Abstract
We have developed a UHPLC-MS/MS method for the detection and quantitation of aflatoxins in smokeless tobacco products and used it to determine aflatoxin B1 concentrations in 32 smokeless tobacco products commercially available in the US. Smokeless tobacco products were dried, milled and amended with 13C17-labelled internal standards, extracted in water/methanol solution in the presence of a surfactant, isolated through use of immunoaffinity column chromatography and reconstituted in mobile phase prior to UHPLC-MS/MS analysis. Our method was capable of baseline separation of aflatoxins B1, B2, G1 and G2 in a 2.5 min run by use of a fused core C18 column and a water/methanol gradient. MS/MS transition (m/z) 313.3>241.2 was used for aflatoxin B1 quantitation, with 313.3>285.1 used for confirmation. The limit of detection (LOD) for aflatoxin B1 was 0.007 parts per billion (ppb). Method imprecision for aflatoxin B1 (expressed as coefficient of variation) ranged from 5.5% to 9.4%. Spike recoveries were 105–111%. Aflatoxin B1 concentrations in the smokeless tobacco products analysed ranged from <LOD to 0.271 ppb (dry mass). Aflatoxin B1 was most frequently detected in dry snuffs and chews, whereas all moist snuff products tested were below LOD. The amounts of aflatoxin B1 we detected were low relative to the 20 ppb regulatory limit established by the Food and Drug Administration for foods and feeds.
Keywords: aflatoxins, smokeless tobacco, chewing tobacco, UHPLC-MS/MS, immunoaffinity column chromatography
Introduction
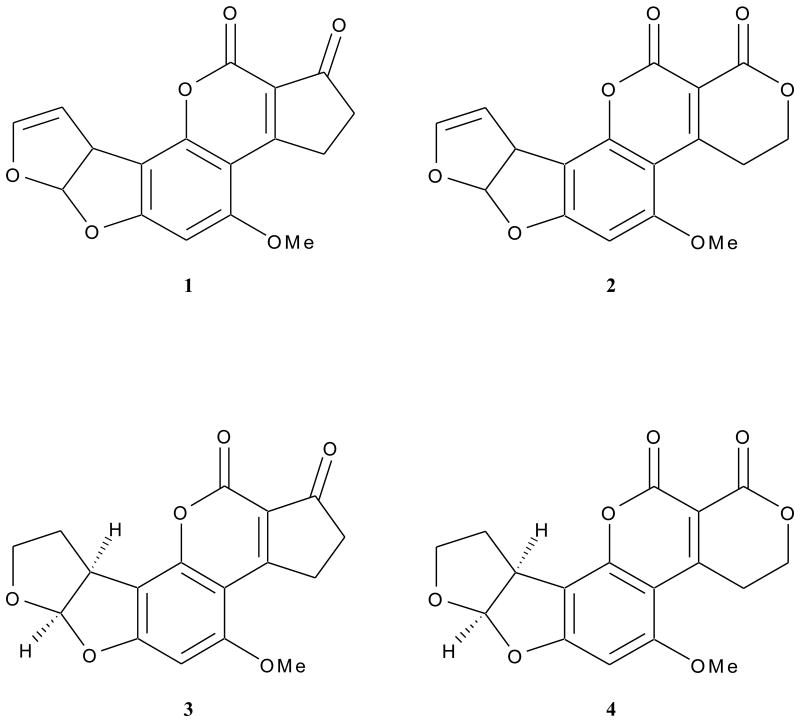
Mycotoxins are secondary metabolites produced by fungi that frequently contaminate agricultural commodities. Aflatoxins, produced by common mold fungi in the genus Aspergillus, are one of the most important groups of mycotoxins (Figure 1). Of the commonly occurring forms of aflatoxins (B1, G1, B2, G2) (1–4), aflatoxin B1 (AFB1) (1) is the most toxic and has been the subject of regulation in foods and feeds in many countries, including the United States.1 AFB1 has been shown to be hepatotoxic, carcinogenic, and teratogenic, as well as causing other less specific symptoms such as weight loss and impaired immune systems.2 AFB1 is typically associated with oil-rich seeds and crops such as nuts and grains, although contamination can occur in a varied range of products due to improper storage conditions.2 In addition to its regulation in foods and feeds, AFB1 is included as a carcinogen on the list of Harmful and Potentially Harmful Constituents (HPHCs) in tobacco products as regulated by the Food and Drug Administration (FDA).3
Figure 1.
Chemical structures of aflatoxins: aflatoxin B1 (AFB1) (1), aflatoxin G1 (AFG1) (2), aflatoxin B2 (AFB2) (3), aflatoxin G2 (4).
Oral and nasal smokeless tobacco encompasses a diverse group of products that includes traditional chewing tobacco (loose leaf, plug, twist), dry snuff, fermented US-type moist snuff, heat-treated moist snuff (snus), and dissolvable tobacco products. Smokeless tobacco products have been identified as containing human carcinogens causing cancer of the oral cavity and pancreas.4,5 Known carcinogens present in these products include N-nitroso compounds such as tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons, heavy metals, aldehydes, and radionuclides.4,6,7 Limited information is available, however, on AFB1 concentrations in tobacco and tobacco products. Aspergillus flavus growth and the production of AFB1 on flue-cured tobacco have been observed8 and Aspergilli have been found in stored leaves of tobacco.9 AFB1 has been reported in tobacco products such as cigarettes10 and chewable tobacco mixtures including other plant material such as betel leaf.11 Exposure to aflatoxins in cigarettes may be mitigated by their potential destruction in the combustion process;12 however, aflatoxins present in a smokeless product are likely to be introduced into the body via ingestion.13
AFB1 has traditionally been detected in commodities by use of HPLC with fluorescence detection,14 with HPLC-MS/MS and UHPLC-MS/MS methods becoming more common.15,16,17 The use of mass spectrometry for AFB1 detection is not new,18 but advances in these technologies have resulted in methods for AFB1 with limits of detection (LOD) in the low parts per trillion (ppt) range.15 In this study, we have developed and validated a method for the determination of AFB1 in smokeless tobacco products and applied it to the analysis of a selection of smokeless tobacco products commercially available in the US. Although our method was optimized for AFB1 detection and quantitation, we have also validated it for detecting and quantifying aflatoxins B2 (AFB2), G1 (AFG1) and G2 (AFG2). To ensure the highest possible degree of specificity and sensitivity towards AFB1 measurement, we employed immunoaffinity chromatography (IAC) to selectively bind AFB1 from product sample extracts, and UHPLC-MS/MS with 13C17-labeled internal standards for detection.
