Abstract
BACKGROUND
The American College of Cardiology Foundation (ACCF) and the Society of Thoracic Surgeons (STS) Collaboration on the Comparative Effectiveness of Revascularization Strategies (ASCERT) was a large observational study designed to compare the long-term effectiveness of coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI) to treat coronary artery disease (CAD) over 4 to 5 years.
OBJECTIVES
We examined the cost effectiveness of CABG compared to PCI for stable ischemic heart disease.
METHODS
The STS and ACCF databases were linked to the Centers for Medicare and Medicaid Services claims data. Costs for the index and observation period (2004 to 2008) hospitalizations were assessed by diagnosis-related group Medicare reimbursement rates; costs beyond the observation period were estimated from average Medicare participant per capita expenditure. Effectiveness was measured via mortality and life expectancy data. Cost and effectiveness comparisons were adjusted using propensity score matching with the incremental cost-effectiveness ratio (ICER) expressed as cost per quality-adjusted life year (QALY) gained.
RESULTS
CABG patients (n = 86,244) and PCI patients (n = 103,549) were at least 65-yearsold with 2 or 3-vessel CAD. Adjusted costs were higher for CABG for the index hospitalization, study period, and lifetime by $10,670, $8,145, and $11,575, respectively. Patients undergoing CABG gained an adjusted average of 0.2525 and 0.3801 life-years relative to PCI over the observation period and lifetime, respectively. The life-time ICER of CABG compared to PCI was $30,454/QALY gained.
CONCLUSIONS
Over a period of 4 years or longer, patients undergoing CABG had better outcomes but at higher costs than those undergoing PCI.
Keywords: coronary artery bypass graft, incremental cost-effectiveness ratio, percutaneous coronary intervention, stable ischemic heart disease
While death rates attributable to coronary artery disease (CAD) have declined in recent years, CAD remains (as it has for decades) the leading cause of death and disability in the United States and the Western world (1). While randomized controlled trials (RCTs) continue as the gold standard for comparing therapeutic choices, the available data may not be sufficient to support many therapeutic decisions. Additionally, the cost and time constraints of developing RCTs that cover all variables in all populations render their ubiquitous use impossible. Thus, in the field of comparative effectiveness research (CER), nonrandomized data must often be considered.
Comparisons from registry databases, while subject to treatment selection bias, can supplement randomized trials based on specific advantages, such as greater numbers, greater generalizability, and more contemporary data that can be regularly updated. Because of their size, representativeness, and reflection of real-world practice, observational databases are becoming major contributors to CER (2,3).
In the area of cardiovascular disease, two prominent registries, the American College of Cardiology Foundation (ACCF) National Cardiovascular Data Registry (NCDR®) and The Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD), have for several years individually supplied data for several CER outcomes studies (4–9). The National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) awarded a grant to The ACCF in partnership with the STS to study the comparative effectiveness of percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery for treating stable ischemic heart disease (SIHD).
The resulting study, known as ASCERT (American College of Cardiology Foundation-The Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies), compared long-term outcomes of PCI and CABG using these professional society databases as well as the Centers for Medicare and Medicaid Services (CMS) 100% denominator file data (10). To further examine the comparative efficacy of CABG and PCI, we studied the relative costs and cost effectiveness of revascularization strategies using ASCERT data.
METHODS
STUDY SUMMARY
A complete description of the ASCERT study design and methods has been published previously (11). Briefly, ASCERT was a large observational study designed to compare the long-term effectiveness of CABG and PCI to treat CAD over 4 to 5 years. The outcomes used to compare PCI and CABG were death rates; need for additional or repeat procedures; rehospitalization; and presentation of new cardiac disease conditions, such as stroke or myocardial infarction (MI).
Patients were enrolled in ASCERT based on their first eligible revascularization record (index revascularization). Data from 644 sites were available for 1,943,653 patients who underwent nonemergent isolated CABG or PCI between 2004 and 2007. The CMS database was linked to the NCDR CathPCI® and ACSD registries, yielding an analytic population of 189,793 patients, including 86,244 CABG and 103,549 PCI patients (Supplement Figure 1) (11).
ECONOMIC ANALYSIS PLAN AND ASSESSMENT OF COST
The source for determining costs was patient-level resource utilization from the ASCERT study. Direct medical care costs associated with index hospitalizations were derived from resource use captured in the ACCF and STS databases; subsequent hospitalizations and subsequent outpatient procedures were obtained from the CMS dataset. The index and subsequent hospitalizations were assigned a diagnosis-related group (DRG) in accordance with U.S. Medicare diagnostic standards. Costs for each DRG were estimated using average Medicare reimbursement rates obtained from the Medicare Part A data file. Physician costs were estimated by Current Procedural Terminology (CPT) coding of procedures and assigned a cost based on the Medicare fee schedule.
Lifetime costs beyond the observation period and costs not included in the hospitalization were estimated from average Medicare participant per capita expenditure, stratified by age group. Average Medicare participant per capita expenditure was $5,276 in 2004, the base year and the latest date with available data by age. The average costs for patients 65–74 years of age, 75–84, and 85 plus were, $10,778, $16,389, and $25,691, respectively (12). Costs (also for life years and quality-adjusted life years) beyond the first year of follow-up were discounted 3% annually (13). Drug-eluting stent (DES) cost was reduced by 70%, given the DES cost is substantially reduced compared to the study period time.
LIFE EXPECTANCY ESTIMATION
If death occurred during the follow-up period, life years lost were obtained by subtracting the survival times recorded in the ASCERT database from estimated age- and sex-specific life expectancy estimates for patients (14). We assumed that the relationship between observed age- and sex-specific mortality rates calculated from the ASCERT patients and expected corresponding mortality rates from US-Life Table would continue to apply to the still alive ASCERT patients in the future (Supplement-Life Expectancy Estimation).
For patients with nonfatal MI and/or stroke events during follow-up, additional age- and sex-specific life expectancy adjustments were made using Framingham Heart Study (13,14) risk estimations and added to the results obtained in the previous step (Supplement Table 1) (15–17).
EuroSCORE II (European System for Cardiac Operative Risk Evaluation II) (18) risk was calculated to adjust the estimation of life years lost for each treatment group and reflect the uncertainties in long-term mortality. The EuroSCORE II for each patient was estimated based on patient factors, cardiac factors, and operation factors.
