Synopsis
Endocytic trafficking is a highly organized process regulated by a network of proteins, including the Rab family of small GTP-binding proteins and the C-terminal EHDs (Eps15 homology-domain-containing proteins). Central roles for Rab proteins have been described in vesicle budding, delivery, tethering and fusion, whereas little is known about the functions of EHDs in membrane transport. Common effectors for these two protein families have been identified, and they facilitate regulation of sequential steps in transport. By comparing and contrasting key aspects in their modes of function, we shall promote a better understanding of how Rab proteins and EHDs regulate endocytic trafficking.
Keywords: collapsin response mediator protein-2 (CRMP2), endocytic trafficking, Eps15 homology (EH), Eps15 homology-domain-containing protein (EHD), GTPase-activating protein (GAP), Rab, transferrin receptor (TfR)
INTRODUCTION
Endocytic trafficking is a basic requirement for all cells, as it is essential for the internalization and transport of nutrients, segments of plasma membrane and cell surface receptors [1–4]. It is a highly organized process regulated by a network of proteins, among which are the Rab family of small GTP-binding proteins and the EHDs (Eps15 homology-domain-containing proteins).
Rab proteins constitute the largest of the Ras superfamily of small GTP-binding proteins. There are 11 Rab members in budding yeast, termed Ypts, and more than 60 members have been identified in humans. Besides their central role in membrane trafficking, Rabs and their effector proteins are also implicated in various processes including cell signalling [5,6], cell migration [7], cytokinesis [8,9], ciliogenesis [10–12], apoptosis [13,14] and autophagy [15–18]. Dysfunction of Rab proteins is associated with cancer, pathogen-related diseases and inherited disorders [19].
The human EHD family was initially characterized about a decade ago (reviewed in [20,21]). This family consists of four highly homologous mammalian members, EHD1–4. The single orthologue of human EHD1 in Caenorhabditis elegans is known as Rme-1 [22], but there are no real homologues in Saccharomyces cerevisiae. All four mammalian EHDs are involved in the regulation of endocytic transport, albeit at distinct but partially overlapping steps [20,23]. EHDs exert many of their regulatory functions through various binding partners. The majority of these interactions are through the C-terminal EH domain of the EHDs, which bind to an NPF (asparagine-proline-phenylalanine) motif present in EHD-binding proteins [20,23]. Although the aberrant expression of EHDs has been documented in various diseases, it remains unclear as to whether this altered expression has any physiological significance.
Interestingly, some EHD-binding partners are also Rab effectors. To date, however, it remains unclear as to whether they coordinately control transport or merely take advantage of the same effectors. In the present review, we shall compare key aspects of the EHD and Rab family proteins and provide a better understanding of how they each control endocytic transport.
FUNCTIONS IN ENDOCYTIC TRAFFICKING
Rab GTP-binding proteins, together with their diverse effectors, are implicated in all steps of endocytic transport, including vesicle formation, motility, tethering/docking and fusion [19,24]. A classic example of Rab protein involvement in vesicle formation is provided by Rab5. The Rab5–GDI (GDP dissociation inhibitor) complex is required for clathrin-coated vesicle formation in vitro [25] and overexpression of a Rab5-GAP (GTPase-activating protein), RN-Tre, inhibits epidermal growth factor receptor internalization [26]. Other examples of Rab involvement in vesicle budding include Rab4 and Rab7; an in vitro assay indicates that vesicle formation is regulated by Rab4 and its effector, rabaptin-5/rabex-5 [27], whereas Rab7 is involved in macropinosome formation [28]. Rab regulation of vesicle transport along the actin filaments and microtubules is through direct or indirect interaction with motor proteins, which will be discussed subsequently in this review.
Among the most widely recognized roles for Rabs is the tethering and fusion of vesicles to target organelles. Rabs recruit tethering factors that form bridges between vesicles and target membranes, which in turn lead to membrane fusion through Rab effector–SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) interactions [19,24,29]. For example, Rab5 serves as a regulator of early endosomal tethering and fusion. The Rab5 effector EEA1 (early endosome antigen 1) bridges Rab5-positive vesicles through two Rab5-binding sites on both the N- and C-termini [30], and subsequently mediates membrane fusion by direct interactions with the SNARE proteins syntaxin 6 and syntaxin 13 [31,32].