Materials and Methods
Samples, standards and reagents
Reverse osmosis deionized water, HPLC grade solvents, and high-purity reagents were used throughout. Stock solutions of AFB1 (2.0 ng/μL) as well as the internal standard 13C17-AFB1 (0.5 ng/μL) in acetonitrile were purchased (Romer Labs, Union, MO) and stored at -20 °C, from which calibrators were made as needed. Internal standard (100 μL) was diluted into 1900 μL acetonitrile to generate a solution of 12.5 pg/μL. 2S3 Kentucky Reference Moist Snuff (University of Kentucky, College of Agriculture, Lexington, KY) was used as a matrix for blanks, calibrators and quality control (QC) samples. A convenience sample of 32 commercially available smokeless tobacco products was acquired by purchasing products at the retail point-of-sale in the Washington, DC and Atlanta, GA metropolitan areas in 2013. The convenience sample consisted of 12 loose moist snuffs, 4 pouched moist snuffs, 6 dry snuffs, 7 chewing tobaccos, and 3 snus. A total of 17 product brands produced by 8 manufacturers were sampled. Tobacco products purchased in the Washington, DC area were shipped to CDC at room temperature and arrived within 48 h following shipment. Products were inventoried and then stored at -70 °C until further use.
Sample preparation
Tobacco products stored at -70 °C were first transferred to a -20 °C freezer for 1 h, and then allowed to acclimate to room temperature. Once at room temperature, an entire retail package (i.e., tin, pouch, etc.) of each product was weighed to determine their fresh weight. Products were then dried for at least 10 h at 55 °C. After drying, the products were reweighed to obtain a dry weight. The difference between the two values was then used to determine the relative moisture (i.e. oven volatiles) content (%) of the products. The dried products were next milled to a fine powder under liquid nitrogen by use of a model 6770 cryomill (SPEX Sample Prep, Metuchen, NJ). The resulting powder was then thoroughly mixed to assure uniform distribution, and 2-g aliquots (± ≤0.0015 g) were weighed out in 15-mL polypropylene centrifuge tubes (Becton Dickinson, Franklin Lakes, NJ). This process was also performed on the reference smokeless tobacco product used as the matrix for blanks, calibrators, and QC samples. Aliquots of the reference smokeless tobacco product were then amended with 40 μL of the 13C17-AFB1 and AFB1 to generate 10 calibrators (0.005, 0.010, 0.020, 0.050, 0.100, 0.200, 0.500, 1.000, 2.000, and 5.000 ppb), and 3 QC samples (0.07 ppb [‘low’], 0.21 ppb [‘medium’], and 0.7 ppb [‘high’]). Test samples were also amended with 40 μL of the 13C17-AFB1 internal standards. Ten mL of 80:20 methanol-water solution, 0.2 % Tween 20 (Fisher, Fair Lawn, NJ) was then added to the blank, calibrator standards, and samples and they were capped and sealed with parafilm, and extracted for 4 h on a rotary mixer at 45° at 30 rpm. After extraction, the tubes were transferred to a centrifuge equipped with a swing-bucket rotor and centrifuged at 3700 rpm for 10 min to separate the tobacco samples from their respective extraction solutions. The clarified sample extracts were then transferred to new 15-mL polypropylene centrifuge tubes, and stored overnight at 4 °C.
IAC column cleanup
Samples were first allowed to come to room temperature if previously refrigerated. The samples were diluted (1 mL sample in 4 mL of water, 0.2 % Tween 20), loaded onto an Aflatest WB immunoaffinity column (Vicam, Milford, MA) and allowed to elute via gravity. The IAC column was then washed once with 10 mL of methanol:water (20:80, v/v), 0.2% Tween 20, followed by 10mL of methanol:water (20:80, v/v). Analytes were finally eluted with 1.0 mL acetonitrile, into 13 × 100 mm level 2 borosilicate silanized glass collection tubes (Kimble Chase, Vineland, NJ). The collection tubes were then transferred to a Savant SpeedVac Plus SC210A rotary vacuum concentrator (Thermo Fisher, Asheville, NC) and evaporated to dryness under gentle conditions (drying rate set to low). The samples were next reconstituted with 400 μL of methanol:water (60:40, v/v), and transferred to 2 mL autosampler vials with a PTFE/silicone septa for UHPLC-MS/MS analysis.
