Abstract
This article seeks to determine whether factors related to autoimmunity risk remain significant after the initiation of two or more diabetes-related autoantibodies and continue to contribute to type 1 diabetes (T1D) risk among autoantibody-positive children in The Environmental Determinants of Diabetes in the Young (TEDDY) study. Characteristics included are age at multiple autoantibody positivity, sex, selected high-risk HLA-DR-DQ genotypes, relationship to a family member with T1D, autoantibody at seroconversion, INS gene (rs1004446_A), and non-HLA gene polymorphisms identified by the Type 1 Diabetes Genetics Consortium (T1DGC). The risk of progression to T1D was not different among those with or without a family history of T1D (P = 0.39) or HLA-DR-DQ genotypes (P = 0.74). Age at developing multiple autoantibodies (hazard ratio = 0.96 per 1-month increase in age; 95% CI 0.95, 0.97; P < 0.001) and the type of first autoantibody (when more than a single autoantibody was the first-appearing indication of seroconversion [P = 0.006]) were statistically significant. Female sex was also a significant risk factor (P = 0.03). Three single nucleotide polymorphisms were associated with increased diabetes risk (rs10517086_A [P = 0.03], rs1534422_G [P = 0.006], and rs2327832_G [P = 0.03] in TNFAIP3) and one with decreased risk (rs1004446_A in INS [P = 0.006]). The TEDDY data suggest that non-HLA gene polymorphisms may play a different role in the initiation of autoimmunity than they do in progression to T1D once autoimmunity has appeared. The strength of these associations may be related to the age of the population and the high-risk HLA-DR-DQ subtypes studied.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease preceded by the onset of one of more islet autoantibodies (IA). The presence of two or more autoantibodies is generally felt to increase that risk significantly, especially among young children (1,2). Previous studies have shown that the incidence of T1D is increased in individuals with another family member known to have the disease (3,4). The risk of T1D is on the order of 10-fold higher in first-degree relatives (FDRs) of an individual with T1D as compared with the general population (GP). In addition, it is fairly well established that the incidence of autoimmunity and T1D in individuals with certain HLA loci varies considerably with a gradient that spans the range of highly susceptible to protective loci (5,6). This article examines T1D risk among those individuals who already have developed two or more IA in The Environmental Determinants of Diabetes in the Young (TEDDY) study, a large cohort of genetically at-risk individuals followed from birth with uniform sampling from 3 months of age onward (7,8). It seeks to determine whether factors significant for autoimmunity risk remain significant after the initiation of autoimmunity and continue to contribute to our understanding of the highly variable rate of progression to T1D among autoantibody-positive children.
Research Design and Methods
Participants
TEDDY is a prospective cohort study funded by the National Institutes of Health with the primary goal to identify environmental causes of T1D. It includes six clinical research centers—three in the U.S. (Colorado, Georgia/Florida, and Washington) and three in Europe (Finland, Germany, and Sweden). Detailed study design and methods have been previously published (7–9). Written informed consents were obtained for all study participants from a parent or primary caretaker, separately, for genetic screening and participation in the prospective follow-up. The high-risk genotypes for participants screened from the GP were as follows: DRB1*04-DQA1*03-DQB1*03:02/DRB1*03-DQA1*05-DQB1*02:01 (DR3/4), DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*03:02 (DR4/4), DRB1*04-DQA1*03-DQB1*03:02/DRB1*08-DQA1*04-DQB1*04:02 (DR4/8), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*03-DQA1*05-DQB1*02:01 (DR3/3). Additional genotypes were included for FDRs of a subject with T1D: DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*02:02 (DR4/4b), DRB1*04-DQA1*03-DQB1*03:02/DRB1*01-DQA1*01-DQB1*05:01 (DR4/1), DRB1*04-DQA1*03-DQB1*03:02/DRB1*13-DQA1*01-DQB1*06:04 (DR4/13), DRB1*04-DQA1*03-DQB1*03:02/DRB1*09-DQA1*03-DQB1*03:03 (DR4/9), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*09-DQA1*03-DQB1*03:03 (DR3/9). The HLA-DR-DQ genotype abbreviations shown in parentheses will be used throughout this article. Genotyping was confirmed by reverse blot hybridization at the central HLA Reference Laboratory at Roche Molecular Systems, Oakland, CA (9), along with the INS-23Hph1 (rs689), CTLA4 T17A (rs231775), and PTPN22 R620W (rs2476601) single nucleotide polymorphism (SNP) primer pairs. The study was approved by local institutional review or ethics boards and is monitored by an external evaluation committee formed by the National Institutes of Health.