Although Rabs are primarily involved in vesicle tethering and fusion, EHDs may function as ‘pinchases’ in vesicle scission, thus regulating vesicle transport from endosomes to downstream compartments [33]. Despite a high level of sequence identity between the four paralogues (67.9–86.5%), EHDs regulate different steps of endocytic traffic. However, loss of expression of one EHD can apparently be partially compensated by other EHDs ([34–36], and reviewed in [23]). EHD1 is the most widely studied family member, and its best-characterized role is in regulating the recycling of membrane cargo from the ERC (endocytic recycling compartment) to the plasma membrane. EHD1 regulates both clathrin-dependent and -independent pathways, including TfR (transferrin receptor) [37], MHCI [38] and β1 integrins [39]. On the other hand, the precise function of EHD2 is yet to be elucidated. It is involved in the internalization of TfR and the glucose transporter GLUT4 [40] and displays functional redundancy with EHD1 in some tissues [35]. EHD3 is the closest paralogue to EHD1, sharing 86% amino acid identity and it regulates retrograde transport from the EE (early endosome) to the Golgi [41,42]. EHD4 negatively regulates Rab5 and has been implicated in vesicle transport from the EE to the ERC, as well as from the EE to the lysosomal degradation pathway [35,43].
Choose your nucleotide
Rab proteins function as molecular switches between active GTP-bound forms and inactive GDP-bound forms. GDP-bound Rab is stabilized in its inactive cytosolic form by GDI [44–46], and its targeting to specific membranes is mediated by the membrane-bound GDFs (GDI displacement factors) [47]. Subsequently, GEFs (guanine-nucleotide-exchange factors) catalyse the exchange of GDP for GTP and convert Rabs into membrane-bound active forms that are recognized by various effector proteins. Finally, GAPs stimulate the hydrolysis of GTP, and convert Rabs back into the GDP-bound inactive form [48,49].
Surprisingly, despite the similarity of the EHD nucleotide-binding G-domain to those found in Ras and dynamin family GTPases, EHDs display a much higher affinity for ATP than GTP. Nonetheless, the rate of ATP hydrolysis stimulated by EHDs is 600-fold slower than GTP hydrolysis by dynamin [33]. The structure of EHDs consists of two helical domains with a nucleotide-binding domain in between, a linker region and a C-terminal EH domain [33] (Figure 1). Cytoplasmic-localized EHDs bind ATP and oligomerize along tubular membranes. Unlike Rab proteins, which depend on extrinsic GTPase activity via GAPs, EHDs have intrinsic ATPase activity that is stimulated by membrane binding. ATP hydrolysis induces a conformational change that destabilizes the membrane, which in turn leads to vesicle scission [33].
Figure 1. Schematic diagram of EHD domain architecture.
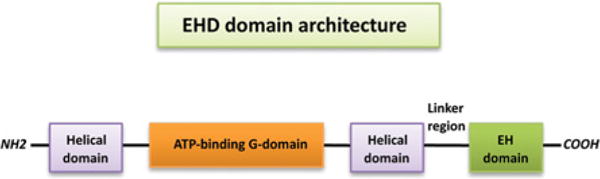
EHDs contain two helical regions with an ATP-binding G domain in between. The C-terminal EH domain is connected with the helical domain by a 40-residue linker region.
Hanging on
Rab GTPases are reversibly associated with membranes by prenylation, whereas membrane binding of EHDs is mediated by their lipid-binding helical regions [33] or through direct binding of C-terminal EH domains to phosphatidylinositols [50]. In addition, EH domain binding to an array of NPF-containing interaction partners might facilitate either recruitment of EHD to membranes or stabilization on the membranes [51]. Cytosolic GDP-bound Rabs are recognized by REP (Rab escort protein), which presents Rabs to GGT (geranylgeranyltransferase) [52]. Rab proteins are then prenylated on one or two C-terminal cysteine residues by GGT and the prenylated Rabs are bound to GDIs. The GDI–Rab complex is recognized by a membrane-bound GDF, which promotes the release of the GDI [47]. After fulfilling their various functions at the membrane through the GTP–GDP cycle regulated by GEFs and GAPs, Rab proteins return to the inactive GDP-bound form and then can be extracted from the membrane by GDIs [44–46,53,54].