UHPLC-MS/MS analysis
Our UHPLC-MS/MS system consisted of an Acquity I-Class Binary Solvent Manager, Acquity I-Class Sample Manager, and Acquity I-Class Column Compartment coupled to a Waters Xevo TQ-S tandem quadrupole mass spectrometer (Waters, Milford MA). The UHPLC-MS/MS was controlled by Acquity UPLC Console Software 1.51.3347 and MassLynx Software 4.1 (Waters). Analytes of interest were separated using a 100 × 3 mm, 2.7 μm particle Supelco Ascentis Express C18 column (Supelco Analytical, Bellefonte, PA, USA) with a 0.5 μm × 0.004 in. ID KrudKatcher Ultra HPLC in-line filter (Phenomenex, Torrance, CA). A gradient consisting of water with 0.1 mM ammonium formate (solvent A) and 99:1 methanol-water with 0.1 mM ammonium formate (solvent B) at a constant flow rate of 1.0 mL per min was used. The solvent gradient program was as follows: 0 min, 40% B; 1.0 min, 55% B; 1.25 min, 100% B; 1.75 min, 100% B; 2.0 min, 40% B; 2.5 min, 40% B. Injection volume used was 10 μL. Column temperature was maintained at 70 °C. MS/MS transitions (m/z) for AFB1 were as follows: 313.32>241.22 (quantitation); 313.32>285.08 (confirmation); 330.37>301.09 (13C17-labeled internal standard). Global mass spectrometer settings were as follows: capillary voltage, 3.5 kV; cone voltage, 75 V; source offset voltage, 50 V; source temperature, 150 °C; desolvation temperature, 650 °C; cone gas, nitrogen (600 L/h); desolvation gas, nitrogen (600 L/h); collision gas, argon (0.20 mL/min); nebulizer gas, nitrogen (6.00 bar). Analytes were quantified by interpolation of peak area ratios of the analyte over its 13C17-labeled internal standard for MS/MS transitions against a ten-point calibration curve (1/x weighting).
Validation
QC samples were prepared at three distinct concentrations levels across the calibration range. QC samples were generated for each run via direct spiking of blank reference tobacco. All QC samples were run at the beginning and end of each analysis batch. Data from the first 20 independent runs (days) were used to establish the QC limits as part of a multi-rule procedure for determining whether instrument runs were in or out of control.19 QC samples were also used for determining the recovery of the method, extraction efficiency, and other validation tests as indicated. The within-run and between-run coefficients of variation from the 20-d duplicate analysis of the three QC samples were also used to assess within-run and between run imprecision, respectively.
Method robustness was tested by varying the solvent extraction conditions from the proposed method to different percentages of water and methanol (30:70 and 10:90, v/v) as well as varying the mass of tobacco extracted from the method 2 g to 1 g or 3 g. The robustness of the IAC step was also tested by varying the volume of sample extract loaded on the IAC column, the volume of solvent used to elute the analytes as well as substitution of the IAC column specified in the method with similar IAC column products. In all cases 7 replicates of the test condition were performed on a single day.
LODs were determined by performing repeat measurements in samples spiked with concentrations near the LOD. Plotting the standard deviation of these samples as a function of concentration, an estimate of the standard deviation at a concentration of zero (σ0) was obtained by extrapolation, and the LOD was calculated as 3σ0.20
Product analysis
A minimum of 7 replicate measurements were made on each commercial product tested. Each replicate consisted of an independent sample that was prepared and run on a different day. QC samples and calibrators were prepared and analyzed in duplicate, bracketing the product samples in the analysis sequence. Calibrations were determined as the average from these duplicate injections. Run performance was determined by comparing QC values from each run versus acceptance rules determined from QC limits.19
Results and Discussion
Method development and optimization
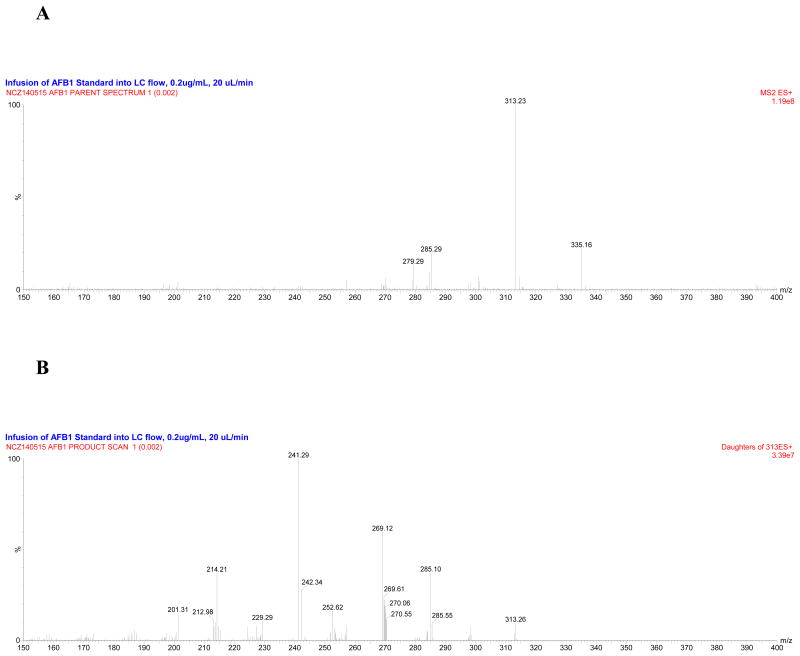
We infused a standard of AFB1 and were able to generate a mass spectrum dominated by a [M + H]+ molecular ion of m/z 313.2, with evidence of a [M + Na]+ adduct at m/z 335.16. Similarly, we found a [M + H]+ molecular ion of m/z 330.21 and a [M + Na]+ adduct at m/z 352.19 when infusing the 13C17-AFB1 internal standard (Figure 2). Using the [M + H]+ molecular ion as our precursor ion for both the standard and internal standard, we observed predominant MS/MS fragment product ions at m/z 241.29, 269.12, and 285.10 for AFB1, and m/z 255.32, 284.50, and 301.15 for 13C17-AFB1. After optimizing the MS/MS parameters for each of the fragments, we selected m/z 313.32>241.22 for AFB1 quantitation, 313.32>285.08 for AFB1 confirmation, and 330.37>301.09 for internal standardization with 13C17-AFB1. These transitions were selected because they represented the best available choices in terms of signal intensity, signal-to-noise ratio, and blank baseline in the chromatographic method, and the product ions did not appear to be the product of non-specific losses (e.g., H2O, NH3). Our selection of the m/z 313.32>241.22 transition for AFB1 quantitation is consistent with its use in other LC-MS/MS methods.16,21,22 Using an analogous approach we also identified quantitation and confirmation MS/MS transitions for AFB2, G1, G2, as well as internal standardization MS/MS transitions for their respective 13C17-labeled analogues.
