the infection (i.e. renal failure, etc) | 5 (24%) | 4 (21%) | 0 (0%) | 9 | |
| Testing practices vary by individual clinician at our GeoSentinel Site | 8 (38%) | 9 (47%) | 6 (38%) | 23 | |
|
| |||||
| C. difficile testing method2 | No. responses3 | Europe | North America | Other | Total |
|
| |||||
| C. difficile toxin antigen detection by EIA | 40 | 8 (53%) | 7 (50%) | 8 (73%) | 23 |
| C. difficile toxin PCR | 44 | 9 (56%) | 17 (94%) | 6 (60%) | 32 |
| C. difficile toxin assay by monolayer cell culture | 37 | 3 (19%) | 1 (8%) | 0 (0%) | 4 |
| C. difficile rapid bacterial antigen plus toxin assay combination testing | 40 | 9 (50%) | 4 (31%) | 5 (56%) | 18 |
| C. difficile toxin immunoassay | 36 | 1 (6%) | 1 (9%) | 1 (11%) | 3 |
Note that these answers are not mutually exclusive, thus percentages do not add up to 100.
Note that these testing methods are not mutually exclusive, since many sites have more than 1 testing method.
Responses could be Yes, No, or Unknown.
The most common method of diagnostic testing used was the C. difficile toxin gene PCR (used by 94% of sites in North America and 56% of sites in Europe). Other common methods included the C. difficile toxin antigen detectixon by enzyme immunoassay (EIA), and the C. difficile rapid combination bacterial antigen plus toxin immunoassays. These testing methods are not mutually exclusive, since many sites reported use of more than one testing method.
Discussion
The epidemiology of CDI is changing, and a broader array of risk factors is becoming apparent.8,14 Our study summarizes CDI diagnoses amongst travellers who visited clinics in the GeoSentinel Global Surveillance Network during a 19-year period. Our data show an increase in reported CDI cases in the GeoSentinel network over time, though one that parallels the increases in reported cases of other etiological intestinal disease diagnoses as our surveillance network expanded.
While CDI is a commonly diagnosed diarrhoeal pathogen in high-income countries, there is little data on its epidemiology in low- or middle-income countries.15 In our analysis, nearly all regions of the world were represented as destinations from which travellers returned. However, we are unable to determine the timing of C. difficile acquisition in our study. It is possible that a proportion of these travellers were colonized prior to departure, and then developed clinical CDI after various insults leading to microbiota disturbances, or that they became infected after their return. It is also possible that pre-travel C. difficile colonization may be protective against disease by stimulating antibody production.1
The pathogenesis of CDI involves both pathogen and host factors, but the most widely recognized risk factor is antibiotic use. Antibiotic use is associated with disruption of the indigenous gut microbiota, which can lead to long-lasting or even permanent loss of organisms.16 There are a number of possible mechanisms by which such alterations in the gut microbiota contributes to CDI. The pathogenic mechanisms of such disruptions include altering the composition of various bile salts in the gut, leading to C. difficile spore germination and vegetative growth; destruction of healthy bacteria decreasing competition for other nutrient resources in the gut; and immune dysregulation with a consequent proinflammatory state, which is often seen with C. difficile colitis.17,18 Aside from antibiotic use, other factors that influence the gut microbiota include changes in diet, both intestinal and extra-intestinal infections, use of non-antimicrobial medications (including agents that affect gut motility), and changes in environment, many of which may be encountered during international travel. In addition to diarrhoea, travellers may experience many other health problems (fever, respiratory tract infection or skin infection) that may lead to antibiotic exposure (by self-medication or by local caregivers). While chemoprophylaxis for malaria with antimicrobials such as doxycycline is common, there is insufficient data to support its association with CDI.6 Nevertheless, antimicrobial use during travel has been associated with other adverse outcomes such as increased risk of colonization with multi-drug resistant bacteria.19 Thus, prevention of CDI is another reason to optimize the judicious use of antimicrobials in travellers. Defining more specific criteria for travellers to self-administer antibiotics for travel-associated diarrhoea, and counseling on preventative measures, such as basic hygiene measures20 may be the key to preventing dysbiosis and CDI.