Compared with the membrane-association cycle of Rab proteins controlled by Rab regulatory proteins, the mechanism of the EHD interaction with membranes remains to be elucidated. Although EHDs are capable of tubulating liposomes in vitro [33], their interaction with membranes in vivo may be more complex. It has been shown that depletion of EHD1 has no effect on the tubular membrane localization of the double-palmitoylated and -farnesylated C-terminus of H-Ras [55]. Furthermore, MICAL-L1 (molecule interacting with CAsL-like 1), an EHD1-binding partner, recruits and/or stabilizes EHD1 on tubular membranes [51]. These results suggest that EHDs associate with pre-existing tubular membranes in vivo, and may exert their roles in membrane tubulation co-operatively with their diverse binding partners, including the BAR (Bin/amphiphysin/Rvs)-domain-containing protein AMPH-1/amphiphysin/Bin1 [56]. Accordingly, EHDs may behave similarly to dynamin, which tubulates membranes in vitro [57] and in vivo [58], whereas its main role is to form a spiral at the neck of budding vesicles and stimulate membrane scission [59–62].
EHDs against Rabs: united or alone
While Rabs function as monomers, oligomerization is required for EHD ATPases to function. Dimerization of EHDs is mediated by a hydrophobic interface in the nucleotide-binding G domain [33]. Where this dimerization occurs is not clear. One possibility is that it occurs in the cytoplasm and is required for membrane association. A different possibility is that dimerization might ensue on interaction with the membrane to stabilize binding. Indeed, EHD mutants impaired in their ability to homo- or hetero-oligomerize display a cytoplasmic distribution [42], suggesting that oligomerization is required for association or continued contact with tubular membranes. Subsequently, it has been predicted that the EHD ring structures which are generated as a result of advanced oligomerization stimulate ATP hydrolysis, thus leading to membrane fission and the release of vesicles [33].
Membrane bending activity by EHDs
Similar to the dynamin superfamily of GTPases, EHD2 is capable of tubulating liposomes in vitro in a nucleotide-independent manner [33]. Moreover, it has been predicted that EHD2 oligomerizes in ring-like structures around lipid tubules, and that the membrane curvature imposed by the proposed oligomer is perpendicular to the curved membrane interface of the EHD2 dimer [33]. Mathematical modelling of EHD2 ring-like structures along the membrane tube suggests that EHD2 induces membrane bending through a scaffolding mechanism that can be described by the Helfrich model of membrane elasticity [63]. This model formulates the membrane curvature as a mathematical surface so that the energy needed for membrane fission can be calculated [63,64]. The model also contends that additional protein players may be required to reduce the energy barrier and facilitate fission of the membrane [63]. To date, there is no membrane bending function reported for Rab proteins.
Interaction with motors
The movement of vesicles along actin microfilaments or microtubules is propelled by motor proteins, which include the actin-dependent motors of the myosin family and the microtubule-dependent motors: kinesin superfamily proteins and cytoplasmic dynein.
Rabs are involved in regulating all three types of motors [19,29] (Figure 2B). One example of a Rab interaction with actin motors is via the Rab effector protein, Rab11-FIP2 (Rab11-family interacting protein 2), which is also an EHD-binding partner [42]. Rab11-FIP2 connects Rab11 and myosin Vb and regulates vesicle recycling [65]. Another example is the recent discovery that Rab6 controls the fission of Rab6 vesicles from the Golgi complex through its interaction with myosin II [66].
Figure 2. Interaction with motor proteins.
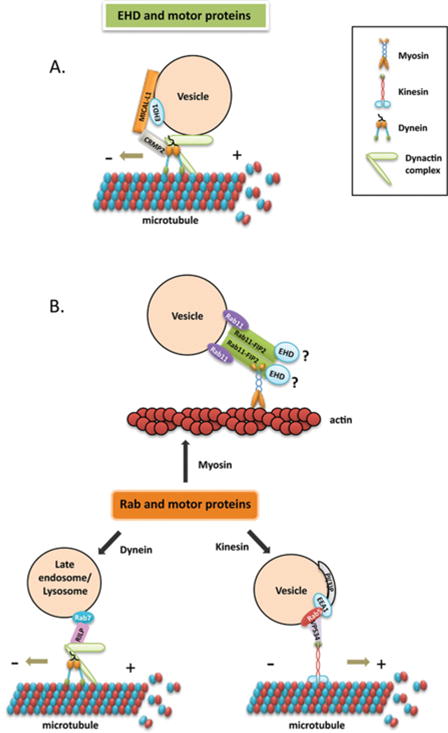
(A) EHD and dynein. CRMP2 serves as a link between MICAL-L1-EHD1-associated vesicles and cytoplasmic dynein, and mediates microtubule-dependent vesicle transport. (B) Interactions between Rab proteins and either myosin, kinesin or dynein motors through their respective effectors. Rab11 interacts with myosin Vb through Rab11-FIP2, which is also an EHD-binding partner. Rab5 and its effector hVPS34 (human VPS34) regulate KIF16B (kinesin 16B) transportation of PI(3)P [PtdIns(3)P]-positive early endosomes. RILP binds to Rab7 and mediates vesicle transport by recruiting the dynein–dynactin complex.