Figure 2.
(A) Precursor and (B) product ion scans for AFB1 (1).
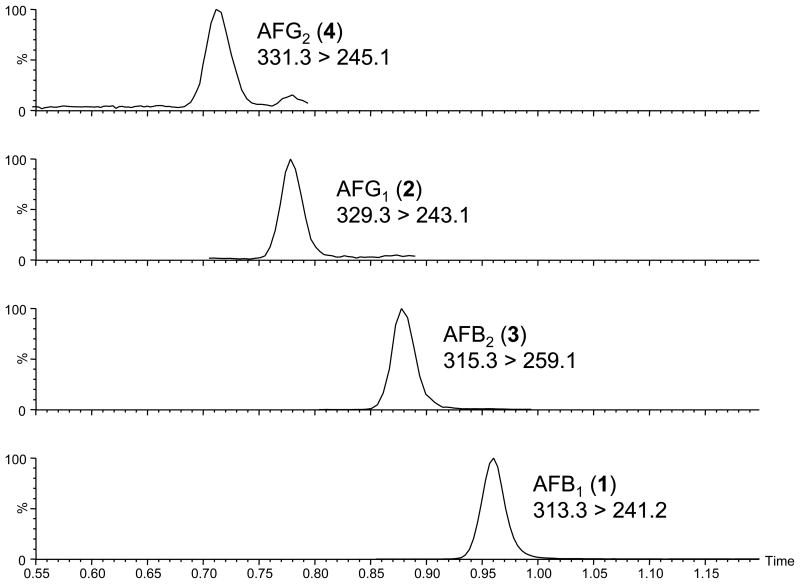
Chromatographic separations based on C18 stationary phases have frequently been used in LC-MS/MS methods for aflatoxins.16,23 After testing a sampling of C18 columns as well as other stationary phases we decided upon a fused-core C18 column for our method. Using this column and a methanol-water gradient with 0.1 M ammonium formate, we were able to develop a separation that resolved AFB1, AFB2, AFG1 and AFG2 in less than 1 min (Figure 3) with performance optimized for AFB1 quantitation and confirmation (Figure 4). Using our QC sample data, we found that the average retention time for AFB1 was 0.94 min and varied by <2% CV over 15 d. Our selection of a fused-core particle stationary phase over traditional porous particles proved to be advantageous in that it allowed us to perform the chromatographic separation at a relatively high flow rate (1 mL/min) with very high peak resolution while still operating at the lower end of LC pressures (∼450 bar). This relatively low operating pressure permits the possible adaptation of our method beyond UHPLC systems to mid-pressure HPLC systems capable of operating pressures of up to 600 bar.
Figure 3.
MS/MS chromatograms of aflatoxins (1–4).
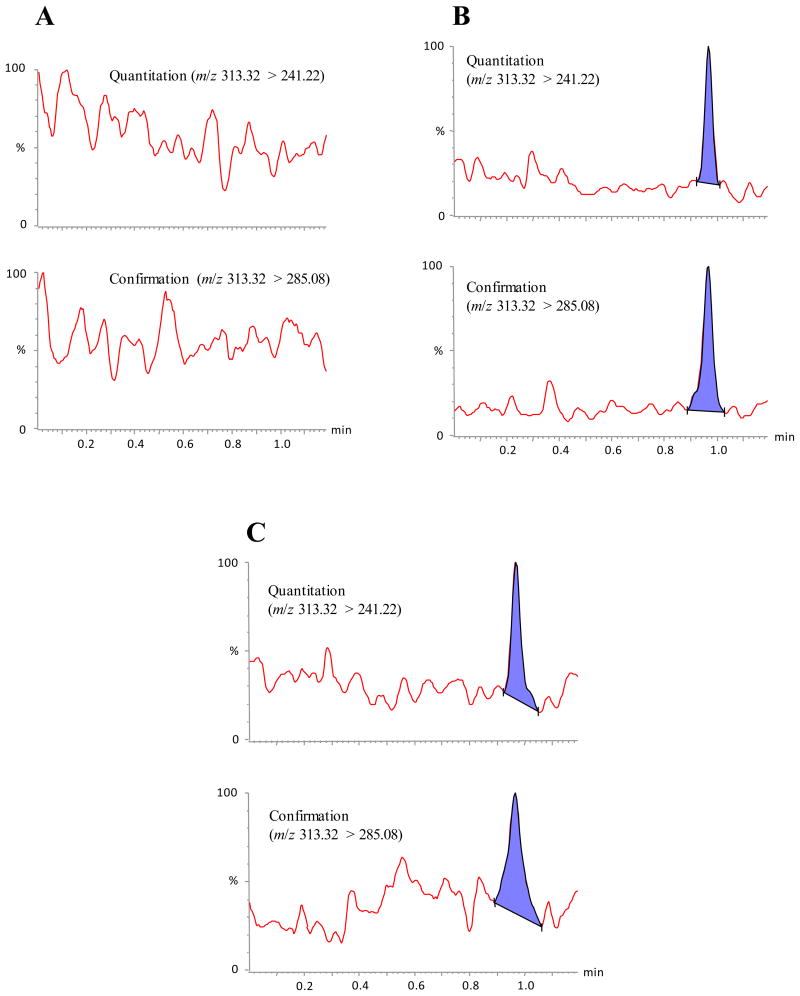
Figure 4.