We demonstrate a high heterogeneity across sites in diagnostic testing practices for CDI. The most common reported indications for testing were acute or chronic diarrhoea with the risk factors of antibiotic use or healthcare exposure during travel. These data suggest inter- and intra-site variability in clinician awareness of CDI as a cause of travellers’ diarrhoea, potentially leading to under diagnosis of CDI. Recent studies have highlighted the prevalence of community acquired CDI, and the relative importance of risk factors other than antibiotic exposure, including proton pump inhibitors and exposure to infants.4 This broadening of clinical risk assessment may not yet be utilized or reflected in clinical consideration of CDI as a cause of travellers’ diarrhoea. Prospective studies that involve testing all travellers for C. difficile are needed for determination of independent risk factors.
The variability of laboratory diagnostic testing practices found in our study illustrates the lack of standardization in the diagnostic approach for travellers’ diarrhoea. The toxin gene PCR assay was the most commonly used diagnostic test by clinicians at GeoSentinel clinics, although a number of other testing strategies were used. Toxin gene PCR assays are regarded as a rapid, sensitive and specific testing method,12 and are commonly used either alone or in combination with toxin EIA and glutamate dehydrogenase testing. We also noted differences in testing strategies between our European and North American sites.21 The variability of diagnostic strategies may affect the identification and reporting of CDI as the cause of travel-associated diarrhoea in individual travellers.
This study has a number of limitations. First, because the findings are limited to travellers who present for care to GeoSentinel sites, the data are not necessarily representative of all international travellers. Second, the lack of denominator data limits our ability to evaluate the risk of travel-associated CDI. Third, despite the use of standard diagnosis codes, data coding and entry practices might vary by clinician and site, and over time, and we are limited by our reliance on the reporting provider’s clinical judgement and their interpretation of microbiologic results. Fourth, changes in GeoSentinel data collection methods and the number of GeoSentinel sites limit conclusions drawn from direct longitudinal comparisons. A fifth limitation is that GeoSentinel has not routinely collected information on antibiotic exposure among travellers, either during travel or since return from travel. Sixth, current testing methods may not be able to differentiate between colonization with C. difficile bacteria, with or without toxin production, and true clinical disease due to C. difficile;22 thus some patients with C. difficile colonization may be inaccurately diagnosed with CDI. Lastly, this study demonstrated significant variability in clinical testing practices, so we acknowledge the potential for inconsistent capture of CDI cases. It is important to note that multiple new diagnostic testing modalities have been implemented over the study period.
Despite these limitations, we report the largest series of travel-associated CDI to date. Clinicians who evaluate returning international travellers should be aware of CDI as a potential cause of diarrhoeal illness.
Acknowledgments
Additional members of the GeoSentinel Surveillance Network who contributed data but did not author this article are Kevin Kain (Toronto, Ontario), Eric Caumes (Paris, France), Hilmir Asgeirsson (Stockholm,Sweden), Camilla Rothe (Hamburg, Germany), William Stauffer (St. Paul, Minnesota, U.S.A.), Patricia Schlagenhauf (Zurich, Switzerland), Phyllis Kozarsky (Atlanta, Georgia, U.S.A.), Jean Haulman (Seattle, Washington, U.S.A.), Carmelo Licitra (Orlando, Florida, U.S.A.), Yukiriro Yoshimura (Yokohama City, Kanagawa, Japan), Francois Chappuis (Geneva, Switzerland), Frank Mockenhaupt (Berlin, Germany), Sarah Borwein (Hong Kong, China), Mogens Jensenius (Oslo, Norway), Susan Anderson (Palo Alto, California, U.S.A.), Francesco Castelli (Brescia, Italy), Lin Chen (Cambridge, Massachusetts, U.S.A.), Karin Leder (Parkville, Victoria, Australia), Jean Vincelette (Montreal, Quebec, Canada), Marc Shaw (Auckland, New Zealand), Christina Coyle (Bronx, New York, U.S.A.), Paul Kelly (Bronx, New York, U.S.A.), John Cahill (New York, New York, U.S.A.), Poh-Lian Lim (Singapore), Shuzo Kanagawa (Tokyo, Japan), Jan Hajek (Vancouver, British Columbia, Canada).