Kinesins can be directly or indirectly regulated by Rabs. Direct interaction between Rab6 and the kinesin-like protein Rabkinesin 6 is essential for cytokinesis [67,68]. An example of indirect regulation is the kinesin KIF16B (kinesin 16B) that is localized to phosphatidylinositol-3-phosphate-positive endosomal membranes and controlled by Rab5 [69].
Indirect interactions between dynein and Rabs have also been described. RILP (Rab7-interacting lysosomal protein) binds to active Rab7 and mediates minus-end-directed transport of late endosomes by recruiting the dynein–dynactin complex [70]. A new study has shown that the Rab11 effector protein Rab11-FIP3 interacts with the DLIC-1 (dynein light intermediate chain 1) and mediates transport from sorting endosomes to the ERC [71].
Compared with Rab proteins, little is known about the interactions between EHDs and motor proteins. A recent study has shown that CRMP2 (collapsin response mediator protein-2), a novel binding partner of the EHD1-interaction protein MICAL-L1, serves as a link between MICAL-L1-EHD1-associated endosomes and cytoplasmic dynein [72] (Figure 2A). Another report demonstrated that myosin Vb co-localizes with Rab8a on tubules containing EHD1 and EHD3 [73], suggesting that EHDs and Rab proteins may function coordinately in regulating vesicle transport through their interactions with common motor proteins. Further investigation will be required to better understand the mechanism by which EHDs interact with motor proteins and the regulatory effect on vesicle transport.
Effectors and binding partners
Rab effectors are proteins that generally bind to a specific GTP-bound Rab and mediate at least one set of downstream effects. On the other hand, EHD-binding partners are proteins that usually contain an NPF motif flanked by acidic residues [20,23,74]. Both Rab proteins and EHDs appear to have multiple effectors or binding partners through which diverse functions of membrane transport are facilitated. For example, an affinity chromatography approach yielded 22 proteins that potentially interact with Rab5-GTP [75]. A wide range of Rab- and EHD-binding partners have been summarized in several reviews [20,23,29].
The various Rab effectors share little sequence homology, although the binding interfaces usually involve the two switch regions of Rab proteins [76]. Many Rab effectors contain more than one binding domain for distinct binding partners, suggesting these multivalent molecules may play roles in coordinating interrelated endocytic pathways [77,78]. On the contrary, EHD-binding partners almost always contain an NPF tripeptide sequence followed by acidic residues and interact with EHDs via NPF motif-EH domain binding. The highly positively charged surface of the EH domain dictates the requirement for flanking acidic residues [74,79].
So far, three proteins have been identified that serve both as Rab effectors and as EHD-binding partners: Rabenosyn-5, Rab11-FIP2 and MICAL-L1. The identification of these proteins raises the possibility that Rab proteins and EHDs might coordinately regulate intracellular trafficking.
Rabenosyn-5, a divalent effector of Rab4 and Rab5, binds to the EHD1 EH domain through the first two of its five NPF motifs [80]. The distribution patterns of internalized transferrin and MHCI in cells depleted of both Rabenosyn-5 and EHD1 is similar to cells depleted only of Rabenosyn-5, indicating that Rabenosyn-5 acts upstream of EHD1. These results suggest that Rabenosyn-5 may connect with Rab4 on early endosomes, and subsequently with EHD1 at the ERC, thus linking these two consecutive pathways to regulate cargo recycling to the plasma membrane.
Rab11-FIP2, the only known Rab11 effector that contains three NPF motifs, binds to the EHD1 and EHD3 EH domains. EHD3 localizes to early endosomes and may connect with Rab11 through Rab11-FIP2, and subsequently through its homo-oligomerization or hetero-oligomerization with EHD1 at the ERC to regulate the transport from early endosomes to the ERC [42].