QUALITY-ADJUSTED LIFE YEARS
Life years may be limited for comparison across studies and disciplines because a person in perfect health will derive more value for a life year saved than a patient in impaired health. This is addressed by calculating quality-adjusted life years (QALYs). In ASCERT, QALYs were calculated by multiplying survival by utility, a measure of health status scaled from zero (death) to one (perfect health).
QALYs were estimated from published data on utilities and estimates of survival, since utility measures were not available from the ACCF or STS databases. Utility was determined on the basis of whether a patient experienced no nonfatal cardiovascular events or suffered a stroke. The utilities were taken from the Cost-effectiveness Analysis Registry and age-specific utility estimates were applied from population-based studies (19).
ESTIMATION OF COST EFFECTIVENESS
The cost effectiveness of CABG was expressed as the incremental cost-effectiveness ratio (ICER), defined as the additional cost of CABG divided by the life years gained and QALYs gained. Mean costs for each strategy were calculated as well as the mean difference. Direct medical care costs associated with index hospitalizations, subsequent hospitalizations, and total costs were considered in this analysis. Because the distribution of the differences for cost and effectiveness is typically skewed, the distribution of the difference was estimated by bootstrap analysis (17).
To reduce treatment selection bias in this observational study, propensity score bin bootstrapping methods (PSBB) were used to estimate the 95% confidence interval (CI) for the mean differences of cost and effectiveness between the two strategies (10,000 replicates) (20) (Supplements: Propensity Model and included Variables and PSBB Approach). Further, to assure transparency and comparability of the CABG and PCI population, one-to-one matching without replacement was conducted to generate an analytic population, restricted to CABG and PCI patients matching in 3 or more decimals of propensity scores. Baseline characteristics were assessed to ensure the balance of patients for the matched population.
An observational study with a large sample size offers an important source for revealing heterogeneity of different patient characteristics, providing sufficient power to conduct subgroup cost-effectiveness analysis defined by age (≥75 years and <75 years), diabetes status, anginal status (none, stable, or unstable), 2-vessel, or 3 or more vessel disease, and heart failure status. Cost effectiveness was assessed in these subgroups using the same methods as in the overall matched analytic population.
Traditional one-way sensitivity analyses included varying life years gained for the CABG versus PCI group by 10% to 40%. Meanwhile, the impact of unmeasured confounding factors on cost effectiveness has been a crucial uncertainty and was assessed. Probabilistic sensitivity analyses evaluated the impact of simultaneous changes of all the variables involved in the cost and life years gained. Monte Carlo simulation was performed to derive the differences in QALYs and mean cost between the 2 treatment groups (21).
RESULTS
CLINICAL DATA SUMMARY
The study included a total of 189,793 patients, of whom 86,244 patients underwent CABG and 103,549 patients underwent PCI (Table 1). There were significant differences between these groups in both demographic and clinical characteristics prior to adjustment. A matched analytic population (n = 43,084 for each treatment group) was obtained via one-to-one matching in 3 or more decimals of propensity scores. Table 1 shows the comparison of baseline characteristics by treatment group with unadjusted data, adjusted by inverse probability weighted (IPW), and matched analytic population. Age, sex, and most clinical covariates were well balanced between the CABG and PCI groups for both IPW-adjusted data and matched analytic data. Note that number of vessels diseased and urgent status were not balanced by IPW adjusted, but were similar in the matched analytic population.
TABLE 1.
Baseline Characteristics
| Unadjusted | Inverse Probability Weighted Adjusted |
Matched Data |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CABG | PCI | p Value | CABG | PCI | p Value |
CABG | PCI | p Value |
|
| (n = 86,244) |
(n = 103,549) |
(n = 86,244) |
(n = 103,549) |
(n = 43,084) |
(n = 43,084) |
||||
| Age, yr | 73 ± 6 | 75 ± 7 | <0.0001 | 74 ± 9 | 74 ± 8 | 0.49 | 74 ± 6 | 74 ± 6 | 0.62 |
| Male | 69 | 58 | <0.0001 | 62 | 63 | 0.17 | 64 | 64 | 0.69 |
| History of heart failure | 12 | 10 | <0.0001 | 11 | 11 | 0.07 | 11 | 11 | 0.31 |
| History of MI | 25 | 25 | 0.0001 | 25 | 25 | 0.51 | 24 | 24 | 0.89 |
| Diabetes | 39 | 34 | <0.0001 | 36 | 36 | 0.97 | 37 | 37 | 0.63 |
| Insulin RDM | 10.2 | 9.8 | 0.0069 | 9.7 | 9.9 | 0.35 | 10.1 | 10.0 | 0.66 |
| Hypertension | 85 | 83 | <0.0001 | 84 | 84 | 0.58 | 84 | 84 | 0.37 |
| Renal failure | 6.1 | 6.2 | 0.57 | 6.1 | 6.1 | 0.80 | 6.2 | 6.2 | 0.84 |
| Chronic lung disease | 21 | 19 | <0.0001 | 19 | 20 | 0.50 | 20 | 20 | 0.99 |
| Cerebrovascular disease | 18 | 16 | <0.0001 | 17 | 17 | 0.86 | 17 | 17 | 0.93 |
| Peripheral artery disease | 18 | 15 | <0.0001 | 16 | 16 | 0.97 | 16 | 17 | 0.45 |
| BMI | 29 ± 6 | 29 ± 6 | 0.78 | 29 ± 9 | 29 ± 8 | 0.97 | 29 ± 6 | 29 ± 6 | 0.58 |
| Former smoker | 44 | 43 | <0.0001 | 43 | 43 | 0.45 | 43 | 43 | 0.37 |
| Current smoker | 13 | 12 | <0.0001 | 12 | 12 | 0.74 | 12 | 12 | 0.39 |
| No angina | 22 | 31 | <0.0001 | 26 | 27 | 0.23 | 28 | 28 | 0.94 |
| Stable angina | 50 | 23 | <0.0001 | 35 | 35 | 0.46 | 34 | 34 | 0.65 |
| Unstable angina | 28 | 47 | <0.0001 | 39 | 38 | 0.066 | 38 | 38 | 0.71 |
| Ejection fraction | 53 ± 13 | 55 ± 13 | <0.0001 | 54 | 54 | 0.58 | 54 ± 12 | 54 ± 13 | 0.43 |
| Vessels diseased | |||||||||
| 2 | 20 | 68 | <0.0001 | 47 | 46 | 0.043 | 37 | 37 | 0.88 |
| 3 | 80 | 32 | 53 | 54 | 63 | 63 | |||
| Status urgent | 35 | 36 | <0.0001 | 36 | 35 | 0.051 | 36 | 36 | 0.43 |
Values are % or mean ± standard deviation Values are % or median ± standard deviation.