Sample MS/MS AFB1 (1) chromatograms. (A) reference tobacco blank (2S3 Kentucky Reference Moist Snuff); (B) low QC sample (2S3 Kentucky Reference Moist Snuff spiked with 0.07 ppb AFB1); (C) commercial smokeless tobacco product (endogenous concentration of 0.007 ppb AFB1).
Since smokeless tobacco products can occur in a variety of heterogeneous forms, we dried and cryogenically milled all products prior to analysis in order to generate homogenous, powdered samples that were of similar consistency across all product types. The drying step also enabled us to determine the moisture content (i.e. oven volatiles) of the products as part of the sample preparation process as opposed to performing a separate moisture determination. Our drying conditions for the smokeless tobacco products (55 °C, >10 h) differed from those typically used for determining moisture content (100 °C, 3 h).24,25 We evaluated our drying step for possible aflatoxin losses by calculating analyte recoveries based on the addition of the internal standard both before and after the drying step. We observed quantitative recovery in replicate analyses (n = 5) of a dry snuff product with ∼0.3 ppb of endogenous AFB1 present, as well as samples of 2S3 Kentucky Reference Moist Snuff to which a net concentration of 0.7 ppb of AFB1 was added. Although our testing of a selection of smokeless tobacco products dried at 100 °C did not show any noticeable aflatoxin losses, we decided to dry products at 55 °C as a more conservative approach. We also observed no differences in the moisture content determinations of several smokeless tobacco products performed under both drying conditions.
We used a methanol-water extraction followed by IAC column cleanup to isolate aflatoxins from smokeless tobacco samples. Our extraction methodology is similar to that developed for analyses of aflatoxins from corn, peanuts, and peanut butter (AOAC Method 991.31).26 In optimizing the extraction step we found that acetonitrile-water mixtures14 were as effective as methanol-water mixtures in terms of analyte recovery; however we decided to use a methanol-water extraction as it was consistent with the IAC column manufacturer recommendations as well as other published methods.26 The use of IAC, or a comparable post-extraction cleanup technique such as SPE, was necessary in order to analyze the tobacco extracts for AFB1. Attempts that we made to perform direct analyses on the tobacco extracts without a post-extraction cleanup step resulted in complete suppression of all analyte and internal standard signals. We also found that the use of a surfactant was essential to the IAC process in order to mitigate nonspecific binding. When we omitted the surfactant from the IAC process we frequently encountered discoloration and clogging of the IAC column bed. In addition to IAC, SPE has been used for post-column cleanup of aflatoxin extracts.15,27 We tested an SPE substrate used for determining aflatoxins, but in our application we were unable to attain SPE recoveries as high as those we observed with IAC (∼70% recovery for SPE vs. ∼100% for the IAC). In light of the apparently better analyte recovery, and the specificity and selectivity inherent to the immunoaffinity process, we decided to use IAC over SPE in our method.
Through the course of method development, we noted steps in the sample preparation process that were particularly susceptible to analyte losses with the potential to greatly impact analyte recovery and overall method performance. First, we observed that aflatoxins were susceptible to apparent binding during the dry-down and reconstitution step if untreated borosilicate glass was used vs. silanized glass. When using untreated borosilicate glass tubes, we found that analyte recoveries were ∼5% lower in acetonitrile samples and ∼70% lower in methanol samples than when silanized glass was used with either solvent type. Consequentially, we used acetonitrile and silanized glass for all dry-down steps. Second, we observed that prolonged exposure to air during the dry down step resulted in analyte losses, presumably to oxidation. In dried samples that were exposed to air, we found that ∼95% of aflatoxins were lost after 8 h, with complete loss occurring after 72 h. By comparison, we observed no analyte losses in dried samples that were purged with nitrogen and capped for the same timeframe. For this reason, care had to be taken to perform the dry down step under gentle conditions and promptly reconstitute the dried samples to minimize analyte losses due to oxidation.
Method validation
We prepared QC samples for each run at three levels (‘low’, ‘medium’, and ‘high’) in order to track instrument run control as well as to perform certain aspects of method validation. The QC pools were assigned target values and uncertainty limits by duplicate analysis over 20 d. These data were also used to determine method imprecision for AFB1 (Table 1). Between run imprecision for AFB1 was 4.5–8.4% and within run imprecision was 4.4–6.0%, depending on the QC level. Overall imprecision of the method was 5.5–9.4%. Recovery of the target amount in our QC samples was generally within 10% of being quantitative (100%), ranging from 105–111%.
Table 1. Analytical Performance.
| MS/MS transition (m/z) | LOD (ppb)a | QC Samples | Characterization mean and standard deviation (SD)b | Recovery of target concentration (%)b | |||
|---|---|---|---|---|---|---|---|
| Level | Target (ppb) | Mean (ppb) | SD (ppb) | CV (%) | |||
| Quantitation: 313.32 > 241.22 | 0.007 | Low | 0.07 | 0.078 | 0.007 | 9.4 | 111 |
| Medium | 0.21 | 0.220 | 0.014 | 6.5 | 105 | ||
| High | 0.70 | 0.738 | 0.041 | 5.5 | 105 | ||
| Confirmation: 313.32 > 285.08 | 0.009 | Low | 0.07 | 0.079 | 0.009 | 10.9 | 113 |
| Medium | 0.21 | 0.220 | 0.014 | 6.2 | 105 | ||
| High | 0.70 | 0.730 | 0.040 | 5.5 | 104 | ||
Measured over 15 d (1 replicate/d).
Measured over 20 d (2 replicates/d).