The EHD1-binding partner MICAL-L1 is also a Rab8a effector [81,82]. Unlike most Rab effectors, association of MICAL-L1 with tubular membranes is independent of Rab8a. MICAL-L1 may recruit or stabilize EHD1 to tubular membranes and provide a link between EHDs and Rab proteins to coordinately regulate recycling to the plasma membrane [51]. The identification of additional proteins involved in the recycling pathways will be required to elucidate the exact sequence of events that occur in the course of recycling.
In addition to these three proteins, we have identified Rabankyrin-5/ANKFY1, a Rab5 effector required for macropinocytosis [83], as a novel EHD1-binding partner that contains the amino acid sequence NPFED [84]. Rabankyrin-5 partially co-localized with EHD1 on punctate membrane structures to regulate cargo internalization and recycling through both clathrin-dependent and -independent pathways [84].
It remains an open question, however, as to whether there is really significant cross-talk between Rabs and EHDs. One possibility is that the EHDs, whose absence from yeast indicates an evolutionary appearance later than the Rabs, evolved to take advantage of pre-existing effectors used by Rab family members. In this manner, EHD1 would have evolved to interact specifically with the Rab effectors Rabenosyn-5, MICAL-L1 and Rab11-FIP2. Additional studies will be necessary to elucidate whether these two endocytic regulatory protein families coordinate trafficking events or merely function in parallel.
SUMMARY
Over the past decade, EHDs have become known as integral regulators of endocytic trafficking, along with the Rab family of GTP-binding proteins. EHD and Rab protein families share some common features, such as nucleotide binding, transient association with membranes and interactions with motor proteins. However, they display distinct functions in regulating vesicle transport. The well-established model of Rab function is in membrane tethering and fusion (Figure 3B). Rab proteins recruit tethering factors, which in turn interact with SNARE proteins and activate the formation of SNARE complexes, leading to membrane fusion [19,29].
Figure 3. Models of EHDs and Rab proteins.
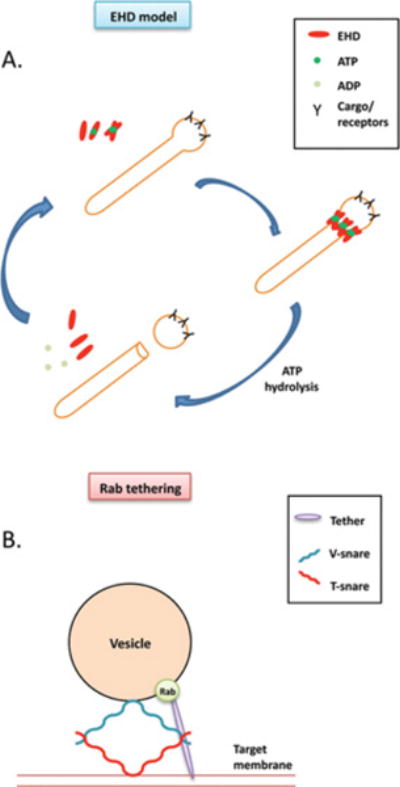
(A) EHD ‘pinchase’ model. EHD-ATP dimers oligomerize along the tubular membrane. ATP hydrolysis induces a conformational change that destabilizes the membrane, which in turn leads to vesicle scission.
(B) Rab tethering model. Rabs recruit tethering factors, which in turn interact with SNARE proteins and activate the formation of SNARE complexes, leading to membrane fusion. Scale and stoichiometry are simplified for the illustration.
In comparison, one of the models proposed for EHD function is the regulation of membrane fission (Figure 3A). According to this model, EHD-ATP dimers insert themselves into the hydrophobic lipid bilayer and induce membrane bending, while ATP hydrolysis induces a conformational change, which destabilizes the membrane and leads to membrane fission [33]. It is interesting that despite differences in function, Rab proteins and EHDs interact with each other through common effectors. These proteins interact with Rabs and EHDs through distinct domains and link Rab- and EHD-mediated pathways controlling endocytic trafficking.