SNP analysis was performed by the Center for Public Health Genomics at University of Virginia, using the Illumina Immunochip, which is a custom array for genotyping of SNPs selected from regions of the human genome firmly associated with autoimmune diseases (10). The final selection of SNPs containing ∼186,000 SNPs in 186 regions for 12 autoimmune diseases was decided by the Immunochip Consortium. TEDDY previously examined whether any of 41 non-HLA SNPs previously shown to be associated with T1D conferred risk for IA (11). These SNPs were reexamined in relation to the risk of T1D from the time of development of multiple IA.
IA
Islet autoantibodies to insulin (IAA), glutamic acid decarboxylase (GADA), or insulinoma antigen-2 (IA-2A) were measured in two laboratories by radiobinding assays (7,8). In the U.S., all sera were assayed at the Barbara Davis Center for Childhood Diabetes at the University of Colorado, Aurora, CO; in Europe, all sera were assayed at the University of Bristol, Bristol, U.K. Both laboratories demonstrated high sensitivity and specificity as well as concordance (12). All positive IA and 5% of negative samples were retested in the other reference laboratory and deemed confirmed if concordant. Persistent islet autoimmunity was defined as confirmed positive autoantibodies to insulin, GAD65, or IA-2A in at least two consecutive samples.
Statistical Methods
Characteristics of those who progressed to T1D and those who did not are presented for descriptive purposes. Cox proportional hazards models were applied to examine factors related to the risk of progression from the detection of multiple autoantibodies to T1D. The magnitudes of the associations were described by hazard ratios (HR) with 95% CI. Adjustments for population stratification were made by using the top two principal components from the Immunochip SNP data as covariates in the proportional hazards model (13). Data were analyzed using the Statistical Analysis System software (version 9.4; SAS Institute, Cary, NC). Two-tailed P values less than 0.05 were considered to be statistically significant. No adjustment in type 1 error was made for multiple comparisons except in the context of the multiple Cox regression model.
Results
TEDDY enrolled 8,676 children at birth and has followed them quarterly for the appearance of autoantibodies and T1D. Follow-up of children with one or more IA continued on this schedule, whereas children who were autoantibody negative were followed semiannually after 4 years of age. Excluded from this analysis are 172 children who were either ineligible or whose autoantibody status was indeterminate. The median (interquartile range [IQR]) age at last follow-up was 8.0 (6.7–9.3) years.