The LOD of our method is estimated to be 0.007 ppb for AFB1. Although direct comparison of LODs among different methods is not straightforward because of differences and/or missing details in how LODs were calculated, we believe that our detection limit of 0.007 ppb for AFB1 is among the lowest of comparable LC-MS/MS methods. For instance, Huang et al.15 demonstrated a LOD of 0.009 ppb for AFB1 in peanuts by LC-MS/MS. An LOD in the low ppt range is beneficial, as our method can be used not only for potential regulatory level investigation work which is typically in the ppb range but also for characterization of products at much lower AFB1 concentrations.
We examined the confirmation ratio, i.e., the peak area of the confirmation MS/MS transition divided by the area of the quantitation MS/MS transition, for AFB1 across all calibrators and QC samples during the 20-d characterization period. The confirmation ratio was 1.03–1.04 with a CV of <10% at concentrations ≥0.07 ppb (the target concentration of the low QC). At concentrations of <0.07 ppb the CV of the ratio gradually increased to ∼20% at 0.02 ppb, and ∼30% at 0.01 ppb.
We investigated method robustness by identifying 5 critical method parameters and evaluating the effect of intentionally varying these parameters on QC sample results. The variables we investigated were the volume of sample extract loaded onto the IAC, elution volume from the IAC, extraction solvent composition, mass of tobacco extracted, and IAC type. We performed seven replicates of each test condition on a single day and compared these results with those obtained from the 20-d characterization. When we increased the volume of sample extract loaded on the IAC column from 1 mL to 2 mL, the accuracy and precision of the AFB1 and AFB2 results appeared to be unaffected; however, AFG1 and AFG2 recovery in the low pool appeared to be slightly lower. We found that decreasing the volume of extract loaded on the IAC column to 0.5 mL resulted in apparent higher imprecision for all aflatoxins and in some cases a spurious effect on recovery of the pool target value was observed. Changing the extraction composition or the IAC products did not appear to have an appreciable effect on the accuracy or precision of the QC analyses. We observed that reducing the amount of tobacco in the extraction from 2 g to 1 g did not seem to affect AFB1 but resulted in lower recoveries for AFG2. Increasing the amount of tobacco to 3 g frequently resulted in the extraction tubes cracking during the centrifugation step. Altering the volume of elution solvent used, as well as substituting different IAC products did not appear to have much effect on the QC values.
Product analyses
We first determined the moisture content of the loose moist snuff, pouched moist snuff, dry snuff, snus, and chewing tobacco products selected for our analyses. We observed that dry snuffs were generally <5% moisture by weight, chews were ∼20% moisture, snus were ∼30% moisture, and moist snuffs were ∼50% moisture (Table 2). Our moisture determinations were generally consistent with the expected values for these product classes reported in the literature.5,28
Table 2. Aflatoxin B1 in Commercially Available Smokeless Tobacco Products.
| Category | ID | Moisture content (%) | Aflatoxin B1 (AFB1) analysis | ||
|---|---|---|---|---|---|
| Replicates (n) | AFB1 concentration ± SD (ppb)a | CV (%) | |||
| Chewb | Chew 1a | 21.3 | 7 | 0.029 ± 0.002 | 6 |
| Chew 1b | 23.6 | 7 | 0.013 ± 0.002 | 16 | |
| Chew 2a | 20.7 | 7 | 0.015 ± 0.003 | 17 | |
| Chew 2b | 23.2 | 9 | 0.016 ± 0.002 | 10 | |
| Chew 3 | 22.0 | 9 | <LOD | N/A | |
| Chew 4 | 22.1 | 9 | <LOD | N/A | |
| Chew 5 | 21.3 | 8 | <LOD | N/A | |
| Dry snuff | Dry snuff 1 | 2.0 | 9 | 0.032 ± 0.009 | 27 |
| Dry snuff 2 | 5.1 | 8 | 0.050 ±0.006 | 11 | |
| Dry snuff 3 | 4.4 | 8 | 0.026 ±0.007 | 27 | |
| Dry snuff 4 | 4.0 | 9 | 0.271 ±0.051 | 19 | |
| Dry snuff 5 | 3.7 | 9 | 0.013 ±0.005 | 38 | |
| Dry snuff 6 | 4.6 | 9 | 0.153 ±0.024 | 16 | |
| Moist snuff (loose) | All products tested (n= 12) | 51–56 | 7–8 | <LOD | N/A |
| Moist snuff (pouched) | All products tested (n=4) | 46–54 | 7–8 | <LOD | N/A |
| Snus | All products tested (n=4) | 26–32 | 8 | <LOD | N/A |
Concentration is based on dry mass of product.
Chew 1a and Chew 1b were different lots from the same brand. Chew 2a and Chew 2b were different lots from the same brand.
Using our LC-MS/MS method, we then analyzed samples of the selected smokeless tobacco products for AFB1 and reported these data as the mean concentration found in the dry product (n = 7–9, depending on the product) (Table 2). We observed that AFB1 concentrations were less than our method LOD of 0.007 ppb in all of the loose moist snuff, pouched moist snuff and snus products we tested. All of the dry snuff products and a majority of the chew products (5 of 7) we tested had detectable concentrations of AFB1. The dry snuffs had the highest AFB1 concentrations among all product types we tested, with the highest mean AFB1 concentration among the dry snuffs tested being 0.271 ± 0.051 ppb. By comparison, the highest concentration of AFB1 we observed among the chew products tested was approximately an order of magnitude lower (0.029 ± 0.002 ppb). For two chew products we tested different lot numbers of the same product to gain insight into within-product variability (Table 2). We observed a high degree of variability among the two lots tested for one of the chew products (Chew 1: 0.029 ± 0.002 ppb vs. 0.013 ± 0.002 ppb), whereas the other product (Chew 2) showed much closer agreement among tested lots (0.015 ± 0.003 ppb vs 0.016 ± 0.002 ppb).