BMI = body mass index; CABG = coronary artery bypass graft; MI = myocardial infarction; PCI = percutaneous coronary intervention; RDM = requiring diabetes mellitus
EFFECTIVENESS: LIFE YEARS LOST, LIFE YEARS GAINED, AND QALYS LOST
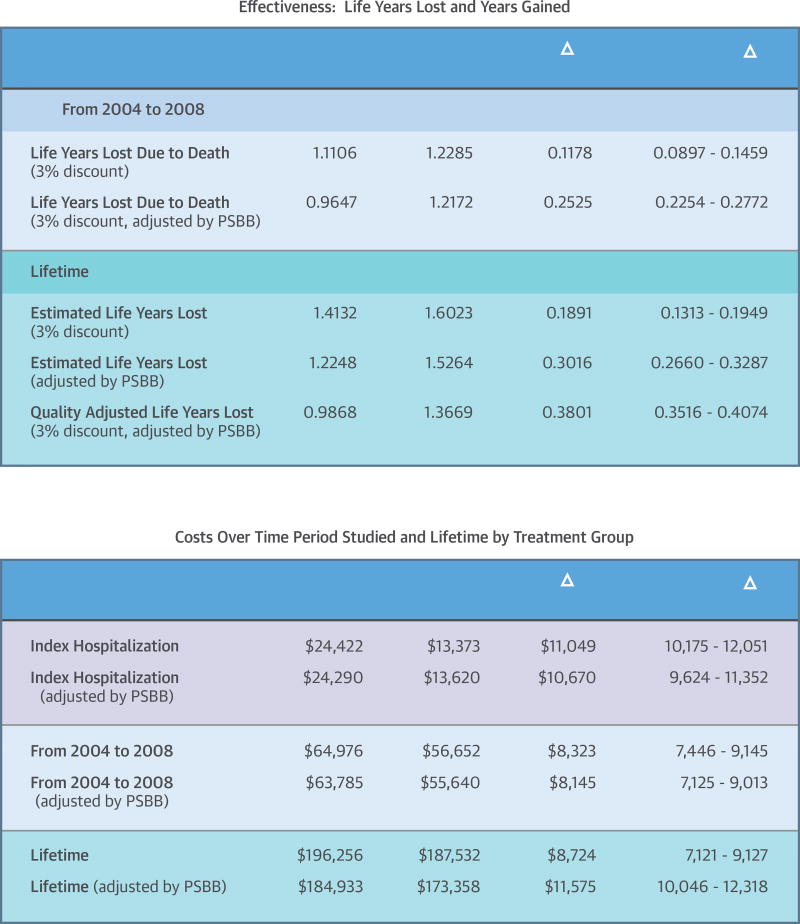
The Central Illustration shows effectiveness: life years lost in the CABG and PCI groups, life years gained with CABG compared to PCI plus PSBB and quality-adjusted survival results over the follow-up period from 2004 through 2008, and estimated life-time results. Over the follow-up period, before adjustment, there was a significant difference in life years lost between the groups (1.1106 and 1.2283 life years lost for the CABG and PCI group, respectively), resulting in 0.1178 life years gained for CABG compared to PCI; the PSBB-adjusted years lost were 0.9647 and 1.2172 in the CABG and PCI groups, respectively, resulting in 0.2525 life years gained with CABG versus PCI.
CENTRAL ILLUSTRATION. Tables Demonstrating Effectiveness And Cost Of Index Over The Study Period.
Upper Panel: Effectiveness: Life Year Lost for each Procedure and Life Year Gained with CABG compared with PCI unadjusted and PSBB adjusted
Lower Panel: Costs of Index, period over 2004 through 2008 and lifetime by treatment group unadjusted and PSBB adjusted. PSBB = propensity score bin bootstrapping; CABG = coronary artery bypass graft.
For lifetime, unadjusted life years lost was 1.4132 and 1.6023 in the CABG and PCI group, respectively; the quality- and PSBB-adjusted years lost were 0.9868 and 1.3669 in the CABG and PCI groups respectively, resulting in 0.3801 PSBB-adjusted QALYs gained for CABG compared to PCI. Similar results were shown for the matched analytic population (Supplement Table 2).
COSTS: 2004 THROUGH 2008 AND LIFETIME
As seen in the Central Illustration, index hospitalization cost remained significantly higher for the CABG group compared to PCI, by $11,049 before and $10,670 after adjustment. The adjusted average total cost over the follow-up period was higher in the CABG group by $8,145 ($63,783 in CABG vs. $55,640 in PCI). The difference in cost during the follow-up period (2004 through 2008) was mainly due to the difference in index hospitalization cost. This trend remained for estimated lifetime cost.
The unadjusted lifetime cost in the CABG group was $8,742 higher than in the PCI group ($196,256 vs. $187,532). The PSBB-adjusted lifetime cost was $184,933 in the CABG group and $173,358 in the PCI group, still significantly higher for CABG by $11,575. A similar trend was seen in the matched analytic population (Supplement Table 3).
COST-EFFECTIVENESS ANALYSIS FOR FOLLOW-UP AND LIFETIME. Table 2
Table 2.
Cost-Effectiveness Analysis: PSBB Adjusted
| Δ CABG - PCI |
Life Years or QALY Gained with CABG |
ICER | % CABG Dominated |
% CABG Dominant |
% <$30,000 /LYG |
% <$50,000 /LYG |
% <$100,000 /LYG |
|
|---|---|---|---|---|---|---|---|---|
| Life years from 2004 through 2008 | $8,323 | 0.1178 | $70,647 | 0 | 0 | 0 | 0 | 99.0 |
| Life years from 2004 through 2008 (3% discount and PSBB adjusted) | $8,088 | 0.3088 | $26,192 | 0 | 0 | 70 | 97.0 | 100.0 |
| Lifetime (3% discount and PSBB adjusted) | $11,575 | 0.3016 | $38,379 | 0 | 0 | 3.0 | 91.0 | 99.0 |
| Quality-adjusted lifetime (3% and PSBB adjusted) | $11,575 | 0.3801 | %30,454 | 0 | 0 | 47.0 | 98.0 | 100.0 |
ICER = incremental cost-effectiveness ratio; LYG = life years gained; other abbreviations as in Tables 1 and 2.