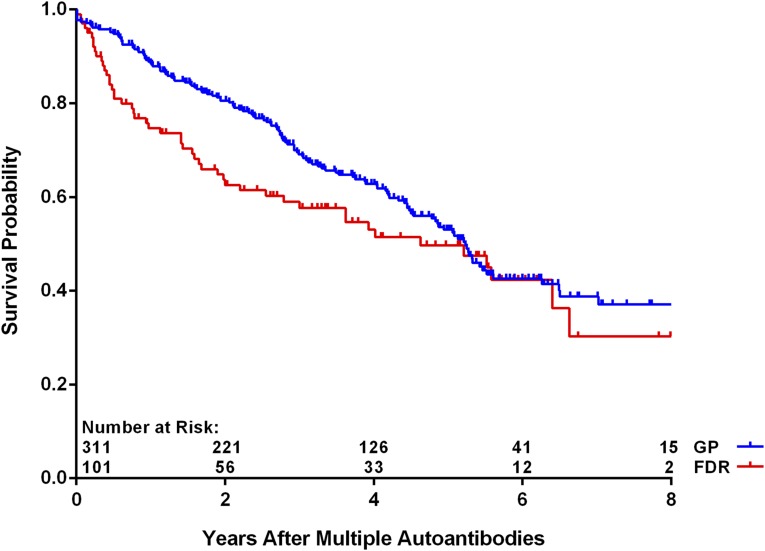
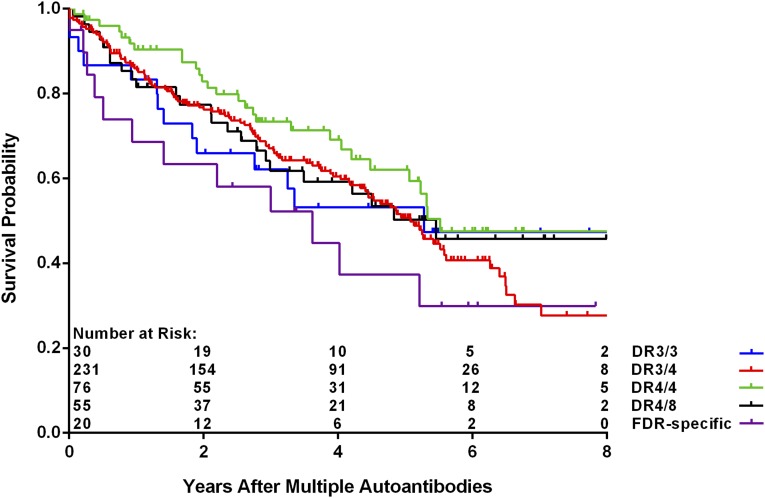
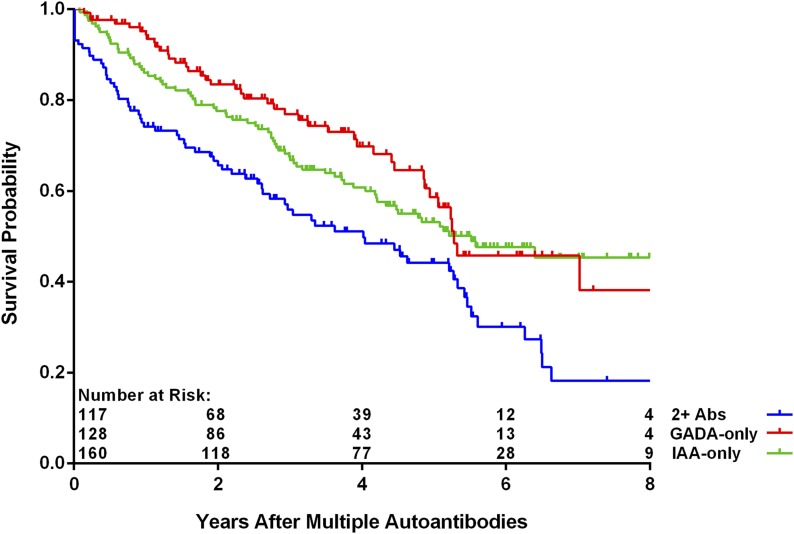
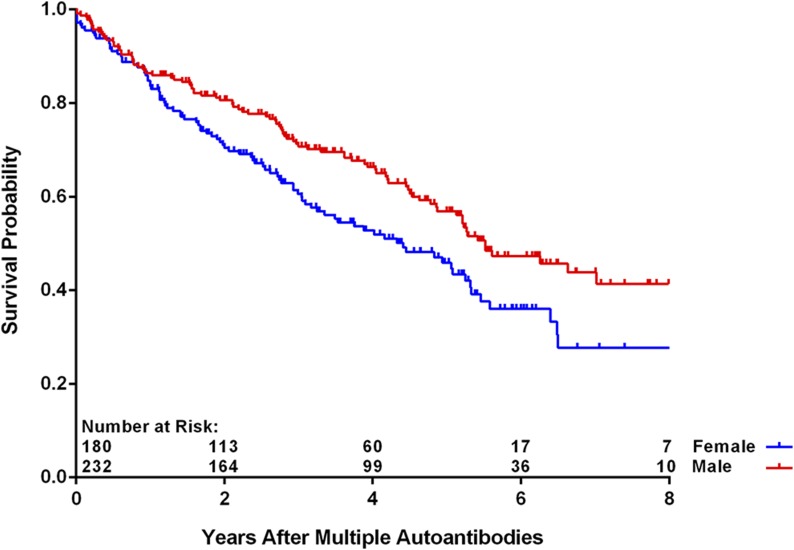
As of 30 June 2016, 412 children (4.8%) have developed multiple persistent confirmed IA, and of these, 190 (46.1%) have progressed to T1D (Table 1). The median (IQR) duration of follow-up from the appearance of multiple autoantibodies was 3.0 (1.4–5.1) years. The age at which multiple autoantibodies first appeared was associated with increased risk of progression to T1D (P < 0.001), as was the appearance of multiple autoantibodies at first appearance (P = 0.006). The risk to progress to T1D was not significantly different when the data were analyzed by country of residence, family history, sex, and HLA-DR-DQ genotype (P = NS). A multiple Cox regression analysis of these same characteristics confirmed the lack of statistical significance associated with family history (FDR vs. GP) (Fig. 1) (P = 0.39) or HLA-DR-DQ genotype (P = 0.74) (Fig. 2). Relationship of the TEDDY child to the family member with T1D among the FDRs compared with GP was also not significantly different (offspring of father with T1D [P = 0.29], mother [P = 0.42], or sibling [P = 0.96]) (Table 2). Age at multiple autoantibodies (HR = 0.96 per 1-month increase in age; 95% CI 0.95, 0.97; P < 0.001) and when more than a single autoantibody was first-appearing indication of seroconversion (HR = 1.66 compared with IAA only [P = 0.006]) were statistically significant (Fig. 3). In the multiple Cox regression, female (as compared with male) sex became a significant risk factor (HR = 1.43; 95% CI 1.04, 1.96; P = 0.03) (Fig. 4).