Overall, the concentrations of AFB1 we found in the smokeless tobacco products we surveyed were much lower than the 20 ppb regulatory limit typical for foods and feeds.1 We did not detect AFB2, AFG1, or AFG2 in any of the smokeless tobacco products we tested. AFB1 concentrations tend to be higher than other aflatoxins when contamination is present, and given the AFB1 concentrations we detected were already in the ppt range, it is not surprising that we did not detect any other aflatoxins. Furthermore, the LODs for AFB2 (0.01 ppb), AFG1 (0.02 ppb), and AFG2 (0.1 ppb) with our method were higher than that of AFB1 (0.007 ppb).
Interpretation of our findings is not straightforward. First, although we were able to confirm the presence of AFB1 in several smokeless tobacco products, we have no way to identify the source of the AFB1 contamination. While AFB1 contamination can occur directly in tobacco leaves,8,9 it is also possible that AFB1 contamination found in a smokeless tobacco product may result from an additive. For example, heavy casings, such as molasses, licorice and fruit extracts that are added during processing are potential sources of AFB1 contamination. The amounts of these additives can be significant, such as in chewing tobacco where added sugar content is typically 35%.5 Conversely, the absence of AFB1 in many of the smokeless tobacco products we tested may also be due to additives introduced during processing, such as the addition of sodium propionate as a fungicide. Additives raising the pH of smokeless tobacco products as a means of increasing nicotine potency may also affect AFB1 concentrations.5 Second, we noted with interest that the smokeless tobacco products with the lowest moisture content (dry snuffs) had the highest AFB1 concentrations, whereas we were unable to detect AFB1 in any of the products with the highest moisture content (moist snuff). This seems counter-intuitive, as from a simplistic standpoint we would expect moisture encountered in storage to contribute to higher aflatoxin concentrations, and suggests to us that the mechanism by which AFB1 contamination is introduced into smokeless tobacco products is much more complex. Finally, the potential health effects of AFB1 in a smokeless tobacco depend not only upon the amount of AFB1 present, but also the different ways in which a smokeless tobacco product can be used. This may be particularly important in the case of dry snuffs, as not only did we observe the highest AFB1 concentrations in these products, but it is also the only class of smokeless tobacco product we tested that is used both orally and nasally. Even among smokeless tobacco products that are strictly used orally, product class and cultural practices can determine whether a product is used for minutes vs. hours, and consequentially influence potential AFB1 exposure.5
We believe that our work has two key strengths: we believe it to be the first published report of an LC-MS/MS method specifically designed for determining aflatoxin concentrations in tobacco products; and that the data we presented is also the first published survey of AFB1 concentrations in a subset of smokeless tobacco products commercially available in the U.S. We do acknowledge, however, that there are limitations to our work that warrant further investigation. While we were able to apply our method to a variety of smokeless tobacco products (moist and dry snuffs, snus, chews), there exist other product classes that we did not test (e.g., dissolvables, “sticks”) and these products may not be amenable to our procedure without modification and additional validation. We were able to identify a reference smokeless tobacco product (2S3 Kentucky Reference Moist Snuff) that was free of detectable aflatoxin and successfully used it as a matrix for blanks, calibrators, and QC samples. The availability, however, of a reference material with a detectable endogenous concentration of AFB1 would be beneficial as it could potentially be used as a QC material without the need for analyte spiking and could possibly be developed as a positive AFB1 reference material. We were successful in using the IAC process to isolate AFB1 from the complex sample extracts with a great degree of specificity; however, this process was performed manually in our study and was time-consuming. Automating the IAC process as well as other steps in the sample extraction process should be investigated in the future as a means of improving sample throughput.
Supplementary Material
Supplemental Table 1. UHPLC-MS/MS Analysis Parameters for AFB2, AFG1, and AFG2
Supplemental Table 2. Analytical Performance for AFB1, AFB2, AFG1, and AFG2
Supplemental Table 3. Robustness Testing: Volume of Sample Extract Loaded on IAC Column
Supplemental Table 4. Robustness Testing: Extraction Composition
Supplemental Table 5. Robustness Testing: Mass of Tobacco Extracted
Supplemental Table 6. Robustness Testing: Volume of Elution Solvent Used in IAC Step
Supplemental Table 7. Robustness Testing: Substituting IAC Products
Acknowledgments
We thank Stephen Stanfill, Dr. Clifford Watson and Tameka Lawler, of the Centers for Disease Control and Prevention, National Center for Environmental Health, and Jeannie Jeong-Im of the Food and Drug Administration, Center for Tobacco Products, for their collegial input on this project.
Funding: This study was funded by the Food and Drug Administration, Center for Tobacco Products.
Abbreviations used
- AFB1
aflatoxin B1
- AFB2
aflatoxin B2
- AFG1
aflatoxin G1
- AFG2
aflatoxin G2
- CV
coefficient of variation
- HPHCs
Harmful and Potentially Harmful Constituents
- IAC
immunoaffinity chromatography
- LC
liquid chromatography
- SPE
solid phase extraction
Footnotes
Notes: The authors declare no competing financial interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the CDC/Agency for Toxic Substances and Disease Registry, the FDA, or the Department of Health and Human Services.