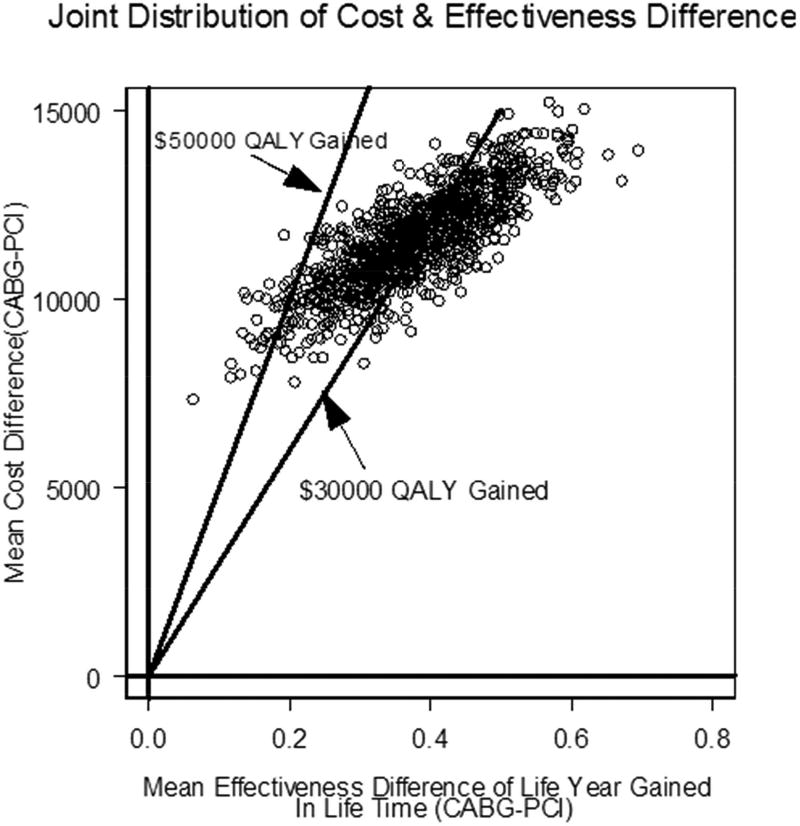
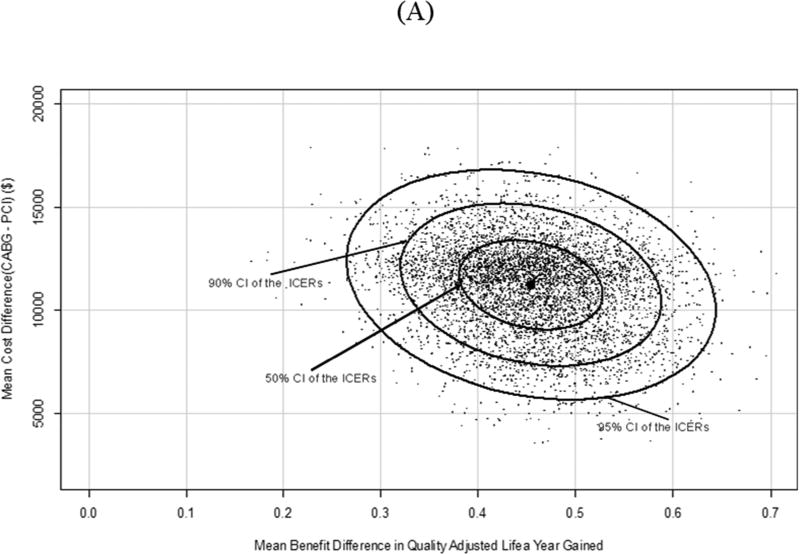
(PSBB-adjusted) and Table 4 (matched analytic populations) show the cost-effectiveness analysis for the follow-up period and lifetime. For lifetime, the PSBB-adjusted ICER of CABG compared to PCI was $38,379 per life year gained (LYG), with 3.0% and 91.0% of observations below $30,000 and $50,000 per LYG, respectively, and 99.0% below $100,000 per LYG; the further quality-adjusted ICER of CABG compared to PCI was $30,454 QALY gained, with 47.0% and 98.0% of observations below $30,000 and $50,000 per QALY gained, respectively, and 100% below $100,000 per QALY gained (Figure 1A).
Table 4.
Cost-Effectiveness Analysis for Matched Analytic Population Subgroups
| Δ CABG - PCI |
Life Years or QALY Gained with CABG |
ICER | % CABG Dominated |
% CABG Dominant |
% <$30,000 /LYG |
% < $50,000 /LYG |
% <$100,000 /LYG |
|
|---|---|---|---|---|---|---|---|---|
| Diabetes | $14,069 | 0.5525 | $25,467 | 0 | 0 | 100.0 | 100.0 | 100.0 |
| No diabetes | $11,075 | 0.3051 | $36,298 | 0 | 0 | 0 | 100.0 | 100.0 |
| Age >75 yr | $12,407 | 0.3863 | $32,118 | 0 | 0 | 1.0 | 100.0 | 100.0 |
| Age ≤75 yr | $11,806 | 0.4046 | $29,182 | 0 | 0 | 70.0 | 100.0 | 100.0 |
| White | $12,021 | 0.3999 | $30,060 | 0 | 0 | 46.0 | 100.0 | 100.0 |
| Non-white | $13,377 | 0.3511 | $38,102 | 0 | 0 | 0 | 95.0 | 100.0 |
| Male | $11,496 | 0.3922 | $29,309 | 0 | 0 | 72.0 | 100.0 | 100.0 |
| Female | $13,432 | 0.3987 | $33,693 | 0 | 0 | 0 | 100.0 | 100.0 |
| No angina | $14,914 | 0.5842 | $25,527 | 0 | 0 | 99.0 | 100.0 | 100.0 |
| Stable angina | $9,600 | 0.2262 | $42,443 | 0 | 0 | 0 | 94.0 | 100.0 |
| Unstable angina | $12,439 | 0.4066 | $30,484 | 0 | 0 | 37.0 | 100.0 | 100.0 |
| 2-vessel disease | $10, 943 | 0.2589 | $42,269 | 0 | 0 | 0 | 95.0 | 100.0 |
| ≥3-vessel disease | $12,883 | 0.4758 | $27,080 | 0 | 0 | 99.0 | 100.0 | 100.0 |
| HF | $20,213 | 0.8216 | $24,602 | 0 | 0 | 100.0 | 100.0 | 100.0 |
| No HF | $11,090 | 0.3376 | $32,848 | 0 | 0 | 0 | 100.0 | 100.0 |
FIGURE 1. Scatterplot of Cost and Effectiveness Differences.