Although significant progress has been made in understanding how Rabs and EHDs function in membrane trafficking, there are still many unanswered questions for both protein families. The diversity of Rabs reflects the complexity and multitude of endocytic traffic pathways in mammalian cells. Reconstitution of the Rab machinery in vitro may clarify our understanding of this complex process [85]. On the other hand, with the increasing number of Rab regulators and effectors being identified, it is important not only to characterize their individual roles but also to examine how they integrate cross-talk between multiple pathways. Less is known about how EHDs function in vesicle transport. A model has been proposed for EHD function in membrane fission [33], yet the mechanism remains to be proved. Another area that is still poorly understood is how EHDs interact with motor proteins. Identification of new EHD-binding partners may provide the necessary link by which EHD is connected with motor proteins to regulate microtubule-mediated vesicle transport [72].
In summary, Rabs and EHDs are key regulators of intracellular membrane trafficking. Further investigation of their distinct but complementary functions in regulating endocytic transport will improve our understanding of underlying mechanisms of endocytic transport.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health (NIH) [grant numbers R01GM074876, 1R01GM087455] and the Nebraska Center for Cellular Signaling NIH [grant number P20 RR018759].
Abbreviations
- CRMP2
collapsin response mediator protein-2
- EE
early endosome
- EH
Eps15 homology
- EHD
Eps15 homology-domain-containing protein
- ERC
endocytic recycling compartment
- GAP
GTPase-activating protein
- GDI
GDP dissociation inhibitor
- GDF
GDI displacement factor
- GEF
guanine-nucleotide-exchange factor
- GGT
geranylgeranyltransferase
- MICAL-L1
molecule interacting with CAsL-Like 1
- NPF
asparagine-proline-phenylalanine
- Rab11-FIP2
Rab11-family interacting protein 2
- RILP
Rab7-interacting lysosomal protein
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor
- TfR
transferrin receptor
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 3.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr Opin Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Mathijssen IM, Morton JE, et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Albertson R, Riggs B, Field CM, Sullivan W. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J Cell Biol. 2008;182:301–313. doi: 10.1083/jcb.200712036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellinen T, Tuomi S, Arjonen A, Wolf M, Edgren H, Meyer H, Grosse R, Kitzing T, Rantala JK, Kallioniemi O, et al. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell. 2008;15:371–385. doi: 10.1016/j.devcel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 11.Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo P, Hu T, Zhang J, Jiang S, Wang X. Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation. Proc Natl Acad Sci USA. 2010;107:18016–18021. doi: 10.1073/pnas.1008946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snider MD. A role for rab7 GTPase in growth factor-regulated cell nutrition and apoptosis. Mol Cell. 2003;12:796–797. doi: 10.1016/s1097-2765(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 15.Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell. 2008;19:2916–2925. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci USA. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munafo DB, Colombo MI. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic. 2002;3:472–482. doi: 10.1034/j.1600-0854.2002.30704.x. [DOI] [PubMed] [Google Scholar]
- 18.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 20.Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? J Cell Sci. 2005;118:4093–4101. doi: 10.1242/jcs.02595. [DOI] [PubMed] [Google Scholar]
- 22.Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- 23.Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122–131. doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 25.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 26.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 27.Pagano A, Crottet P, Prescianotto-Baschong C, Spiess M. In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Mol Biol Cell. 2004;15:4990–5000. doi: 10.1091/mbc.E04-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rupper A, Lee K, Knecht D, Cardelli J. Sequential activities of phosphoinositide 3-kinase, PKB/Akt, and Rab7 during macropinosome formation in dictyostelium. Mol Biol Cell. 2001;12:2813–2824. doi: 10.1091/mbc.12.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI3K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 31.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen A, Gaullier JM, D’Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- 33.Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 34.George M, Rainey MA, Naramura M, Ying G, Harms DW, Vitaterna MH, Doglio L, Crawford SE, Hess RA, Band V, Band H. Ehd4 is required to attain normal prepubertal testis size but dispensable for fertility in male mice. Genesis. 2010;48:328–342. doi: 10.1002/dvg.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George M, Ying G, Rainey MA, Solomon A, Parikh PT, Gao Q, Band V, Band H. Shared as well as distinct roles of EHD proteins revealed by biochemical and functional comparisons in mammalian cells and C. elegans. BMC Cell Biol. 2007;8:3. doi: 10.1186/1471-2121-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapaport D, Auerbach W, Naslavsky N, Pasmanik-Chor M, Galperin E, Fein A, Caplan S, Joyner AL, Horowitz M. Recycling to the plasma membrane is delayed in EHD1 knockout mice. Traffic. 2006;7:52–60. doi: 10.1111/j.1600-0854.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 38.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates β1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 40.Guilherme A, Soriano NA, Bose S, Holik J, Bose A, Pomerleau DP, Furcinitti P, Leszyk J, Corvera S, Czech MP. EHD2 and the novel EH domain binding protein EHBP1 couple endocytosis to the actin cytoskeleton. J Biol Chem. 2004;279:10593–10605. doi: 10.1074/jbc.M307702200. [DOI] [PubMed] [Google Scholar]