Supporting Information: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.USDHHS. Chemical Contaminants, Metals, Natural Toxins & Pesticides > Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed. U.S Food and Drug Administration; 2000. [Accessed: 8 September 2015]. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/ucm077969.htm. [Google Scholar]
- 2.Mycotoxins: Risks in Plant, Animal and Human Systems Task Force Report No 139. Council for Agricultural Science and Technology (CAST); Ames, IA, USA: 2003. [Google Scholar]
- 3.USDHHS. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. U.S. Food and Drug Administration; 2012. [Accessed: 8 September 2015]. http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf. [Google Scholar]
- 4.Rodu B, Jansson C. Smokeless tobacco and oral cancer: A review of the risks determinants. Crit Rev Oral Biol Med. 2004;15:252–263. doi: 10.1177/154411130401500502. [DOI] [PubMed] [Google Scholar]
- 5.IARC working group on the evaluation of carcinogenic risks to humans. Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2007;89:1–592. [PMC free article] [PubMed] [Google Scholar]
- 6.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10:773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products. Am J Prev Med. 2007;33:368–378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Pattee HE. Production of aflatoxins by Aspergillus flavus cultured on flue-cured tobacco. Appl Microbiol. 1969;18:952–953. doi: 10.1128/am.18.5.952-953.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma SK, Verma RA, Jha AK. Ecotoxicological aspects of Aspergilli present in the phylloplane of stored leaves of chewing tobacco (Nicotiana tobaccum) Mycopathologia. 1991;113:19–23. doi: 10.1007/BF00436380. [DOI] [PubMed] [Google Scholar]
- 10.Edinboro LE, Karnes HT. Determination of aflatoxin B1 in sidestream cigarette smoke by immunoaffinity column extraction coupled with liquid chromatography/mass spectrometry. J Chromatogr A. 2005;1083:127–132. doi: 10.1016/j.chroma.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Verma RJ, Kolhe AS, Dube HC. Aflatoxin contamination in chewing products. Proc Natl Acad Sci, India, Sect B. 1995;65:167–170. [Google Scholar]
- 12.Tso TC, Sorokin T. Examination of aflatoxin B1 in leaf tobacco and in cigarette smoke condensate. Beitr Tabakforsch. 1967;4:18–20. [Google Scholar]
- 13.Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci. 2011;120:S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trucksess MW, Weaver CM, Oles CJ, Fry FS, Jr, Noonan GO. Determination of aflatoxins B1, B2, G1, and G2 and ochratoxin A in ginseng and ginger by multitoxin immunoaffinity column cleanup and liquid chromatographic quantitation: collaborative study. J AOAC Int. 2008;91:511–523. [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B, Han Z, Cai Z, Wu Y, Ren Y. Simultaneous determination of aflatoxins B1, B2, G1, G2, M1, and M2 in peanuts and their derivative products by ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2010;662:62–68. doi: 10.1016/j.aca.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Herrman TJ, Dai SY. Determination of aflatoxins in animal feeds by liquid chromatography/tandem mass spectrometry with isotope dilution. Rapid Commun Mass Spectrom. 2011;25:1222–1230. doi: 10.1002/rcm.4979. [DOI] [PubMed] [Google Scholar]
- 17.Cervino C, Asam S, Knopp D, Rychlik M, Niessner R. Use of isotope-labeled aflatoxins for LC-MS/MS stable isotope dilution analysis of foods. J Agric Food Chem. 2008;56:1873–1879. doi: 10.1021/jf073231z. [DOI] [PubMed] [Google Scholar]
- 18.Plattner RD, Bennett GA, Stubblefield RD. Identification of aflatoxins by quadruple mass spectrometry/mass spectrometry. J - Assoc Off Anal Chem. 1984;67:734–738. [PubMed] [Google Scholar]
- 19.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JK. Quality Assurance of Chemical Measurements. Lewis Publishers; Boca Raton, FL: 1987. pp. 79–82. [Google Scholar]
- 21.Alcaide-Molina M, Ruiz-Jiménez J, Mata-Granados JM, Luque de Castro MD. High through-put aflatoxin determination in plant material by automated solid-phase extraction on-line coupled to laser-induced fluorescence screening and determination by liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr A. 2009;1216:1115–1125. doi: 10.1016/j.chroma.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 22.Delmulle B, De Saeger S, Adams A, De Kimpe N, Van Peteghem C. Development of a liquid chromatography/tandem mass spectrometry method for the simultaneous determination of 16 mycotoxins on cellulose filters and in fungal cultures. Rapid Commun Mass Spectrom. 2006;20:771–776. doi: 10.1002/rcm.2373. [DOI] [PubMed] [Google Scholar]
- 23.Liu R, Jin Q, Tao G, Shan L, Liu Y, Wang X. LC-MS and UPLC-quadrupole time-of-flight MS for identification of photodegradation products of aflatoxin B1. Chromatographia. 2010;71:107–112. [Google Scholar]
- 24.AOAC Official Method 966.02-1968 Loss on Drying (Moisture) in Tobacco. AOAC International; Gaithersburg, MD, USA: 1968. [Google Scholar]
- 25.Coresta Recommended Method N° 76 Determination of Moisture Content (Oven Volatiles of Smokeless Tobacco Products. Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA); Paris, France: 2014. [Google Scholar]
- 26.AOAC Official Method 991.31-1994(2002) Aflatoxins in Corn, Raw Peanuts and Peanut Butter. AOAC International; Gaithersburg, MD, USA: 2002. [Google Scholar]
- 27.Kussak A, Andersson B, Andersson K. Automated ample clean-up with solid-phase extraction for the determination of aflatoxins in urine by liquid chromatography. J Chromatogr. 1993;616:235–241. doi: 10.1016/0378-4347(93)80391-g. [DOI] [PubMed] [Google Scholar]
- 28.Richter P, Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tob Res. 2003;5:885–889. doi: 10.1080/14622200310001614647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. UHPLC-MS/MS Analysis Parameters for AFB2, AFG1, and AFG2
Supplemental Table 2. Analytical Performance for AFB1, AFB2, AFG1, and AFG2
Supplemental Table 3. Robustness Testing: Volume of Sample Extract Loaded on IAC Column
Supplemental Table 4. Robustness Testing: Extraction Composition
Supplemental Table 5. Robustness Testing: Mass of Tobacco Extracted
Supplemental Table 6. Robustness Testing: Volume of Elution Solvent Used in IAC Step
Supplemental Table 7. Robustness Testing: Substituting IAC Products