Table 1.
Characteristics of children who progressed from multiple autoantibodies to T1D and those who did not
| Did not progress to T1D | Progressed to T1D | |
|---|---|---|
| Total, n (%) |
222 (54) |
190 (46) |
| Country of residence, n (%) |
||
| U.S. |
82 (59) |
57 (41) |
| Finland |
53 (48) |
57 (52) |
| Germany |
16 (44) |
20 (56) |
| Sweden |
71 (56) |
56 (44) |
| Family history of T1D, n (%) |
||
| GP |
171 (55) |
140 (45) |
| FDR: mother |
12 (50) |
12 (50) |
| FDR: father |
27 (50) |
27 (50) |
| FDR: sibling |
12 (52) |
11 (48) |
| Sex, n (%) |
||
| Female |
88 (49) |
92 (51) |
| Male |
134 (58) |
98 (42) |
| HLA-DR-DQ genotypes, n (%) |
||
| DR3/4 |
121 (52) |
110 (48) |
| DR4/4 |
46 (61) |
30 (39) |
| DR4/8 |
31 (56) |
24 (44) |
| DR3/3 |
16 (53) |
14 (47) |
| FDR specific |
8 (40) |
12 (60) |
| Age at multiple persistent confirmed IA (months), median (IQR) |
48 (31–74) |
21 (15–31) |
| Type of first autoantibody, n (%) |
||
| GADA only |
85 (66) |
43 (34) |
| IAA only |
84 (53) |
76 (47) |
| Two or more autoantibodies |
49 (42) |
68 (58) |
| IA-2A only | 4 (57) | 3 (43) |
FDR-specific HLA-DR-DQ genotypes are DR4/4b, DR4/1, DR4/13, DR4/9, and DR3/9.
Figure 1.
Progression from multiple autoantibodies to T1D by FDR status (P = 0.39 from Cox regression).
Figure 2.
Progression from multiple autoantibodies to T1D by HLA-DR-DQ genotypes (P = 0.74 from Cox regression). FDR-specific HLA-DR-DQ genotypes are DR4/4b, DR4/1, DR4/13, DR4/9, and DR3/9.