Scatterplot of the joint distribution of cost and effectiveness differences in the cost-effectiveness plane for the lifetime analysis. Results via (A) PSBB adjusted method and (B) the matched analytic population. Abbreviation in Central Illustration.
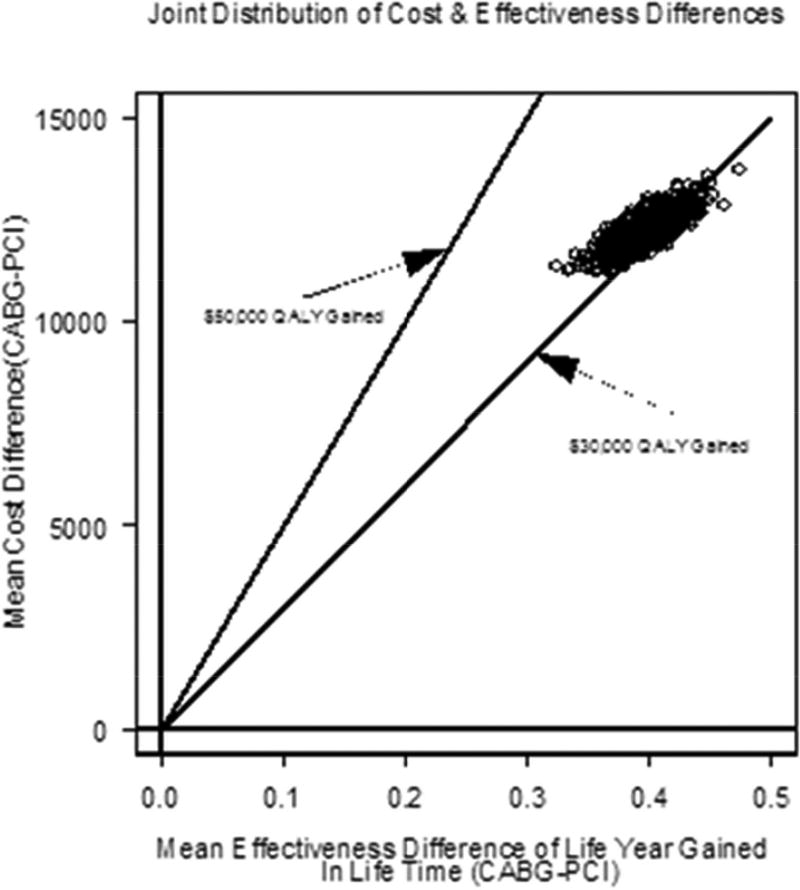
Table 3 shows that the cost-effectiveness results for matched analytic populations were similar to those using the PSBB-adjusted method. For lifetime, the quality-adjusted ICER of CABG compared to PCI was $30,803 QALY gained, with 21.0% and 100.0% of observations below $30,000 and $50,000 per QALY gained, respectively (Figure 1B). Using a common threshold such as $50,000 per QALY gained, results from both the PSBB-adjusted and matched analytic population approaches illustrated that CABG provided moderate-to-high probability of better clinical benefit, which means CABG will often be a cost-effective strategy (Figure 2).
Table 3.
Cost-Effectiveness Analysis: Matched Analytic Population
| Δ CABG PCI |
Life Years or QALY Gained with CABG |
ICER | % CABG Dominated |
% CABG Dominant |
% <$30,000 /LYG |
% <$50,000 /LYG |
% <$100,000 /LYG |
|
|---|---|---|---|---|---|---|---|---|
| Life years from 2004 through 2008 | $8,079 | 0.2674 | $30,217 | 0 | 0 | 45.0 | 100.0 | 100.0 |
| Lifetime (3% discount) | $12,157 | 0.3172 | $38,330 | 0 | 0 | 0 | 100.0 | 100.0 |
| Quality-adjusted lifetime (3% discount) | $12,157 | 0.3947 | $30,803 | 0 | 0 | 21.0 | 100.0 | 100.0 |
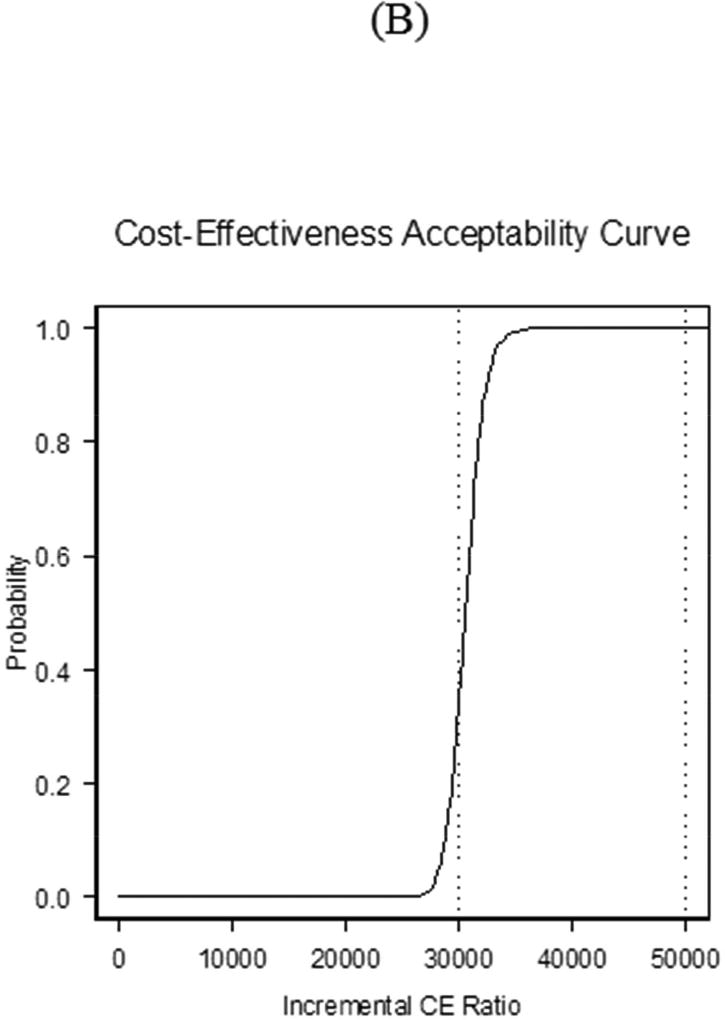
FIGURE 2. Cost-effectiveness Acceptability Curve.