- 41.Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma M, Naslavsky N, Caplan S. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro AD, Pfeffer SR. Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides. J Biol Chem. 1995;270:11085–11090. doi: 10.1074/jbc.270.19.11085. [DOI] [PubMed] [Google Scholar]
- 46.Shisheva A, Chinni SR, DeMarco C. General role of GDP dissociation inhibitor 2 in membrane release of Rab proteins: modulations of its functional interactions by in vitro and in vivo structural modifications. Biochemistry (Moscow) 1999;38:11711–11721. doi: 10.1021/bi990200r. [DOI] [PubMed] [Google Scholar]
- 47.Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab–GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 48.Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- 49.Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol. 2001;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 50.Naslavsky N, Rahajeng J, Chenavas S, Sorgen PL, Caplan S. EHD1 and Eps15 interact with phosphatidylinositols via their Eps15 homology domains. J Biol Chem. 2007;282:16612–16622. doi: 10.1074/jbc.M609493200. [DOI] [PubMed] [Google Scholar]
- 51.Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell. 2009;20:5181–5194. doi: 10.1091/mbc.E09-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andres DA, Seabra MC, Brown MS, Armstrong SA, Smeland TE, Cremers FP, Goldstein JL. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- 53.Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990;265:13007–13015. [PubMed] [Google Scholar]
- 54.Ullrich O, Stenmark H, Alexandrov K, Huber LA, Kaibuchi K, Sasaki T, Takai Y, Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- 55.Jovic M, Kieken F, Naslavsky N, Sorgen PL, Caplan S. Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling. Mol Biol Cell. 2009;20:2731–2743. doi: 10.1091/mbc.E08-11-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pant S, Sharma M, Patel K, Caplan S, Carr CM, Grant BD. AMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recycling. Nat Cell Biol. 2009;11:1399–1410. doi: 10.1038/ncb1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 58.Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 59.Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campelo F, Fabrikant G, McMahon HT, Kozlov MM. Modeling membrane shaping by proteins: focus on EHD2 and N-BAR domains. FEBS Lett. 2010;584:1830–1839. doi: 10.1016/j.febslet.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 64.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 65.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 66.Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12:645–654. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- 67.Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 68.Fontijn RD, Goud B, Echard A, Jollivet F, van Marle J, Pannekoek H, Horrevoets AJ. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol Cell Biol. 2001;21:2944–2955. doi: 10.1128/MCB.21.8.2944-2955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 70.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 71.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 72.Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem. 2010;285:31918–31922. doi: 10.1074/jbc.C110.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kieken F, Sharma M, Jovic M, Giridharan SS, Naslavsky N, Caplan S, Sorgen PL. Mechanism for the selective interaction of C-terminal Eps15 homology domain proteins with specific Asn-Pro-Phe-containing partners. J Biol Chem. 2010;285:8687–8694. doi: 10.1074/jbc.M109.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 76.Lee MT, Mishra A, Lambright DG. Structural mechanisms for regulation of membrane traffic by rab GTPases. Traffic. 2009;10:1377–1389. doi: 10.1111/j.1600-0854.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kieken F, Jovic M, Naslavsky N, Caplan S, Sorgen PL. EH domain of EHD1. J Biomol NMR. 2007;39:323–329. doi: 10.1007/s10858-007-9196-0. [DOI] [PubMed] [Google Scholar]
- 80.Naslavsky N, Boehm M, Backlund PS, Jr, Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol Biol Cell. 2004;15:2410–2422. doi: 10.1091/mbc.E03-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics. 2008;7:1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- 82.Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008;19:971–983. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Reinecke J, Naslavsky N, Caplan S. Rabankyrin-5: a novel interaction partner of the endocytic regulatory protein EHD1. Mol Biol Cell. 2010;21(Suppl) Abstract no. 1258. [Google Scholar]
- 85.Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]