Table 2.
Cox regression analysis of risk factors for progression from multiple autoantibodies to T1D
| HR (95% CI) | P | |
|---|---|---|
| Age at multiple autoantibodies onset (months) |
0.96 (0.95, 0.97) |
<0.001 |
| HLA-DR-DQ genotype |
0.74 |
|
| DR3/4 |
1.24 (0.79, 1.93) |
0.35 |
| DR4/4 |
1 [Reference] |
|
| DR4/8 |
1.22 (0.68, 2.18) |
0.50 |
| DR3/3 |
1.44 (0.70, 2.96) |
0.32 |
| FDR specific |
1.58 (0.73, 3.41) |
0.25 |
| Family history of T1D |
0.69 |
|
| FDR: mother |
1.34 (0.66, 2.75) |
0.42 |
| FDR: father |
1.30 (0.80, 2.09) |
0.29 |
| FDR: sibling |
0.98 (0.48, 2.01) |
0.96 |
| GP |
1 [Reference] |
|
| Type of first autoantibody |
0.02 |
|
| GADA only |
1.16 (0.76, 1.78) |
0.49 |
| IAA only |
1 [Reference] |
|
| Two or more autoantibodies |
1.66 (1.15, 2.39) |
0.006 |
| Sex |
||
| Female |
1.43 (1.04, 1.96) |
0.03 |
| Male |
1 [Reference] |
|
| Country of residence |
0.84 |
|
| U.S. |
1 [Reference] |
|
| Finland |
1.05 (0.53, 2.10) |
0.89 |
| Germany |
1.13 (0.59, 2.14) |
0.71 |
| Sweden |
0.88 (0.58, 1.34) |
0.55 |
| SNP rs1004446_A (INS) |
0.71 (0.55, 0.91) |
0.006 |
| SNP rs10517086_A |
1.31 (1.03, 1.67) |
0.03 |
| SNP rs1534422_G |
1.39 (1.10, 1.76) |
0.006 |
| SNP rs2327832_G (TNFAIP3) |
1.34 (1.03, 1.74) |
0.03 |
| PC1 |
1.11 (0.91, 1.35) |
0.32 |
| PC2 | 0.96 (0.72, 1.28) | 0.78 |
The top two principal components (PC1 and PC2) from the principal components analysis on Immunochip data were included as covariates to correct for population stratification. FDR-specific HLA-DR-DQ genotypes are DR4/4b, DR4/1, DR4/13, DR4/9, and DR3/9.
Figure 3.
Progression from multiple autoantibodies to T1D by type of first autoantibody (Ab) (P = 0.02 from Cox regression).
Figure 4.
Progression from multiple autoantibodies to T1D by sex (P = 0.03 from Cox regression).
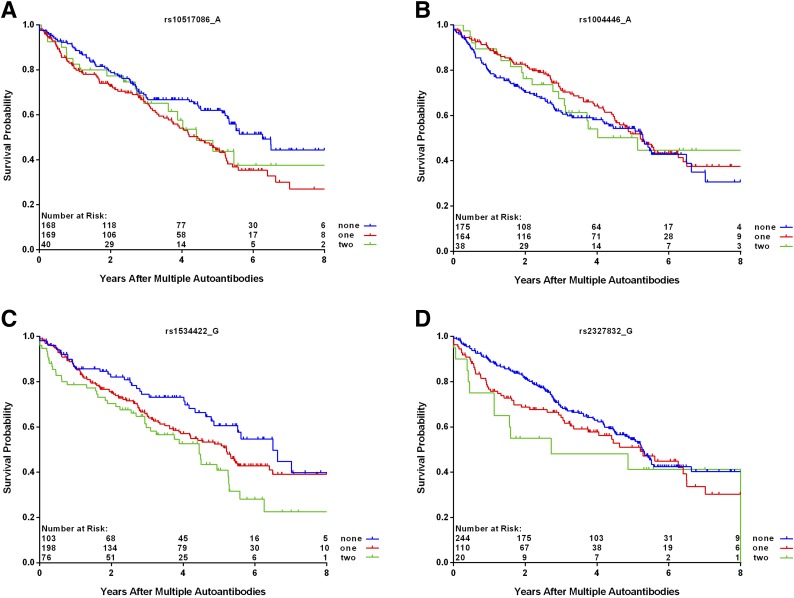
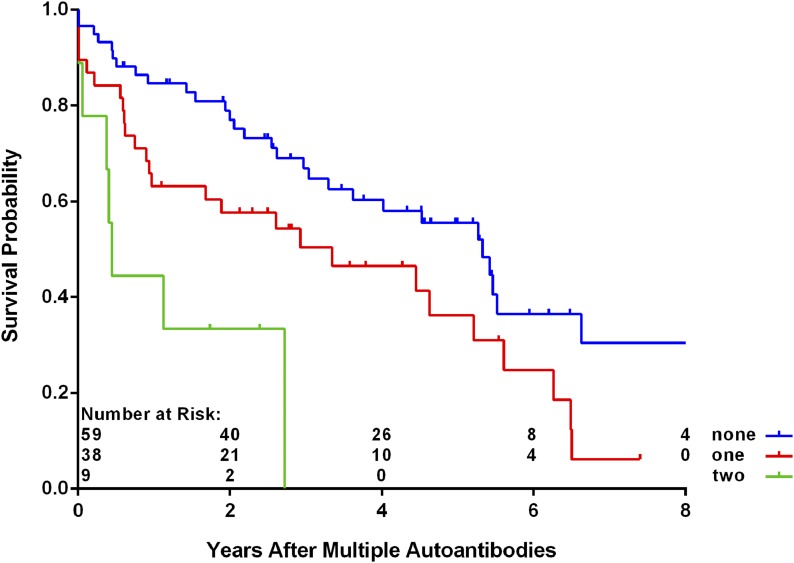