The cost-effectiveness acceptability (CEA) curve for CABG compared to PCI demonstrates that for the lifetime analysis, the matched analytic population curve rises more steeply than that for PSBB adjusted initially, but the two converge over a lifetime. The y axis corresponds to the probability of observations below corresponding incremental cost-effectiveness (CE) ratio. PCI = percutaneous coronary intervention; other abbreviations as in Figure 2 and Central Illustration.
SUBGROUP ANALYSIS
Subgroup lifetime cost-effectiveness analyses were performed for the matched analytic population. Similar results were obtained for all patients via PSBB (not shown). Results in Table 4 demonstrate differences in terms of patient features (Supplement: Subgroup Analysis). For instance, ICERs were $42,443 per QALY and $42,269 per QALY for stable angina and 2-vessel disease patients, respectively, and both with 0% of observation below $30,000 per QALY.
SENSITIVITY ANALYSIS
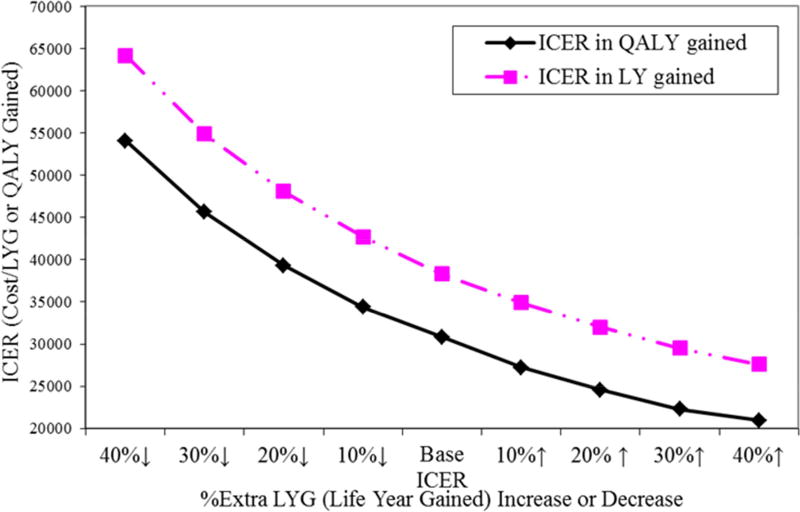
There is uncertainty regarding years of life lost due to early death and the impact of nonfatal MI or stroke on subsequent life expectancy. Thus, for the matched analytic population over a lifetime, if the quality-adjusted benefit of CABG relative to PCI decreased by 10%, 20%, 30%, and 40%, the ICERs would increase from $30,803 to $34,400, $39,330, $45,660, and $54,100 per QALY gained, respectively. In contrast, if the estimated and adjusted QALY gained with CABG relative to PCI increased by 10%, 20%, 30%, and 40%, the ICERs would decrease from $30,803 to $27,240, $24,550, $22,280, and $20,930 per QALY gained, respectively (Figure 3).
FIGURE 3. Sensitivity Analyses.
Additional 10% to 40% increase or decrease of life years gained for CABG compared to PCI for the lifetime for the Matched Analytic Population.
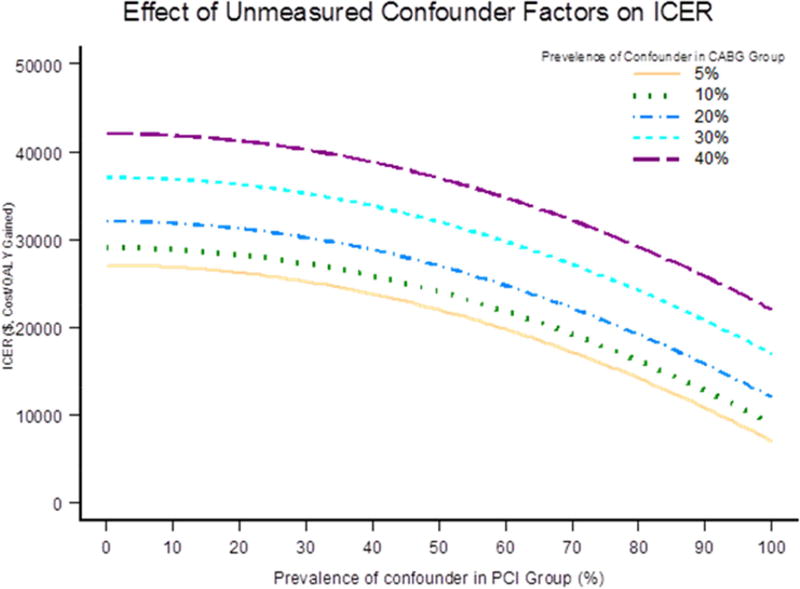
Figure 4 represents the impact of a single unmeasured confounder on the ICER as a combination of cost and effectiveness, to account for the advantage of CABG over PCI detected in the study. From the adjusted survival analysis in ASCERT, if an unmeasured risk factor was present in 10% of the patients in the CABG group and in 20%, 35%, or 50% of the PCI patients, then the hazard ratio that would be required for the observed decreased risk with CABG would be 4.25, 2.09, and 1.65, respectively (11). The unmeasured confounder could also have affected the observed cost difference, ranging from a decrease of 20% to an increase of 10% (23). If the prevalence due to the confounder in PCI was 50%, the ICER of CABG versus PCI would change from $24,000, to $28,000, to $33,000, to $38,000, and $41,000 per QALY gained for the prevalence of the confounder in CABG with 5%, 10%, 20%, 30%, 40%, respectively.
FIGURE 4. Unmeasured Confounder Factor.
The impact of unmeasured confounder factor.