Funding
GeoSentinel, the Global Surveillance Network of the International Society of Travel Medicine (ISTM), is supported by a cooperative agreement (U50CK00189) from the CDC as well as ISTM and the Public Health Agency of Canada.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention.
Authors’ Contributions
Study Design: AMS, DHE, RJS, DHH, JFF, EB, BAC, EGK, AG, NH, ML, RLV, AEM, FVS, ES, PJJVG, LSB, DTL.
Data collection: DHE, RJS, DHH, JFF, EB, BAC, EGK, AG, NH, ML, RLV, AEM, FVS, ES, PJJVG, LSB, DTL.
Analysis: AMS, DHE, RJS, DHH, DTL.
Interpretation: AMS, DHE, RJS, DHH, JFF, EB, BAC, EGK, AG, NH, ML, RLV, AEM, FVS, ES, PJJVG, LSB, DTL.
Conflict of interest
None declared.
References
- 1.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–67. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KA, Fisman DN, Moineddin R, Daneman N. The magnitude and duration of Clostridium difficile infection risk associated with antibiotic therapy: a hospital cohort study. PLoS ONE. 2014;9:e105454. doi: 10.1371/journal.pone.0105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA. 2015;313:71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 7.LaRocque RC, Rao SR, Lee J, et al. Global travepinet: a national consortium of clinics providing care to international travelers–analysis of demographic characteristics, travel destinations, and pretravel healthcare of high-risk US international travelers, 2009–2011. Clin Infect Dis. 2012;54:455–62. doi: 10.1093/cid/cir839. [DOI] [PubMed] [Google Scholar]
- 8.David LA, Materna AC, Friedman J, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenungsson B, Lagergren A, Ekwall E, et al. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin Infect Dis. 2000;30:770–8. doi: 10.1086/313770. [DOI] [PubMed] [Google Scholar]
- 10.Neuberger A, Saadi T, Shetern A, Schwartz E. Clostridium difficile infection in travelers–a neglected pathogen? J Travel Med. 2013;20:37–43. doi: 10.1111/j.1708-8305.2012.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Norman FF, Pérez-Molina J, de Ayala AP, Jimacute BC, Navarro M, López-Vélez R. Clostridium difficile–associated diarrhea after antibiotic treatment for traveler’s diarrhea. Clin Infect Dis. 2008;46:1060–3. doi: 10.1086/529380. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of america (SHEA) and the infectious diseases society of america (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 13.Harvey K, Esposito DH, Han P, et al. Surveillance for travel-related disease–geosentinel surveillance system, United States, 1997–2011. MMWR Surveill Summ. 2013;62:1–23. [PubMed] [Google Scholar]
- 14.Youmans BP, Ajami NJ, Jiang ZD, et al. Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes. 2015;6:110–9. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010 Jul;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–33. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146:1547–53. doi: 10.1053/j.gastro.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seekatz AM, Young VB. Clostridium difficile and the microbiota. J Clin Invest. 2014;124:4182–9. doi: 10.1172/JCI72336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantele A, Lääveri T, Mero S, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing enterobacteriaceae. Clin Infect Dis. 2015;60:837–46. doi: 10.1093/cid/ciu957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor BA, Keystone JS. Editorial commentary: antibiotic self-treatment of travelers’ diarrhea: helpful or harmful? Clin Infect Dis. 2015;60:847–8. doi: 10.1093/cid/ciu961. [DOI] [PubMed] [Google Scholar]
- 21.Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 22.Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792–801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]