Among those with multiple autoantibodies, SNPs rs10517086_A (P = 0.03), rs1534422_G (P = 0.006), and rs2327832_G in TNFAIP3 (P = 0.03) were significantly associated with increased risk of progression to T1D, and SNP rs1004446_A in INS (P = 0.006) was associated with decreased risk (Table 2 and Fig. 5). There was a significant interaction between the SNP rs2327832_G in TNFAIP3 and the type of first autoantibody (P = 0.003), indicating much higher risk of T1D with rs2327832_G polymorphism in the subjects who had the appearance of multiple autoantibodies as the first indication of seroconversion (HR = 2.37; 95% CI 1.52, 3.70; P < 0.001) (Fig. 6). No interaction was found between the other SNPs and first-appearing autoantibody. A table of all SNPs included in this analysis appears in the Supplementary Data.
Figure 5.
Progression from multiple autoantibodies by number of minor alleles of SNPs within panels rs10517086_A (P = 0.03 from Cox regression) (A), rs1004446_A (P = 0.006 from Cox regression) (B), rs1534422_G (P = 0.006 from Cox regression) (C), and rs2327832_G (P = 0.03 from Cox regression) (D).
Figure 6.
Progression from multiple autoantibodies to T1D by number of minor alleles of SNP rs2327832_G in the subset of more than one autoantibody as first-appearing autoantibody (P < 0.001 from Cox regression).
Discussion
Although HLA-DR-DQ haplotypes have been shown to be associated with the incidence of autoimmunity, our data do not show that it continues to be related to progression to T1D in the HLA-selected high-risk TEDDY cohort among those who have multiple diabetes-related autoantibodies. As well, the risk of progression to T1D was not different among those with or without a family history of T1D for the high-risk genotypes followed in TEDDY. TEDDY has previously shown (14) that family history of T1D is a significant risk factor for progression to T1D by 5 years of age among those who initially seroconvert to two autoantibodies, but not among those who initially seroconvert to three autoantibodies. The results reported herein, now with additional follow-up to a median of 8 years of age, indicate that family history is no longer significant among those with two or more antibodies from the time of becoming multiple autoantibody positive.