To account for uncertainties regarding years of life lost due to early death, the impact of nonfatal MI or stroke on subsequent life expectancy and the estimation of cost due to differences in the use of resources, a probabilistic sensitivity analysis was conducted to assess the robustness of the estimates for matched analytic population. The results of EuroSCORE II are shown in the Supplement EuroSCORE II. The distributional assumptions of the cost data were based on the actual data in this study and their ranges come from relevant literature, with probabilities of effectiveness (Supplement Table 4) derived from other cardiovascular studies and resources such as the American Heart Association Heart Disease and Stroke Statistical Update (1). Figure 5A presents the contour plot of simulated distribution of mean differences in cost and effectiveness using matched analytic population and quality adjustment, based on the probabilistic sensitivity analysis over a lifetime. The ellipses indicate 50%, 95%, and 99% confidence intervals of the simulated lifetime ICERs. It reveals that the IPW-adjusted lifetime QALYs gained could range from 0.27 to about 0.65 years for the CABG group compared to the PCI group; on the other hand, the cost for the CABG group would always be significantly larger, varying from $5,500 to $17,800, indicating a better clinical benefit for CABG but at increased cost.
FIGURE 5. Probabilistic Sensitivity Analyses.
Plots based on Probabilistic Sensitivity Analyses for the Matched Analytic Population.
The cost-effectiveness acceptability curve (Figure 5B) illustrates that the variation the ICER considered in this sensitivity analysis was greater than noted purely by the play of chance in the base case; specifically, compared with 21.0% of observations below $30,000 per QALY gained, and 100% below $50,000 per QALY gained for the base case, there was a 33% probability of CABG being cost effective at the $30,000 threshold and 100% at the $50,000 threshold. The probability of CABG being dominated by PCI was <1%. Sensitivity trends were similar for all patients adjusted using the PSBB approach.
DISCUSSION
We performed a cost-effectiveness analysis of ASCERT; the main clinical results showed that there was a long-term survival advantage in older patients with nonemergent multivessel CAD who were selected to have CABG rather than PCI. The present economic analysis shows CABG is more expensive than PCI, almost entirely due to the initial procedural costs (Central Illustration). It is reasonable to conclude that, after the study period, resource utilization and costs would largely track in parallel. A long-term survival advantage among patients in the CABG group was converted into QALYs gained (Central Illustration), but the point estimates for the ICERs for potential CABG benefit were below common benchmarks (Table 2).
To address selection bias in this large observational study, analyses were conducted through both PSBB and a matched analytic population. PSBB captured the information from all patients with balance achieved for measured confounders via propensity score adjustment. The one-to-one matched analytic population indeed had better risk factor balance between the CABG and PCI groups, but approximately one-third of the patients were excluded from the analysis. Nonetheless, the similarity of results from PSBB method and matched analytic population enhances the results’ reliability.
While the greatest uncertainty is in the differential life expectancy, these results are robust to variations in relative gain in life expectancy with CABG, both before and after PSBB adjustment, and for the matched analytic population.
It is natural to evaluate our findings in the context of results from other health economic studies. In 2006, on the basis of a Department of Veterans Affairs Cooperative Study randomizing high-risk patients with medically-refractory myocardial ischemia, a cost-effectiveness assessment of CABG (n = 227) versus PCI (n = 218) for high-risk patients showed that PCI was less costly and at least as effective for the urgent revascularization of medically refractory, high-risk patients over 5 years. After 5 years, average total costs were $81,790 for PCI versus $100,522 for CABG patients, a difference of $18,732 (95% CI: $9873 to $27,831), whereas survival at 5 years was 0.75 for PCI patients versus 0.70 for CABG patients (p = 0.21) (23). FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) was a 5-year trial of 1,800 patients with diabetes and multivessel CAD who were randomly assigned to CABG or PCI with DES at 140 centers throughout the world, between 2005 and 2010. Investigators reported that CABG was associated with 0.66 QALYs gained and higher costs of approximately $5,400 per patient (24). Lifetime cost effectiveness of CABG was therefore $8,132 per QALY gained, significantly lower than the commonly used benchmark of $50,000 per QALY gained for considering a treatment to be cost effective (25,26). The SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) trial randomized 1,800 patients with left-main or 3-vessel CAD to CABG (n = 897) or DES-PCI (n = 903) between 2005 and 2007. Over a lifetime horizon, CABG remained more costly than DES-PCI with a favorable ICER of $16,537/ QALY gained (25). Our ASCERT results demonstrated a similar trend, but exhibited some discrepancy in ICER values. The discrepancy may be due to the change of survival rate of CABG versus PCI, such as no difference in survival between groups at 1 year, but significant better benefit in survival in CABG group at 4 years. The differences over a lifetime may reflect diverse assumptions in estimating life expectancy in both arms of the studies.
Using professional society databases, we demonstrated the distinct advantages of linking clinical and administrative databases. Clinical databases are well-suited to risk adjustment and the identification of clinically important subgroups, but lack long-term outcome information. Administrative datasets have limited capacity for clinical considerations, but they do provide long-term information on outcomes as well as cost of care. Linking the clinical data with administrative data capitalizes on the distinct advantages of each dataset to create a powerful analytic tool for observational studies.
STUDY LIMITATIONS
ASCERT is a nonrandomized observational study, and although PSBB/IPW adjustment created CABG and PCI populations demonstrating excellent balance, enabling a more valid comparison, there remains a potential for unmeasured confounders to have influenced the estimation of both clinical outcomes and cost. While our cost-effectiveness analysis sought a societal basis, costs such as DRG-based values were estimated from a payer perspective. It is also not generally possible to account for all costs and for all utilization. Resource use beyond the trial period is based on a model, not on direct measurement. For instance, costs beyond the period of observation were estimated from average Medicare participant expenditures stratified by age.
For patients still alive at the end of the follow-up period, we applied a Monte Carlo simulation method to estimate the life expectancy on the basis of the observed mortality rate and the U.S. Life Tables, (2009), and converted to life year lost for each procedure. Our methods are an estimate both of patients’ health status (utility) and projected survival. We used Framingham data, an external database, to estimate nonfatal events related to life expectancy. The Framingham database, however, may not reflect multiple advances in medical care, specifically in cardiovascular medicine, that have occurred since that database was created. Projection of life expectancy for disease-specific patients and cost beyond the observational period must be based on models developed from the literature. Evaluation of utility, used to quality adjust survival, is also problematic, with utility estimates not directly from the dataset. Although propensity matching balanced the patients, some important covariates that should have been included in the propensity model could have been missed. It is likely that the accurate estimation of difference in life expectancy was limited. Despite these limitations, the results of our sensitivity analyses were robust, suggesting that the results are unlikely to be severely affected as long as there is a survival benefit of CABG in keeping with the ASCERT results.