Despite the lack of association with HLA-DR-DQ, we did find three SNPs that were associated with increased diabetes risk and one associated with decreased risk. Only SNP rs1004446_A in INS was reported to be a significantly protective SNP of T1D from birth in TEDDY overall and in this multiple autoantibody–positive subset. The other SNPs tested were not significantly related to T1D in the multiple autoantibody–positive population, despite their association with autoimmunity in TEDDY and diabetes in the Type 1 Diabetes Genetics Consortium (T1DGC) (15), suggesting a genetic contribution to progression to diabetes after the appearance of autoantibodies that might be different than in the initiation of autoimmunity.
Of note, the three SNPs associated with an increased diabetes risk in this population of children with multiple diabetes-related autoantibodies were not associated with T1D in TEDDY overall, despite their significant association reported by others (16). This might be due to the fact that the TEDDY study is limited to certain at-risk HLA subgroups or that the SNPs play a role in progression of autoimmunity toward T1D but not in initiation of autoimmunity. SNP rs1534422_G in TNFAIP3 has also been recently shown to be associated with multiple sclerosis risk (17), which is also an autoinflammatory disease with gene–environment risk factors involving the HLA locus. SNP rs2327832_G has been reported to be a risk factor for rheumatoid arthritis (18) and celiac disease (19,20), whereas SNP rs10517086_A has been shown to have an age-related association with IA with increased risk in children under the age of 2 years (21).
Here, we show evidence of increased risk for T1D in multiple autoantibody–positive children. These findings are similar to those reported by Lempainen et al. (22) in the Finnish Diabetes Prediction and Prevention (DIPP) study that also showed a lack of association with FDR status or HLA and progression to diabetes but a positive association with female sex in children positive for two islet autoantibodies. A difference in the findings between the two studies is that the DIPP study reports a significant association of the PTPN22 gene polymorphism with progression from multiple autoantibodies to T1D, whereas the TEDDY study does not. In contrast, the TEDDY study does find an association with the INS gene, but the DIPP does not. Similar to TEDDY, the INS gene, but not PTPN22, was among five genes that, together, stratified progression to disease in the German BABYDIAB and BABYDIET studies (23). The reported differences could be related to the populations, as the Finnish population has a higher prevalence of the PTPN22 gene polymorphism than elsewhere. DIPP, Diabetes Autoimmunity Study in the Young (DAISY), and the German BABYDIAB and BABYDIET studies (2,24) report similar findings with regard to appearance of multiple autoantibodies at a young age and the excess risk associated with female sex. Others have speculated a link between the observed protective effect of the INS gene and immune tolerance through higher levels of expression in the thymus as a plausible mechanism (25).
The TEDDY data suggest that non-HLA gene polymorphisms may play a different role in the initiation of autoimmunity than they do in progression to T1D once autoimmunity has appeared. The strength of these associations and even their direction (increased vs. decreased risk) may vary by population and the nature of the other characteristics included in multivariate models. Although these results extend earlier TEDDY findings by providing additional years of follow-up, it may be that the relationships described are all age related. Cases of T1D diagnosed among older children may share the same mechanisms and strengthen these findings or may be the result of other immunological insults involving other exposures and gene–environment interactions. Having already published an age effect on the initiation of autoimmunity and differences in the pattern of the types of autoantibodies that arise first (26), it is not inconceivable that there is also an age-related association of exposures and both HLA and non-HLA genes. Caution should be exercised in generalizing the results presented here beyond the age range in which they have been discovered and the selected HLA subgroups that constitute the TEDDY population. As well, caution should be exercised in interpreting statistically significant findings due to the number of comparisons that have been made. Adjusting the significance level for multiple comparisons when conducting epidemiological research, especially in the context of a multivariate analysis, has both supporters (27) and detractors (28,29). No matter what side of the argument the reader falls on, the associations reported here should be viewed in the larger context of the results of other studies and other populations to be properly interpreted.
Supplementary Material
Article Information
Acknowledgments. A special acknowledgment to the TEDDY families for their continued participation in this wonderful study.