Finally, there is no scientific basis for a threshold to establish whether a therapy is cost effective. The commonly used threshold of $50,000 is an approximation of societal ‘willingness to pay.’
CONCLUSIONS
This study shows that over a period of 4 years or longer, CABG is associated with better outcomes but at higher cost than PCI among older patients with 2- or 3-vessel CAD. Under the assumption that our analysis has fully accounted for both measured and unmeasured confounding, in patients with stable ischemic heart disease, CABG will often be considered cost effective at thresholds of $30,000 or $50,000/QALY.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Comparison of percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery allow assessment if the effectiveness of the two procedures for treatment of patients with multi-vessel disease. Observational and clinical trial data have demonstrated a survival advantage associated with CABG, but surgery is more expensive, due almost entirely to the initial costs associated with the operation
Translational Outlook
Analyses that link clinical and administrative databases can be applied to other aspects of patient management to enhance insights from observational studies and clinical registries about the relative value of various options and inform shared decision making between patients and providers.
Acknowledgments
Source of Funding: This article is supported by a grant from the National Heart, Lung, and Blood Institute (RC2HL101489) and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (U54-GM104941) of the National Institutes of Health, Bethesda, MD 20892
Abbreviations and Acronyms
- CABG
coronary artery bypass graft
- CPT
current procedural terminology
- DRG
diagnosis-related group
- ICER
incremental cost-effectiveness ratio
- IPW
inverse probability-weighting
- LYL
life-years lost
- PCI
percutaneous coronary intervention
- PSBB
propensity score bin bootstrapping
- QALY
quality-adjusted life year
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs, coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–41. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 3.Smith PK, Califf RM, Tuttle RH, et al. Selection of surgical or percutaneous coronary intervention provides differential longevity benefit. Ann Thorac Surg. 2006;82:1420–28. doi: 10.1016/j.athoracsur.2006.04.044. discussion 1428-29. [DOI] [PubMed] [Google Scholar]
- 4.Brindis RG, Fitzgerald S, Anderson HV, et al. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 5.Kutcher M, Klein LW, Ou FS, et al. Percutaneous Coronary Interventions in Facilities Without Cardiac Surgery On Site: A Report From the National Cardiovascular Data Registry (NCDR) J Am Coll Cardiol. 2009;54:16–24. doi: 10.1016/j.jacc.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Douglas PS, Brennan JM, Anstrom KJ, et al. Clinical effectiveness of coronary stents in elderly persons: results from 262,700 Medicare patients in the American College of Cardiology-National Cardiovascular Data Registry. J Am Coll Cardiol. 2009;53:1629–41. doi: 10.1016/j.jacc.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson TB, Jr, Dziuban SW, Jr, Edwards FH, et al. The STS National Database: current changes and challenges for the new millennium. Committee to Establish a National Database in Cardiothoracic Surgery, The Society of Thoracic Surgeons. Ann Thorac Surg. 2000;69:680–91. doi: 10.1016/s0003-4975(99)01538-6. [DOI] [PubMed] [Google Scholar]
- 8.Shahian D, Edwards FH, Ferraris VA, et al. Quality Measurement in Adult Cardiac Surgery: Part 1 - Conceptual Framework and Measure Selection. Ann Thorac Surg. 2007;83:S3–12. doi: 10.1016/j.athoracsur.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien S, Shahian DM, Delong ER, et al. Quality Measurement in Adult Cardiac Surgery: Part 2 - Statistical Consideraitons in Composite Measure Scoring and Provider Rating. Ann Thorac Surg. 2007;83:S13–26. doi: 10.1016/j.athoracsur.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Klein LW, Edwards FH, DeLong ER, et al. ASCERT: the American College of Cardiology Foundation--the Society of Thoracic Surgeons Collaboration on the comparative effectiveness of revascularization strategies. JACC Cardiovasc Interv. 2010;3:124–6. doi: 10.1016/j.jcin.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services, National Health Statistics Group. Health Expenditures by Age and Gender: Age and Gender Tables. [accessed: October 24, 2014]; http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/2010GenderandAgeTables.pdf. Page last Modified: 05/07/2014. Page.
- 13.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):2550–2583. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub WS, Zhang Z, Mahoney EM, et al. Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation. 2005;111:1106–13. doi: 10.1161/01.CIR.0000157146.86758.BC. [DOI] [PubMed] [Google Scholar]
- 15.Peeters A, Mamun AA, Willekens F, et al. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J. 2002;23:458–66. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes. 2008;1:12–20. doi: 10.1161/CIRCOUTCOMES.108.798462. [DOI] [PubMed] [Google Scholar]
- 17.Caro J, Ishak KJ, Migliaccio-Walle K. Estimating Survival for Cost-Effectiveness Analyses: A Case Study in Atherothrombosis. Value Health. 2004;7:627–35. doi: 10.1111/j.1524-4733.2004.75013.x. [DOI] [PubMed] [Google Scholar]
- 18.Nashef SAM, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 19.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K. The Beaver Dam Health Outcomes study: Initial Catalog of Health-state Quality Factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Gold M, Siegel JE, Russel LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 456. [Google Scholar]
- 22.Handorf EA, Bekelman JE, Heitjan DF, et al. Evaluating Costs with Unmeasured Confounding: A Sensitivity Analysis for the Treatment Effect. Ann Appl Stat. 2013;7:2062–80. doi: 10.1214/13-AOAS665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroupe KT, Morrison DA, Hlatky MA, et al. Cost-effectiveness of coronary artery bypass grafts versus percutaneous coronary intervention for revascularization of high-risk patients. Circulation. 2006;114:1251–7. doi: 10.1161/CIRCULATIONAHA.105.570838. [DOI] [PubMed] [Google Scholar]
- 24.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N Engl J Med. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 25.Cohen DJ, Osnabrugge RL, Magnuson EA, et al. Cost-Effectiveness of Percutaneous Coronary Intervention with Drug-Eluting Stents vs. Bypass Surgery for Patients with 3-Vessel or Left Main Coronary Artery Disease: Final Results from the SYNTAX Trial. Circulation. 2014;130:1146–57. doi: 10.1161/CIRCULATIONAHA.114.009985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.