Funding. This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, and UC4 DK95300) and by the National Institutes of Health (HHSN267200700014C). This work was also funded by National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, JDRF, and Centers for Disease Control and Prevention. This work was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors attest to meeting the International Committee of Medical Journal Editors uniform requirements for authorship by making substantial contributions to conception and design of this paper; acquisitioning, analyzing, and interpreting the data; drafting or revising the article for intellectual content; and giving final approval of the published version. J.P.K. designed the study, proposed the analysis, interpreted the findings, and wrote the manuscript. X.L. performed the analysis and contributed to the manuscript. Å.L., W.A.H., M.J.R., J.-X.S., J.T., A.-G.Z., and B.A. designed the study and reviewed or edited the manuscript. J.P.K. and X.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0261/-/DC1.
A complete list of the TEDDY Study Group can be found in the Supplementary Data online.
References
- 1.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cernea S, Dobreanu M, Raz I. Prevention of type 1 diabetes: today and tomorrow. Diabetes Metab Res Rev 2010;26:602–605 [DOI] [PubMed] [Google Scholar]
- 4.Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev 2008;29:697–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics 2013;132:1112–1122 [DOI] [PubMed] [Google Scholar]
- 6.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet 2016;387:2331–2339 [DOI] [PubMed] [Google Scholar]
- 7.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 8.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet 2013;14:661–673 [DOI] [PubMed] [Google Scholar]
- 11.Törn C, Hadley D, Lee HS, et al.; TEDDY Study Group . Role of type 1 diabetes associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 2015;64:1818–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Concannon P, Chen WM, Julier C, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide scan for linkage to type 1 diabetes in 2,496 multiplex families from the Type 1 Diabetes Genetics Consortium. Diabetes 2009;58:1018–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett JC, Clayton DG, Concannon P, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38:904–909 [DOI] [PubMed] [Google Scholar]
- 17.Lill CM, Luessi F, Alcina A, et al. Genome-wide significant association with seven novel multiple sclerosis risk loci. J Med Genet 2015;52:848–855 [DOI] [PubMed] [Google Scholar]
- 18.Elsby LM, Orozco G, Denton J, Worthington J, Ray DW, Donn RP. Functional evaluation of TNFAIP3 (A20) in rheumatoid arthritis. Clin Exp Rheumatol 2010;28:708–714 [PMC free article] [PubMed] [Google Scholar]
- 19.Izzo V, Pinelli M, Tinto N, et al. Improving the estimation of celiac disease sibling risk by non-HLA genes. PLoS One 2011;6:e26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 2008;359:2767–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederiksen BN, Steck AK, Kroehl M, et al. Evidence of stage- and age-related heterogeneity of non-HLA SNPs and risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Clin Dev Immunol 2013;2013:417657 [DOI] [PMC free article] [PubMed]
- 22.Lempainen J, Hermann R, Veijola R, Simell O, Knip M, Ilonen J. Effect of the PTPN22 and INS risk genotypes on the progression to clinical type 1 diabetes after the initiation of β-cell autoimmunity. Diabetes 2012;61:963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifacio E, Krumsiek J, Winkler C, Theis FJ, Ziegler AG. A strategy to find gene combinations that identify children who progress rapidly to type 1 diabetes after islet autoantibody seroconversion. Acta Diabetol 2014;51:403–411 [DOI] [PubMed] [Google Scholar]
- 24.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler AG. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med 2004;140:882–886 [DOI] [PubMed] [Google Scholar]
- 25.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15:289–292 [DOI] [PubMed] [Google Scholar]
- 26.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JR. Invited commentary: Re: “Multiple comparisons and related issues in the interpretation of epidemiologic data.” Am J Epidemiol 1998;147:801–806 [DOI] [PubMed] [Google Scholar]
- 28.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol 1995;142:904–908 [DOI] [PubMed] [Google Scholar]
- 29.Savitz DA, Olshan AF. Describing data requires no adjustment for multiple comparisons: a reply from Savitz and Olshan. Am J Epidemiol 1998;147:813–814; discussion 815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.